Abstract
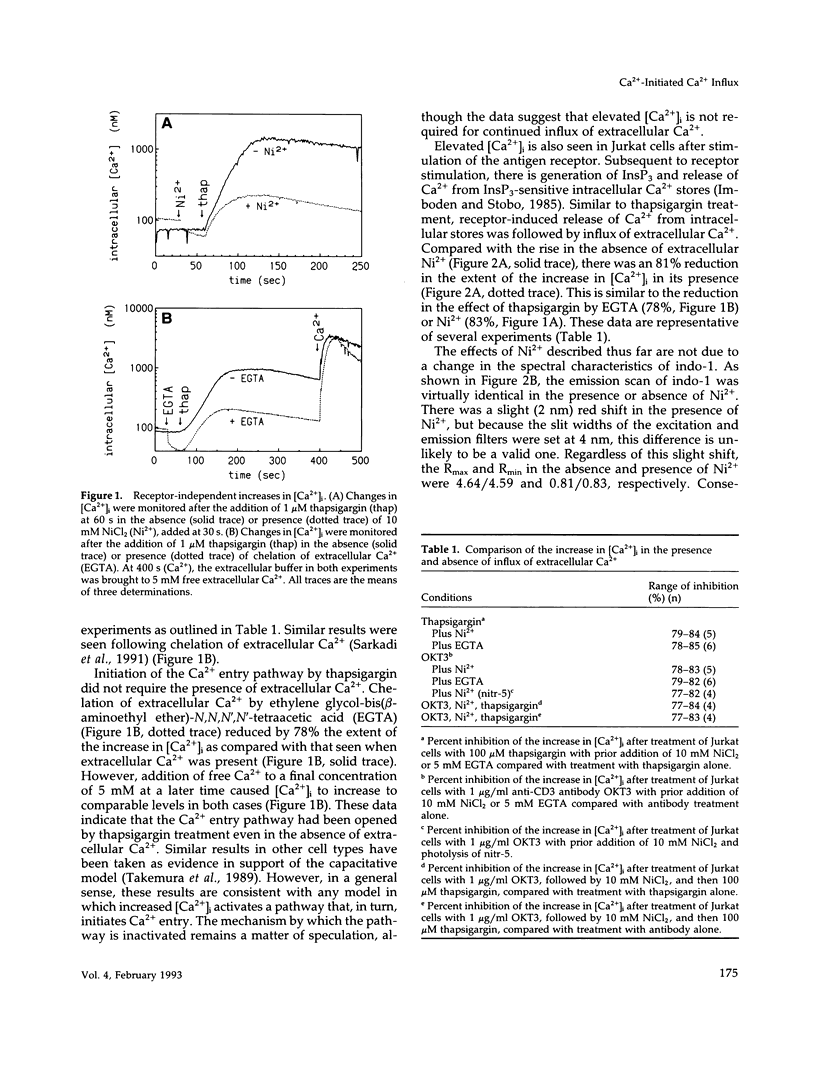
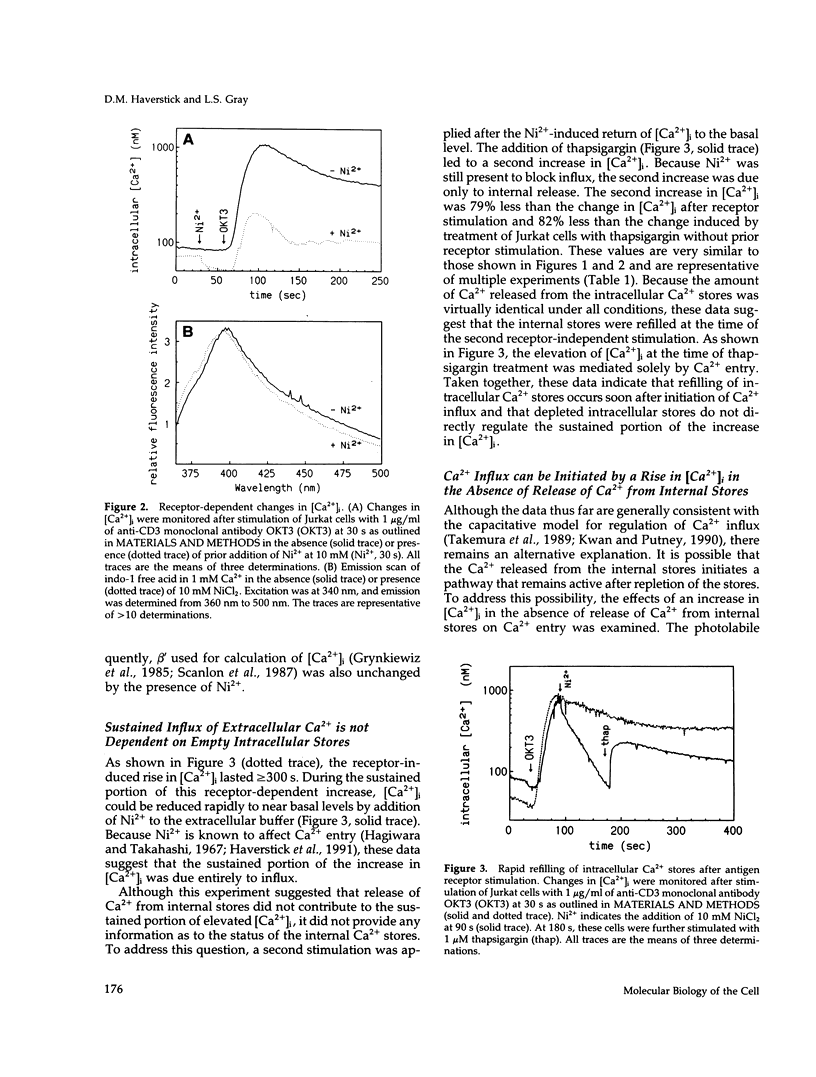
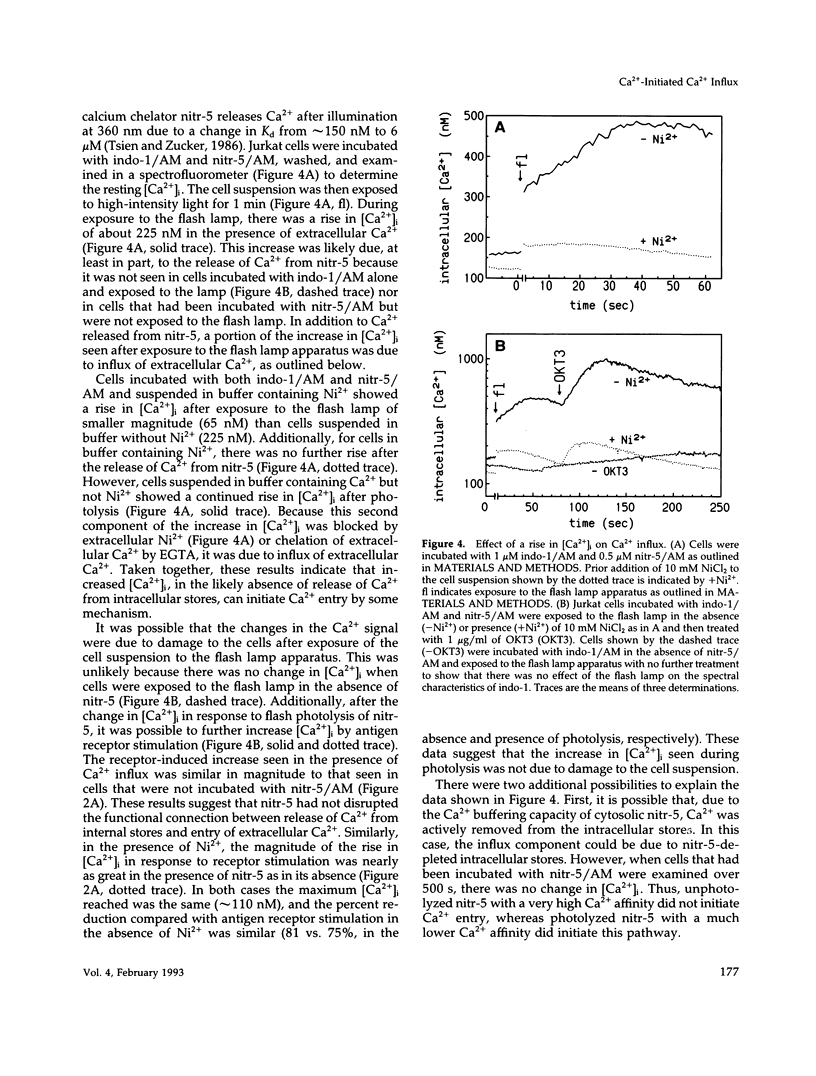
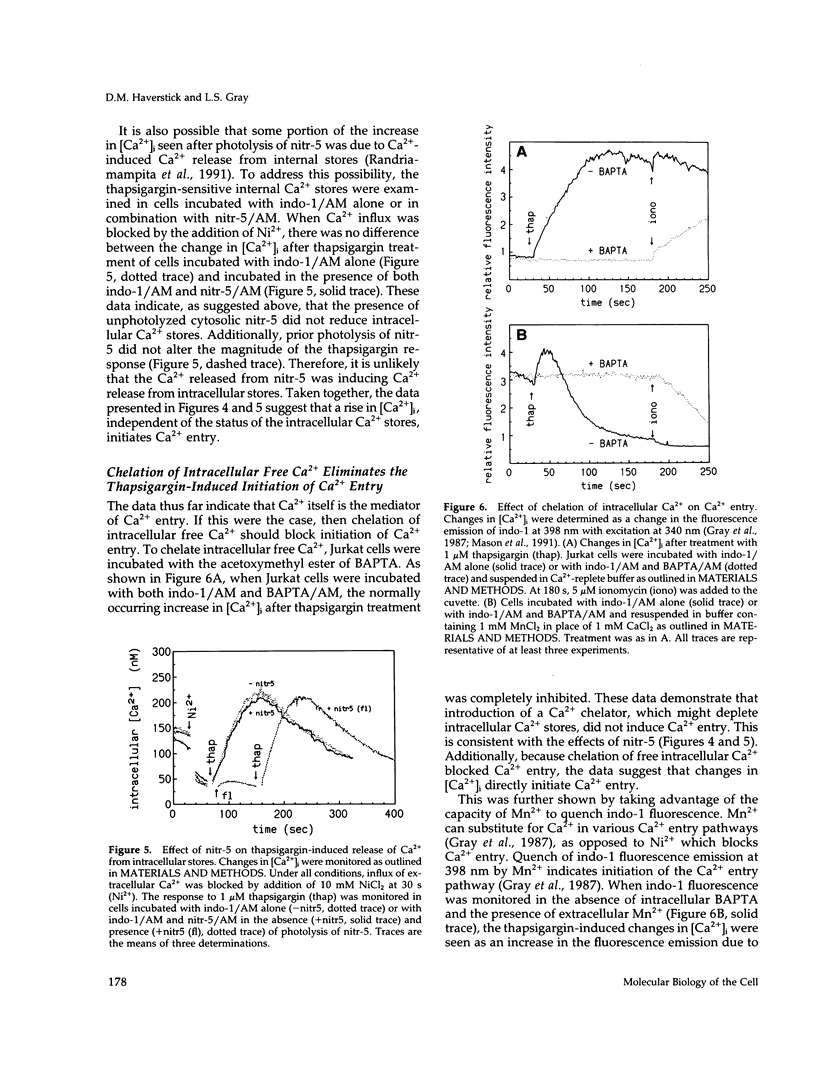
One current hypothesis for the initiation of Ca2+ entry into nonelectrically excitable cells proposes that Ca2+ entry is linked to the state of filling of intracellular Ca2+ stores. In the human T lymphocyte cell line Jurkat, stimulation of the antigen receptor leads to release of Ca2+ from internal stores and influx of extracellular Ca2+. Similarly, treatment of Jurkat cells with the tumor promoter thapsigargin induced release of Ca2+ from internal stores and also resulted in influx of extracellular Ca2+. Initiation of Ca2+ entry by thapsigargin was blocked by chelation of Ca2+ released from the internal storage pool. The Ca2+ entry pathway also could be initiated by an increase in the intracellular concentration of Ca2+ after photolysis of the Ca(2+)-cage, nitr-5. Thus, three separate treatments that caused an increase in the intracellular concentration of Ca2+ initiated Ca2+ influx in Jurkat cells. In all cases, Ca(2+)-initiated Ca2+ influx was blocked by treatment with any of three phenothiazines or W-7, suggesting that it is mediated by calmodulin. These data suggest that release of Ca2+ from internal stores is not linked capacitatively to Ca2+ entry but that initiation is linked instead by Ca2+ itself, perhaps via calmodulin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Cohen N., Weber G., Banner B. L., Welton A. F., Hope W. C., Crowley H., Anderson W. A., Simko B. A., O'Donnell M., Coffey J. W. Analogs of arachidonic acid methylated at C-7 and C-10 as inhibitors of leukotriene biosynthesis. Prostaglandins. 1984 Apr;27(4):553–562. doi: 10.1016/0090-6980(84)90091-1. [DOI] [PubMed] [Google Scholar]
