Abstract
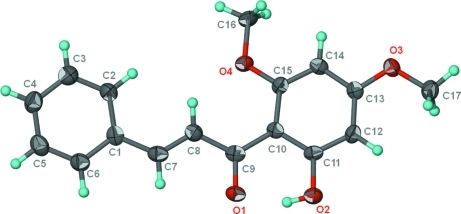
The title compound [systematic name: (E)-1-(2-hydroxy-4,6-dimethoxyphenyl)-3-phenylprop-2-en-1-one], C17H16O4, has an aromatic ring at both ends of the –CH= CH–C(=O)– fragment with the –CH=CH– bond in a trans configuration. The phenyl ring is nearly coplanar with this fragment [dihedral angle 4.8 (3) °] as is the hydroxyldimethoxylphenyl unit [dihedral angle 6.3 (3) °]. The hydroxy group is the donor in an intramolecular hydrogen bond to the double-bonded O atom.
Related literature
For the isolation and spectroscopic characterization of the title compound, see: Flores et al. (2007 ▶); Xuan et al. (2008 ▶).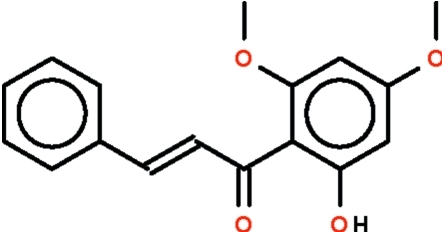
Experimental
Crystal data
C17H16O4
M r = 284.30
Orthorhombic,
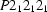
a = 4.9668 (10) Å
b = 12.305 (3) Å
c = 22.552 (5) Å
V = 1378.3 (5) Å3
Z = 4
Mo Kα radiation
μ = 0.10 mm−1
T = 100 K
0.40 × 0.10 × 0.05 mm
Data collection
Bruker SMART APEX diffractometer
10723 measured reflections
1449 independent reflections
1162 reflections with I > 2σ(I)
R int = 0.089
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.128
S = 1.09
1449 reflections
197 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.20 e Å−3
Δρmin = −0.27 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810041395/fl2321sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041395/fl2321Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O2—H2⋯O1 | 0.85 (1) | 1.65 (2) | 2.455 (4) | 157 (4) |
Acknowledgments
We thank the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
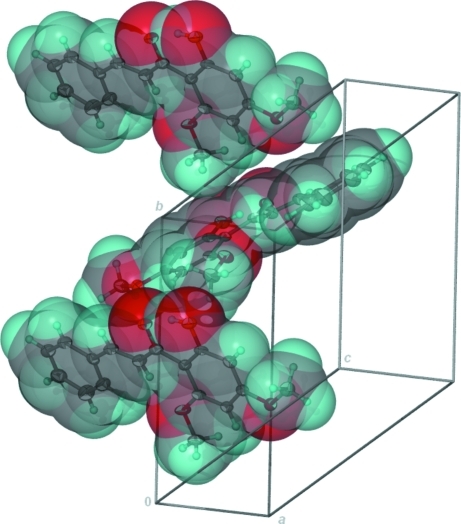
Alpinia mutica is a perennial herb (Zingiberaceae) endemic to southern parts of Malaysia. It is also cultivated as an ornamental plant and the rhizomes are used as an herb for strengthening the stomach. Among the compounds isolated this herb is Flavokavain B, whose structure was elucidated by spectrospic methods (Flores et al., 2007; Xuan et al., 2008) and whose x-ray structure is reported here. The chalcone (Scheme I) has aromatic rings at either ends of the –CH═ CH–C(═O)– linkage; the -CH=CH- double bond has a trans configuration. The phenyl ring is nearly coplanar with this fragment [dihedral angle 4.8 (3) °] as is the hydroxyldimethoxylphenyl ring [dihderal angle 6.3 (3) ° (the dihedral angle between the two rings is 10.0 (2)°) (Fig. 1). The hydroxy group is donor in an intra-molecular H-bond bond with the double-bond oxygen atom of the fragment. Other than a close contact of 3.04 Å between O2 and C16, there are no important intermolecular contacts (Fig. 2).
The compound has been previously isolated and characterized by NMR spectroscopy (Flores et al., 2007; Xuan et al., 2008).
