Abstract
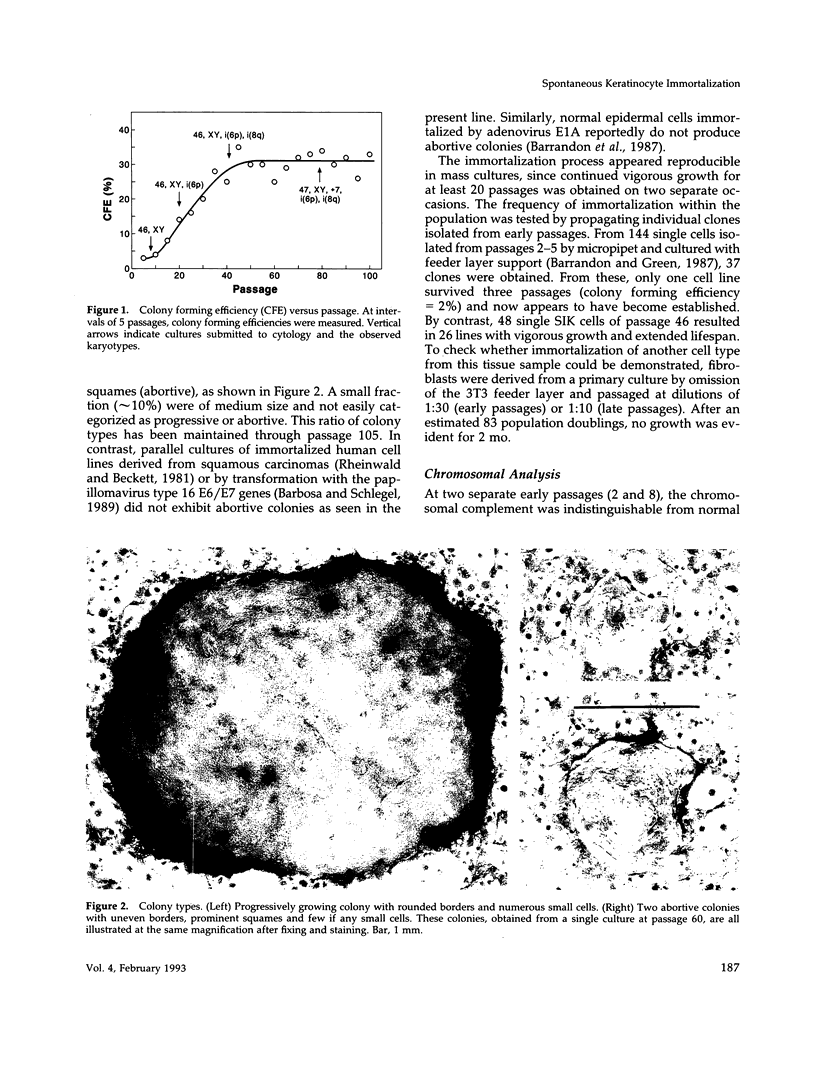
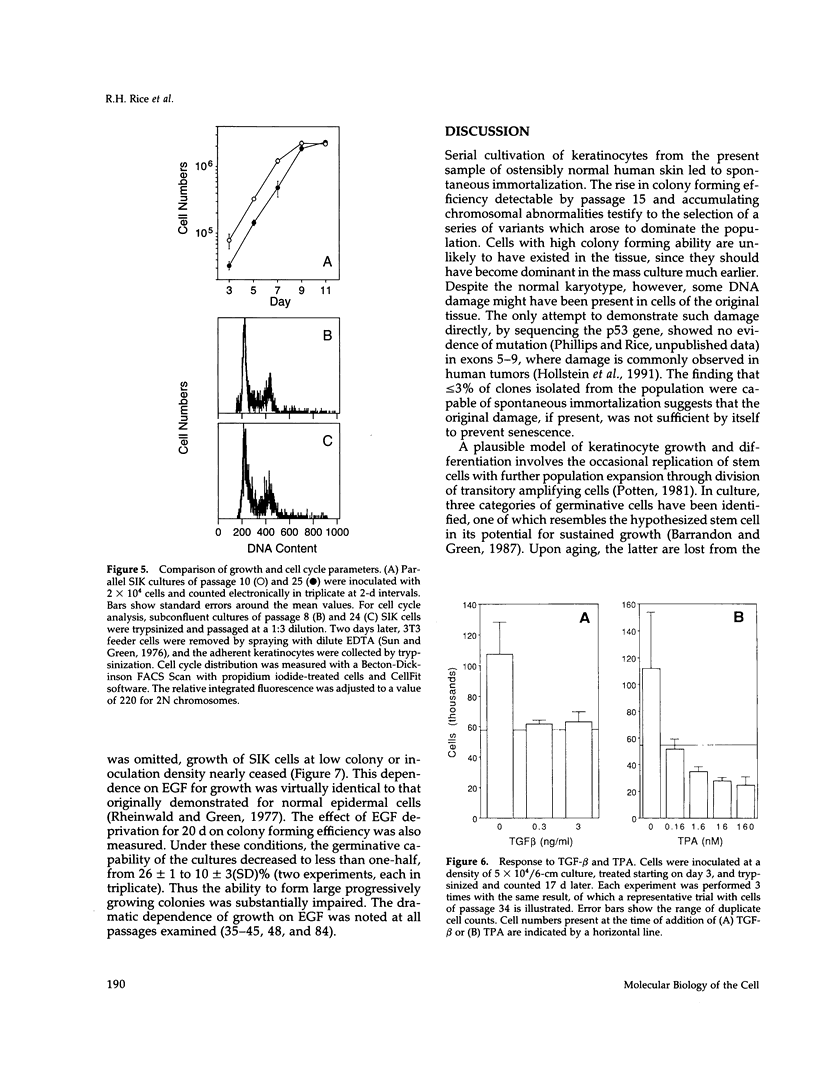
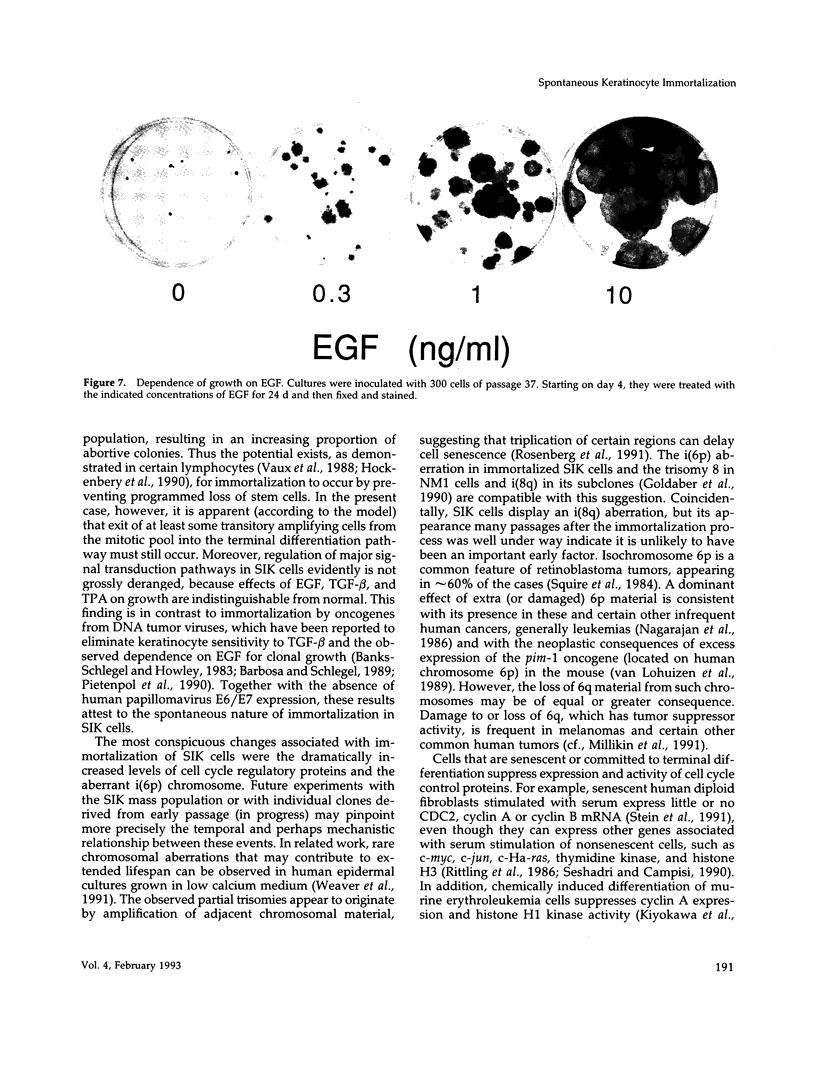
A human line of spontaneously immortalized keratinocytes (SIK cells) has been derived from ostensibly normal epidermis and has proven useful in dissecting molecular changes associated with immortalization. The original cultures had a normal karyotype and a colony forming efficiency of approximately 3% through 10 passages. At passage 15, after which normal strains ordinarily senesce, these cells continued vigorous growth and gradually increased in colony forming efficiency, stabilizing at approximately 30% by passage 40. During the early stage of increasing colony forming efficiency, the cells acquired a single i(6p) chromosomal aberration and 5- to 10-fold increases in expression of the cell-cycle control proteins cyclin A, cyclin B, and p34cdc2. Additional chromosomal aberrations accumulated at later passages (i(8q) and +7), but the i(6p) and the increased expression of cell-cycle proteins were maintained, raising the possibility that these features were important for immortalization. Regulation of cell growth and differentiation in the cultures appeared minimally altered compared with normal keratinocytes as judged by their microscopic appearance and generation of abortive colonies, sensitivity to growth suppression by transforming growth factor-beta and tetradecanoylphorbol acetate, and dependence upon epidermal growth factor for progressive growth.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen-Hoffmann B. L., Rheinwald J. G. Polycyclic aromatic hydrocarbon mutagenesis of human epidermal keratinocytes in culture. Proc Natl Acad Sci U S A. 1984 Dec;81(24):7802–7806. doi: 10.1073/pnas.81.24.7802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baden H. P., Kubilus J., Kvedar J. C., Steinberg M. L., Wolman S. R. Isolation and characterization of a spontaneously arising long-lived line of human keratinocytes (NM 1). In Vitro Cell Dev Biol. 1987 Mar;23(3):205–213. doi: 10.1007/BF02623581. [DOI] [PubMed] [Google Scholar]
- Banks-Schlegel S. P., Howley P. M. Differentiation of human epidermal cells transformed by SV40. J Cell Biol. 1983 Feb;96(2):330–337. doi: 10.1083/jcb.96.2.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa M. S., Schlegel R. The E6 and E7 genes of HPV-18 are sufficient for inducing two-stage in vitro transformation of human keratinocytes. Oncogene. 1989 Dec;4(12):1529–1532. [PubMed] [Google Scholar]