- Densmore J. J., Szabo G., Gray L. S. A voltage-gated calcium channel is linked to the antigen receptor in Jurkat T lymphocytes. FEBS Lett. 1992 Nov 9;312(2-3):161–164. doi: 10.1016/0014-5793(92)80926-8. [DOI] [PubMed] [Google Scholar]
- Gouy H., Cefai D., Christensen S. B., Debré P., Bismuth G. Ca2+ influx in human T lymphocytes is induced independently of inositol phosphate production by mobilization of intracellular Ca2+ stores. A study with the Ca2+ endoplasmic reticulum-ATPase inhibitor thapsigargin. Eur J Immunol. 1990 Oct;20(10):2269–2275. doi: 10.1002/eji.1830201016. [DOI] [PubMed] [Google Scholar]
- Gray L. S., Gnarra J. R., Engelhard V. H. Demonstration of a calcium influx in cytolytic T lymphocytes in response to target cell binding. J Immunol. 1987 Jan 1;138(1):63–69. [PubMed] [Google Scholar]
- Gray L. S., Gnarra J., Hewlett E. L., Engelhard V. H. Increased intracellular cyclic adenosine monophosphate inhibits T lymphocyte-mediated cytolysis by two distinct mechanisms. J Exp Med. 1988 Jun 1;167(6):1963–1968. doi: 10.1084/jem.167.6.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Hagiwara S., Takahashi K. Surface density of calcium ions and calcium spikes in the barnacle muscle fiber membrane. J Gen Physiol. 1967 Jan;50(3):583–601. doi: 10.1085/jgp.50.3.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie R. C., Minke B. The trp gene is essential for a light-activated Ca2+ channel in Drosophila photoreceptors. Neuron. 1992 Apr;8(4):643–651. doi: 10.1016/0896-6273(92)90086-s. [DOI] [PubMed] [Google Scholar]
- Haverstick D. M., Engelhard V. H., Gray L. S. Three intracellular signals for cytotoxic T lymphocyte-mediated killing. Independent roles for protein kinase C, Ca2+ influx, and Ca2+ release from internal stores. J Immunol. 1991 May 15;146(10):3306–3313. [PubMed] [Google Scholar]