Experimental
The rhizome of Alpinia mutica was collected from Pontian, Johor, Malaysia. A voucher specimen was deposited at the Herbarium of the Departmentof Botany, Universiti Putra Malaysia. The n-hexane crude extract of the rhizome (8.97 g) was subjected to silica-gel chromatography and was eluted out by using a gradient mixture of petroleum ether, ethanol and methanol. Twenty three fractions were collected, which were then separated by TLC to afford eight fractions. The sixth fraction was subjected to silica-gel column chromatography to give the title compound (petroleum ether: ether 4/1)which was recrystallized from petroleum ether/ether to afford faint yellow-orange crystals suitable for data collection.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95–0.98 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2–15U(C).
The hydroxy H-atom was located in a difference Fourier map, and was refined with the O–H distance restrained to 0.84±0.01 Å; its temperature factor was refined.
In the absence of heavy atom, Some 976 Friedel pairs were merged.
Figures
Fig. 1.
Thermal ellipsoid plot (Barbour, 2001) of the C17H16O4 at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
Close approach of ajacent molecules.
Crystal data
| C17H16O4 | F(000) = 600 |
| Mr = 284.30 | Dx = 1.370 Mg m−3 |
| Orthorhombic, P212121 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 1135 reflections |
| a = 4.9668 (10) Å | θ = 3.2–21.7° |
| b = 12.305 (3) Å | µ = 0.10 mm−1 |
| c = 22.552 (5) Å | T = 100 K |
| V = 1378.3 (5) Å3 | Prism, faint yellow |
| Z = 4 | 0.40 × 0.10 × 0.05 mm |
Data collection
| Bruker SMART APEX diffractometer | 1162 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.089 |
| graphite | θmax = 25.0°, θmin = 1.8° |
| ω scans | h = −5→5 |
| 10723 measured reflections | k = −14→14 |
| 1449 independent reflections | l = −26→25 |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.049 | H atoms treated by a mixture of independent and constrained refinement |
| wR(F2) = 0.128 | w = 1/[σ2(Fo2) + (0.0736P)2 + 0.021P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.09 | (Δ/σ)max = 0.001 |
| 1449 reflections | Δρmax = 0.20 e Å−3 |
| 197 parameters | Δρmin = −0.26 e Å−3 |
| 1 restraint | Extinction correction: SHELXL, Fc*=kFc[1+0.001xFc2λ3/sin(2θ)]-1/4 |
| Primary atom site location: structure-invariant direct methods | Extinction coefficient: 0.026 (5) |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.5688 (6) | 0.96830 (19) | 0.22125 (11) | 0.0250 (7) | |
| O2 | 0.3291 (6) | 0.9379 (2) | 0.12751 (11) | 0.0260 (7) | |
| H2 | 0.440 (7) | 0.958 (4) | 0.1541 (14) | 0.048 (15)* | |