- Barrandon Y., Green H. Three clonal types of keratinocyte with different capacities for multiplication. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2302–2306. doi: 10.1073/pnas.84.8.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrandon Y., Morgan J. R., Mulligan R. C., Green H. Restoration of growth potential in paraclones of human keratinocytes by a viral oncogene. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4102–4106. doi: 10.1073/pnas.86.11.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bascom C. C., Sipes N. J., Coffey R. J., Moses H. L. Regulation of epithelial cell proliferation by transforming growth factors. J Cell Biochem. 1989 Jan;39(1):25–32. doi: 10.1002/jcb.240390104. [DOI] [PubMed] [Google Scholar]
- Blanquet V., Wang J. A., Chenivesse X., Henglein B., Garreau F., Bréchot C., Turleau C. Assignment of a human cyclin A gene to 4q26-q27. Genomics. 1990 Nov;8(3):595–597. doi: 10.1016/0888-7543(90)90052-v. [DOI] [PubMed] [Google Scholar]
- Boukamp P., Petrussevska R. T., Breitkreutz D., Hornung J., Markham A., Fusenig N. E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J Cell Biol. 1988 Mar;106(3):761–771. doi: 10.1083/jcb.106.3.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson N. L., Myers S., Simpson N. E. A PvuII RFLP detected by pOB231 at CDC2 on human chromosome 10. Nucleic Acids Res. 1991 Jan 11;19(1):196–196. doi: 10.1093/nar/19.1.196-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Urso G., Marraccino R. L., Marshak D. R., Roberts J. M. Cell cycle control of DNA replication by a homologue from human cells of the p34cdc2 protein kinase. Science. 1990 Nov 9;250(4982):786–791. doi: 10.1126/science.2173140. [DOI] [PubMed] [Google Scholar]
- Furukawa Y., Piwnica-Worms H., Ernst T. J., Kanakura Y., Griffin J. D. cdc2 gene expression at the G1 to S transition in human T lymphocytes. Science. 1990 Nov 9;250(4982):805–808. doi: 10.1126/science.2237430. [DOI] [PubMed] [Google Scholar]
- Gautier J., Norbury C., Lohka M., Nurse P., Maller J. Purified maturation-promoting factor contains the product of a Xenopus homolog of the fission yeast cell cycle control gene cdc2+. Cell. 1988 Jul 29;54(3):433–439. doi: 10.1016/0092-8674(88)90206-1. [DOI] [PubMed] [Google Scholar]
- Girard F., Strausfeld U., Fernandez A., Lamb N. J. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991 Dec 20;67(6):1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Goldaber M. L., Kubilus J., Phillips S. B., Henkle C., Atkins L., Baden H. P. Immortal clones of NM1 keratinocytes contain an isochromosome of the long arm of chromosome 8. In Vitro Cell Dev Biol. 1990 Jan;26(1):7–8. doi: 10.1007/BF02624148. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Welty D. J., Strickland J. E., Yuspa S. H. Spontaneous Ha-ras gene activation in cultured primary murine keratinocytes: consequences of Ha-ras gene activation in malignant conversion and malignant progression. Mol Carcinog. 1989;2(4):199–207. doi: 10.1002/mc.2940020406. [DOI] [PubMed] [Google Scholar]