- Haverstick D. M., Sakai H., Gray L. S. Lymphocyte adhesion can be regulated by cytoskeleton-associated, PMA-induced capping of surface receptors. Am J Physiol. 1992 Apr;262(4 Pt 1):C916–C926. doi: 10.1152/ajpcell.1992.262.4.C916. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Ishikawa T. Molecular pharmacology of calmodulin pathways in the cell functions. Cell Calcium. 1992 Jun-Jul;13(6-7):465–472. doi: 10.1016/0143-4160(92)90059-2. [DOI] [PubMed] [Google Scholar]
- Hidaka H., Sasaki Y., Tanaka T., Endo T., Ohno S., Fujii Y., Nagata T. N-(6-aminohexyl)-5-chloro-1-naphthalenesulfonamide, a calmodulin antagonist, inhibits cell proliferation. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4354–4357. doi: 10.1073/pnas.78.7.4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imboden J. B., Stobo J. D. Transmembrane signalling by the T cell antigen receptor. Perturbation of the T3-antigen receptor complex generates inositol phosphates and releases calcium ions from intracellular stores. J Exp Med. 1985 Mar 1;161(3):446–456. doi: 10.1084/jem.161.3.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jan L. Y., Jan Y. N. Tracing the roots of ion channels. Cell. 1992 May 29;69(5):715–718. doi: 10.1016/0092-8674(92)90280-p. [DOI] [PubMed] [Google Scholar]
- Kass G. E., Llopis J., Chow S. C., Duddy S. K., Orrenius S. Receptor-operated calcium influx in rat hepatocytes. Identification and characterization using manganese. J Biol Chem. 1990 Oct 15;265(29):17486–17492. [PubMed] [Google Scholar]