| O3 | −0.3912 (5) | 0.68445 (19) | 0.12028 (10) | 0.0245 (7) | |
| O4 | 0.0995 (6) | 0.72577 (18) | 0.29718 (11) | 0.0272 (7) | |
| C1 | 0.7327 (8) | 0.9257 (3) | 0.40417 (17) | 0.0228 (9) | |
| C2 | 0.5796 (9) | 0.8616 (3) | 0.44201 (16) | 0.0283 (10) | |
| H2A | 0.4369 | 0.8191 | 0.4262 | 0.034* | |
| C3 | 0.6311 (10) | 0.8586 (3) | 0.50210 (18) | 0.0349 (10) | |
| H3 | 0.5230 | 0.8150 | 0.5274 | 0.042* | |
| C4 | 0.8406 (9) | 0.9191 (3) | 0.52537 (17) | 0.0323 (11) | |
| H4 | 0.8762 | 0.9177 | 0.5668 | 0.039* | |
| C5 | 0.9976 (9) | 0.9815 (3) | 0.48823 (18) | 0.0310 (10) | |
| H5 | 1.1418 | 1.0229 | 0.5042 | 0.037* | |
| C6 | 0.9476 (9) | 0.9846 (3) | 0.42798 (17) | 0.0279 (10) | |
| H6 | 1.0594 | 1.0267 | 0.4027 | 0.034* | |
| C7 | 0.6751 (8) | 0.9328 (3) | 0.34039 (17) | 0.0257 (9) | |
| H7 | 0.7994 | 0.9723 | 0.3167 | 0.031* | |
| C8 | 0.4658 (8) | 0.8891 (3) | 0.31275 (16) | 0.0256 (9) | |
| H8 | 0.3381 | 0.8493 | 0.3354 | 0.031* | |
| C9 | 0.4237 (8) | 0.8998 (3) | 0.24859 (16) | 0.0230 (9) | |
| C10 | 0.2169 (8) | 0.8393 (3) | 0.21647 (16) | 0.0196 (8) | |
| C11 | 0.1737 (8) | 0.8639 (3) | 0.15591 (16) | 0.0208 (9) | |
| C12 | −0.0285 (8) | 0.8174 (3) | 0.12220 (16) | 0.0230 (9) | |
| H12 | −0.0586 | 0.8392 | 0.0823 | 0.028* | |
| C13 | −0.1857 (8) | 0.7377 (3) | 0.14850 (16) | 0.0213 (9) | |
| C14 | −0.1454 (8) | 0.7058 (3) | 0.20702 (16) | 0.0221 (8) | |
| H14 | −0.2544 | 0.6504 | 0.2238 | 0.027* | |
| C15 | 0.0513 (8) | 0.7543 (3) | 0.24038 (15) | 0.0211 (9) | |
| C16 | −0.0619 (9) | 0.6404 (3) | 0.32205 (16) | 0.0298 (10) | |
| H16A | −0.0115 | 0.6292 | 0.3636 | 0.045* | |
| H16B | −0.0320 | 0.5731 | 0.2998 | 0.045* | |
| H16C | −0.2525 | 0.6606 | 0.3197 | 0.045* | |
| C17 | −0.4398 (9) | 0.7113 (3) | 0.05989 (15) | 0.0287 (10) | |
| H17A | −0.5907 | 0.6679 | 0.0449 | 0.043* | |
| H17B | −0.2783 | 0.6957 | 0.0364 | 0.043* | |
| H17C | −0.4839 | 0.7887 | 0.0567 | 0.043* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0269 (15) | 0.0177 (12) | 0.0304 (15) | −0.0032 (12) | 0.0027 (13) | 0.0014 (11) |
| O2 | 0.0313 (17) | 0.0199 (13) | 0.0266 (16) | −0.0038 (13) | 0.0000 (14) | 0.0034 (12) |
| O3 | 0.0254 (15) | 0.0218 (13) | 0.0263 (14) | −0.0010 (12) | −0.0042 (13) | −0.0008 (11) |
| O4 | 0.0393 (17) | 0.0180 (12) | 0.0243 (14) | −0.0075 (13) | −0.0017 (13) | 0.0058 (11) |
| C1 | 0.026 (2) | 0.0154 (19) | 0.027 (2) | 0.0059 (17) | −0.0030 (18) | −0.0031 (16) |
| C2 | 0.031 (2) | 0.0254 (19) | 0.028 (2) | −0.006 (2) | −0.002 (2) | −0.0019 (17) |
| C3 | 0.038 (3) | 0.037 (2) | 0.030 (2) | −0.006 (2) | 0.001 (2) | 0.0001 (19) |