- Heimann R., Rice R. H. Rat esophageal and epidermal keratinocytes: intrinsic differences in culture and derivation of continuous lines. J Cell Physiol. 1983 Dec;117(3):362–367. doi: 10.1002/jcp.1041170311. [DOI] [PubMed] [Google Scholar]
- Hockenbery D., Nuñez G., Milliman C., Schreiber R. D., Korsmeyer S. J. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990 Nov 22;348(6299):334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- Hollstein M., Sidransky D., Vogelstein B., Harris C. C. p53 mutations in human cancers. Science. 1991 Jul 5;253(5015):49–53. doi: 10.1126/science.1905840. [DOI] [PubMed] [Google Scholar]
- Inaba T., Matsushime H., Valentine M., Roussel M. F., Sherr C. J., Look A. T. Genomic organization, chromosomal localization, and independent expression of human cyclin D genes. Genomics. 1992 Jul;13(3):565–574. doi: 10.1016/0888-7543(92)90126-d. [DOI] [PubMed] [Google Scholar]
- Kiyokawa H., Ngo L., Kurosaki T., Rifkind R. A., Marks P. A. Changes in p34cdc2 kinase activity and cyclin A during induced differentiation of murine erythroleukemia cells. Cell Growth Differ. 1992 Jun;3(6):377–383. [PubMed] [Google Scholar]
- Kung A. L., Sherwood S. W., Schimke R. T. Cell line-specific differences in the control of cell cycle progression in the absence of mitosis. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9553–9557. doi: 10.1073/pnas.87.24.9553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee M. G., Nurse P. Complementation used to clone a human homologue of the fission yeast cell cycle control gene cdc2. Nature. 1987 May 7;327(6117):31–35. doi: 10.1038/327031a0. [DOI] [PubMed] [Google Scholar]
- Marraccino R. L., Firpo E. J., Roberts J. M. Activation of the p34 CDC2 protein kinase at the start of S phase in the human cell cycle. Mol Biol Cell. 1992 Apr;3(4):389–401. doi: 10.1091/mbc.3.4.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millikin D., Meese E., Vogelstein B., Witkowski C., Trent J. Loss of heterozygosity for loci on the long arm of chromosome 6 in human malignant melanoma. Cancer Res. 1991 Oct 15;51(20):5449–5453. [PubMed] [Google Scholar]
- Motokura T., Bloom T., Kim H. G., Jüppner H., Ruderman J. V., Kronenberg H. M., Arnold A. A novel cyclin encoded by a bcl1-linked candidate oncogene. Nature. 1991 Apr 11;350(6318):512–515. doi: 10.1038/350512a0. [DOI] [PubMed] [Google Scholar]