- Kwan C. Y., Putney J. W., Jr Uptake and intracellular sequestration of divalent cations in resting and methacholine-stimulated mouse lacrimal acinar cells. Dissociation by Sr2+ and Ba2+ of agonist-stimulated divalent cation entry from the refilling of the agonist-sensitive intracellular pool. J Biol Chem. 1990 Jan 15;265(2):678–684. [PubMed] [Google Scholar]
- Li G., Hidaka H., Wollheim C. B. Inhibition of voltage-gated Ca2+ channels and insulin secretion in HIT cells by the Ca2+/calmodulin-dependent protein kinase II inhibitor KN-62: comparison with antagonists of calmodulin and L-type Ca2+ channels. Mol Pharmacol. 1992 Sep;42(3):489–488. [PubMed] [Google Scholar]
- Manger B., Weiss A., Imboden J., Laing T., Stobo J. D. The role of protein kinase C in transmembrane signaling by the T cell antigen receptor complex. Effects of stimulation with soluble or immobilized CD3 antibodies. J Immunol. 1987 Oct 15;139(8):2755–2760. [PubMed] [Google Scholar]
- Mason M. J., Garcia-Rodriguez C., Grinstein S. Coupling between intracellular Ca2+ stores and the Ca2+ permeability of the plasma membrane. Comparison of the effects of thapsigargin, 2,5-di-(tert-butyl)-1,4-hydroquinone, and cyclopiazonic acid in rat thymic lymphocytes. J Biol Chem. 1991 Nov 5;266(31):20856–20862. [PubMed] [Google Scholar]
- McCarron J. G., McGeown J. G., Reardon S., Ikebe M., Fay F. S., Walsh J. V., Jr Calcium-dependent enhancement of calcium current in smooth muscle by calmodulin-dependent protein kinase II. Nature. 1992 May 7;357(6373):74–77. doi: 10.1038/357074a0. [DOI] [PubMed] [Google Scholar]
- Menniti F. S., Bird G. S., Takemura H., Thastrup O., Potter B. V., Putney J. W., Jr Mobilization of calcium by inositol trisphosphates from permeabilized rat parotid acinar cells. Evidence for translocation of calcium from uptake to release sites within the inositol 1,4,5-trisphosphate- and thapsigargin-sensitive calcium pool. J Biol Chem. 1991 Jul 25;266(21):13646–13653. [PubMed] [Google Scholar]