| C4 | 0.044 (3) | 0.026 (2) | 0.027 (2) | 0.001 (2) | −0.006 (2) | 0.0006 (17) |
| C5 | 0.035 (3) | 0.021 (2) | 0.037 (2) | −0.0020 (19) | −0.006 (2) | −0.0040 (17) |
| C6 | 0.032 (2) | 0.0211 (19) | 0.031 (2) | −0.0026 (19) | −0.0017 (19) | 0.0021 (17) |
| C7 | 0.028 (2) | 0.0173 (18) | 0.032 (2) | −0.0015 (18) | 0.005 (2) | 0.0007 (17) |
| C8 | 0.030 (2) | 0.0205 (19) | 0.026 (2) | −0.0034 (17) | −0.0005 (19) | −0.0006 (16) |
| C9 | 0.024 (2) | 0.0154 (16) | 0.029 (2) | 0.0021 (17) | 0.0054 (19) | −0.0024 (16) |
| C10 | 0.022 (2) | 0.0140 (17) | 0.023 (2) | 0.0009 (15) | 0.0026 (17) | −0.0038 (15) |
| C11 | 0.025 (2) | 0.0115 (17) | 0.025 (2) | 0.0016 (16) | 0.0052 (18) | 0.0015 (15) |
| C12 | 0.030 (2) | 0.0202 (19) | 0.0191 (19) | 0.0022 (17) | −0.0020 (18) | −0.0002 (15) |
| C13 | 0.024 (2) | 0.0159 (17) | 0.025 (2) | 0.0029 (16) | −0.0010 (17) | −0.0036 (15) |
| C14 | 0.022 (2) | 0.0158 (16) | 0.029 (2) | 0.0009 (16) | 0.0002 (18) | 0.0005 (15) |
| C15 | 0.030 (2) | 0.0139 (16) | 0.0197 (18) | 0.0015 (16) | 0.0000 (18) | −0.0011 (14) |
| C16 | 0.043 (3) | 0.0207 (18) | 0.026 (2) | −0.0075 (19) | −0.002 (2) | 0.0089 (16) |
| C17 | 0.036 (3) | 0.027 (2) | 0.024 (2) | −0.001 (2) | −0.001 (2) | −0.0043 (16) |
Geometric parameters (Å, °)
| O1—C9 | 1.269 (4) | C7—C8 | 1.326 (5) |
| O2—C11 | 1.354 (4) | C7—H7 | 0.9500 |
| O2—H2 | 0.85 (1) | C8—C9 | 1.468 (5) |
| O3—C13 | 1.370 (5) | C8—H8 | 0.9500 |
| O3—C17 | 1.422 (4) | C9—C10 | 1.461 (5) |
| O4—C15 | 1.349 (4) | C10—C11 | 1.415 (5) |
| O4—C16 | 1.436 (4) | C10—C15 | 1.436 (5) |
| C1—C2 | 1.389 (5) | C11—C12 | 1.384 (5) |
| C1—C6 | 1.397 (5) | C12—C13 | 1.386 (5) |
| C1—C7 | 1.469 (5) | C12—H12 | 0.9500 |
| C2—C3 | 1.380 (5) | C13—C14 | 1.391 (5) |
| C2—H2A | 0.9500 | C14—C15 | 1.370 (5) |
| C3—C4 | 1.383 (6) | C14—H14 | 0.9500 |
| C3—H3 | 0.9500 | C16—H16A | 0.9800 |
| C4—C5 | 1.378 (6) | C16—H16B | 0.9800 |
| C4—H4 | 0.9500 | C16—H16C | 0.9800 |
| C5—C6 | 1.382 (5) | C17—H17A | 0.9800 |
| C5—H5 | 0.9500 | C17—H17B | 0.9800 |
| C6—H6 | 0.9500 | C17—H17C | 0.9800 |
| C11—O2—H2 | 103 (3) | C11—C10—C15 | 115.5 (3) |
| C13—O3—C17 | 117.4 (3) | C11—C10—C9 | 118.4 (3) |
| C15—O4—C16 | 117.5 (3) | C15—C10—C9 | 126.0 (3) |
| C2—C1—C6 | 118.5 (4) | O2—C11—C12 | 115.6 (3) |
| C2—C1—C7 | 121.9 (4) | O2—C11—C10 | 120.9 (4) |
| C6—C1—C7 | 119.6 (4) | C12—C11—C10 | 123.5 (3) |
| C3—C2—C1 | 121.1 (4) | C11—C12—C13 | 117.8 (3) |
| C3—C2—H2A | 119.4 | C11—C12—H12 | 121.1 |
| C1—C2—H2A | 119.4 | C13—C12—H12 | 121.1 |
| C2—C3—C4 | 119.8 (4) | O3—C13—C12 | 124.0 (3) |
| C2—C3—H3 | 120.1 | O3—C13—C14 | 114.4 (3) |
| C4—C3—H3 | 120.1 | C12—C13—C14 | 121.6 (4) |