- Motokura T., Yi H. F., Kronenberg H. M., McBride O. W., Arnold A. Assignment of the human cyclin D3 gene (CCND3) to chromosome 6p----q13. Cytogenet Cell Genet. 1992;61(1):5–7. doi: 10.1159/000133359. [DOI] [PubMed] [Google Scholar]
- Nagarajan L., Louie E., Tsujimoto Y., ar-Rushdi A., Huebner K., Croce C. M. Localization of the human pim oncogene (PIM) to a region of chromosome 6 involved in translocations in acute leukemias. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2556–2560. doi: 10.1073/pnas.83.8.2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazarenko S. A., Ostroverhova N. V., Spurr N. K. Regional assignment of the human cell cycle control gene CDC2 to chromosome 10q21 by in situ hybridization. Hum Genet. 1991 Sep;87(5):621–622. doi: 10.1007/BF00209025. [DOI] [PubMed] [Google Scholar]
- Nishimoto T., Uzawa S., Schlegel R. Mitotic checkpoints. Curr Opin Cell Biol. 1992 Apr;4(2):174–179. doi: 10.1016/0955-0674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkinson E. K., Grabham P., Emmerson A. A subpopulation of cultured human keratinocytes which is resistant to the induction of terminal differentiation-related changes by phorbol, 12-myristate, 13-acetate: evidence for an increase in the resistant population following transformation. Carcinogenesis. 1983;4(7):857–861. doi: 10.1093/carcin/4.7.857. [DOI] [PubMed] [Google Scholar]
- Peterson G. L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal Biochem. 1977 Dec;83(2):346–356. doi: 10.1016/0003-2697(77)90043-4. [DOI] [PubMed] [Google Scholar]
- Phillips M. A., Rice R. H. Convergent differentiation in cultured rat cells from nonkeratinized epithelia: keratinocyte character and intrinsic differences. J Cell Biol. 1983 Sep;97(3):686–691. doi: 10.1083/jcb.97.3.686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietenpol J. A., Stein R. W., Moran E., Yaciuk P., Schlegel R., Lyons R. M., Pittelkow M. R., Münger K., Howley P. M., Moses H. L. TGF-beta 1 inhibition of c-myc transcription and growth in keratinocytes is abrogated by viral transforming proteins with pRB binding domains. Cell. 1990 Jun 1;61(5):777–785. doi: 10.1016/0092-8674(90)90188-k. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990 Aug 23;346(6286):760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Cell replacement in epidermis (keratopoiesis) via discrete units of proliferation. Int Rev Cytol. 1981;69:271–318. doi: 10.1016/s0074-7696(08)62326-8. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Defective terminal differentiation in culture as a consistent and selectable character of malignant human keratinocytes. Cell. 1980 Nov;22(2 Pt 2):629–632. doi: 10.1016/0092-8674(80)90373-6. [DOI] [PubMed] [Google Scholar]