- Merritt J. E., Rink T. J. Regulation of cytosolic free calcium in fura-2-loaded rat parotid acinar cells. J Biol Chem. 1987 Dec 25;262(36):17362–17369. [PubMed] [Google Scholar]
- Nemerow G. R., Cooper N. R. Infection of B lymphocytes by a human herpesvirus, Epstein-Barr virus, is blocked by calmodulin antagonists. Proc Natl Acad Sci U S A. 1984 Aug;81(15):4955–4959. doi: 10.1073/pnas.81.15.4955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet-Brown E., Cheung R. K., Lee J. W., Gelfand E. W. Antigen-dependent increase in cytosolic free calcium in specific human T-lymphocyte clones. Nature. 1985 Aug 8;316(6028):545–547. doi: 10.1038/316545a0. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Schoeffield M. S., Fimmel C. J., Muallem S. The agonist-sensitive calcium pool in the pancreatic acinar cell. Activation of plasma membrane Ca2+ influx mechanism. J Biol Chem. 1987 Dec 15;262(35):16963–16968. [PubMed] [Google Scholar]
- Phillips A. M., Bull A., Kelly L. E. Identification of a Drosophila gene encoding a calmodulin-binding protein with homology to the trp phototransduction gene. Neuron. 1992 Apr;8(4):631–642. doi: 10.1016/0896-6273(92)90085-r. [DOI] [PubMed] [Google Scholar]
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986 Feb;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- Randriamampita C., Bismuth G., Trautmann A. Ca(2+)-induced Ca2+ release amplifies the Ca2+ response elicited by inositol trisphosphate in macrophages. Cell Regul. 1991 Jul;2(7):513–522. doi: 10.1091/mbc.2.7.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage S. O., Rink T. J. The kinetics of changes in intracellular calcium concentration in fura-2-loaded human platelets. J Biol Chem. 1987 Dec 5;262(34):16364–16369. [PubMed] [Google Scholar]