| C5—C4—C3 | 119.7 (4) | C15—C14—C13 | 120.0 (4) |
| C5—C4—H4 | 120.2 | C15—C14—H14 | 120.0 |
| C3—C4—H4 | 120.2 | C13—C14—H14 | 120.0 |
| C4—C5—C6 | 120.7 (4) | O4—C15—C14 | 122.3 (3) |
| C4—C5—H5 | 119.6 | O4—C15—C10 | 116.4 (3) |
| C6—C5—H5 | 119.6 | C14—C15—C10 | 121.3 (3) |
| C5—C6—C1 | 120.1 (4) | O4—C16—H16A | 109.5 |
| C5—C6—H6 | 120.0 | O4—C16—H16B | 109.5 |
| C1—C6—H6 | 120.0 | H16A—C16—H16B | 109.5 |
| C8—C7—C1 | 126.1 (4) | O4—C16—H16C | 109.5 |
| C8—C7—H7 | 117.0 | H16A—C16—H16C | 109.5 |
| C1—C7—H7 | 117.0 | H16B—C16—H16C | 109.5 |
| C7—C8—C9 | 122.6 (4) | O3—C17—H17A | 109.5 |
| C7—C8—H8 | 118.7 | O3—C17—H17B | 109.5 |
| C9—C8—H8 | 118.7 | H17A—C17—H17B | 109.5 |
| O1—C9—C10 | 119.8 (3) | O3—C17—H17C | 109.5 |
| O1—C9—C8 | 117.2 (4) | H17A—C17—H17C | 109.5 |
| C10—C9—C8 | 122.9 (3) | H17B—C17—H17C | 109.5 |
| C6—C1—C2—C3 | 2.3 (6) | C15—C10—C11—C12 | 5.5 (5) |
| C7—C1—C2—C3 | −177.9 (4) | C9—C10—C11—C12 | −175.4 (3) |
| C1—C2—C3—C4 | −0.8 (6) | O2—C11—C12—C13 | 177.2 (3) |
| C2—C3—C4—C5 | −0.4 (6) | C10—C11—C12—C13 | −4.0 (5) |
| C3—C4—C5—C6 | 0.2 (6) | C17—O3—C13—C12 | 1.6 (5) |
| C4—C5—C6—C1 | 1.3 (6) | C17—O3—C13—C14 | −178.6 (3) |
| C2—C1—C6—C5 | −2.5 (6) | C11—C12—C13—O3 | −179.6 (3) |
| C7—C1—C6—C5 | 177.7 (4) | C11—C12—C13—C14 | 0.7 (5) |
| C2—C1—C7—C8 | 6.2 (6) | O3—C13—C14—C15 | −178.9 (3) |
| C6—C1—C7—C8 | −173.9 (4) | C12—C13—C14—C15 | 0.8 (6) |
| C1—C7—C8—C9 | −179.8 (4) | C16—O4—C15—C14 | 0.8 (5) |
| C7—C8—C9—O1 | −12.0 (5) | C16—O4—C15—C10 | −179.6 (3) |
| C7—C8—C9—C10 | 170.8 (4) | C13—C14—C15—O4 | −179.6 (3) |
| O1—C9—C10—C11 | −3.8 (5) | C13—C14—C15—C10 | 0.9 (6) |
| C8—C9—C10—C11 | 173.4 (3) | C11—C10—C15—O4 | 176.6 (3) |
| O1—C9—C10—C15 | 175.3 (3) | C9—C10—C15—O4 | −2.5 (5) |
| C8—C9—C10—C15 | −7.5 (6) | C11—C10—C15—C14 | −3.8 (5) |
| C15—C10—C11—O2 | −175.9 (3) | C9—C10—C15—C14 | 177.1 (4) |
| C9—C10—C11—O2 | 3.3 (5) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O2—H2···O1 | 0.85 (1) | 1.65 (2) | 2.455 (4) | 157 (4) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: FL2321).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Flores, N., Cabrera, G., Jimenez, I. A., Pinero, J., Gimenez, A., Bourdy, G., Cortes-Selva, F. & Bazzocchi, I. (2007). Planta Med.73, 206–211. [DOI] [PubMed]
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
- Xuan, T. D., Fukuta, M., Wei, A. C., Elzaawely, A. A., Khanh, T. D. & Tawata, S. (2008). J. Nat. Med.62, 188–194. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810041395/fl2321sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041395/fl2321Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report