- Rheinwald J. G., Beckett M. A. Tumorigenic keratinocyte lines requiring anchorage and fibroblast support cultured from human squamous cell carcinomas. Cancer Res. 1981 May;41(5):1657–1663. [PubMed] [Google Scholar]
- Rheinwald J. G., Green H. Epidermal growth factor and the multiplication of cultured human epidermal keratinocytes. Nature. 1977 Feb 3;265(5593):421–424. doi: 10.1038/265421a0. [DOI] [PubMed] [Google Scholar]
- Riabowol K., Draetta G., Brizuela L., Vandre D., Beach D. The cdc2 kinase is a nuclear protein that is essential for mitosis in mammalian cells. Cell. 1989 May 5;57(3):393–401. doi: 10.1016/0092-8674(89)90914-8. [DOI] [PubMed] [Google Scholar]
- Rittling S. R., Brooks K. M., Cristofalo V. J., Baserga R. Expression of cell cycle-dependent genes in young and senescent WI-38 fibroblasts. Proc Natl Acad Sci U S A. 1986 May;83(10):3316–3320. doi: 10.1073/pnas.83.10.3316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins B. J., O'Connell T. M., Bennett G., Burton L. E., Stiles C. D., Rheinwald J. G. Environment-dependent growth inhibition of human epidermal keratinocytes by recombinant human transforming growth factor-beta. J Cell Physiol. 1989 Jun;139(3):455–462. doi: 10.1002/jcp.1041390302. [DOI] [PubMed] [Google Scholar]
- Rosenberg C., Stetten G., Kearns W. G., Pearson P. L., Littlefield J. W. Origin of chromosome rearrangements in two long-lived human keratinocyte lines. In Vitro Cell Dev Biol. 1991 Oct;27A(10):823–825. doi: 10.1007/BF02631249. [DOI] [PubMed] [Google Scholar]
- Roy L. M., Swenson K. I., Walker D. H., Gabrielli B. G., Li R. S., Piwnica-Worms H., Maller J. L. Activation of p34cdc2 kinase by cyclin A. J Cell Biol. 1991 May;113(3):507–514. doi: 10.1083/jcb.113.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sartor H., Ehlert F., Grzeschik K. H., Müller R., Adolph S. Assignment of two human cell cycle genes, CDC25C and CCNB1, to 5q31 and 5q12, respectively. Genomics. 1992 Jul;13(3):911–912. doi: 10.1016/0888-7543(92)90190-4. [DOI] [PubMed] [Google Scholar]
- Seabright M. A rapid banding technique for human chromosomes. Lancet. 1971 Oct 30;2(7731):971–972. doi: 10.1016/s0140-6736(71)90287-x. [DOI] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Seto M., Yamamoto K., Iida S., Akao Y., Utsumi K. R., Kubonishi I., Miyoshi I., Ohtsuki T., Yawata Y., Namba M. Gene rearrangement and overexpression of PRAD1 in lymphoid malignancy with t(11;14)(q13;q32) translocation. Oncogene. 1992 Jul;7(7):1401–1406. [PubMed] [Google Scholar]