- Sarkadi B., Tordai A., Homolya L., Scharff O., Gárdos G. Calcium influx and intracellular calcium release in anti-CD3 antibody-stimulated and thapsigargin-treated human T lymphoblasts. J Membr Biol. 1991 Jul;123(1):9–21. doi: 10.1007/BF01993958. [DOI] [PubMed] [Google Scholar]
- Scanlon M., Williams D. A., Fay F. S. A Ca2+-insensitive form of fura-2 associated with polymorphonuclear leukocytes. Assessment and accurate Ca2+ measurement. J Biol Chem. 1987 May 5;262(13):6308–6312. [PubMed] [Google Scholar]
- Takemura H., Hughes A. R., Thastrup O., Putney J. W., Jr Activation of calcium entry by the tumor promoter thapsigargin in parotid acinar cells. Evidence that an intracellular calcium pool and not an inositol phosphate regulates calcium fluxes at the plasma membrane. J Biol Chem. 1989 Jul 25;264(21):12266–12271. [PubMed] [Google Scholar]
- Thastrup O., Cullen P. J., Drøbak B. K., Hanley M. R., Dawson A. P. Thapsigargin, a tumor promoter, discharges intracellular Ca2+ stores by specific inhibition of the endoplasmic reticulum Ca2(+)-ATPase. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2466–2470. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsien R. Y., Zucker R. S. Control of cytoplasmic calcium with photolabile tetracarboxylate 2-nitrobenzhydrol chelators. Biophys J. 1986 Nov;50(5):843–853. doi: 10.1016/S0006-3495(86)83525-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss A., Imboden J., Shoback D., Stobo J. Role of T3 surface molecules in human T-cell activation: T3-dependent activation results in an increase in cytoplasmic free calcium. Proc Natl Acad Sci U S A. 1984 Jul;81(13):4169–4173. doi: 10.1073/pnas.81.13.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]