- Squire J., Phillips R. A., Boyce S., Godbout R., Rogers B., Gallie B. L. Isochromosome 6p, a unique chromosomal abnormality in retinoblastoma: verification by standard staining techniques, new densitometric methods, and somatic cell hybridization. Hum Genet. 1984;66(1):46–53. doi: 10.1007/BF00275185. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmann K. E., Belinsky G. S., Lee D., Schlegel R. Chemically induced premature mitosis: differential response in rodent and human cells and the relationship to cyclin B synthesis and p34cdc2/cyclin B complex formation. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6843–6847. doi: 10.1073/pnas.88.15.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T. T., Green H. Differentiation of the epidermal keratinocyte in cell culture: formation of the cornified envelope. Cell. 1976 Dec;9(4 Pt 1):511–521. doi: 10.1016/0092-8674(76)90033-7. [DOI] [PubMed] [Google Scholar]
- TODARO G. J., GREEN H. Quantitative studies of the growth of mouse embryo cells in culture and their development into established lines. J Cell Biol. 1963 May;17:299–313. doi: 10.1083/jcb.17.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Th'ng J. P., Wright P. S., Hamaguchi J., Lee M. G., Norbury C. J., Nurse P., Bradbury E. M. The FT210 cell line is a mouse G2 phase mutant with a temperature-sensitive CDC2 gene product. Cell. 1990 Oct 19;63(2):313–324. doi: 10.1016/0092-8674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Wang J., Chenivesse X., Henglein B., Bréchot C. Hepatitis B virus integration in a cyclin A gene in a hepatocellular carcinoma. Nature. 1990 Feb 8;343(6258):555–557. doi: 10.1038/343555a0. [DOI] [PubMed] [Google Scholar]
- Wang J., Zindy F., Chenivesse X., Lamas E., Henglein B., Bréchot C. Modification of cyclin A expression by hepatitis B virus DNA integration in a hepatocellular carcinoma. Oncogene. 1992 Aug;7(8):1653–1656. [PubMed] [Google Scholar]
- Weaver J. D., Stetten G., Littlefield J. W. Partial trisomies in two spontaneously arising long-lived human keratinocyte lines. In Vitro Cell Dev Biol. 1991 Aug;27A(8):670–675. doi: 10.1007/BF02631112. [DOI] [PubMed] [Google Scholar]
- Withers D. A., Harvey R. C., Faust J. B., Melnyk O., Carey K., Meeker T. C. Characterization of a candidate bcl-1 gene. Mol Cell Biol. 1991 Oct;11(10):4846–4853. doi: 10.1128/mcb.11.10.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Menninger J., Beach D., Ward D. C. Molecular cloning and chromosomal mapping of CCND genes encoding human D-type cyclins. Genomics. 1992 Jul;13(3):575–584. doi: 10.1016/0888-7543(92)90127-e. [DOI] [PubMed] [Google Scholar]
- Yamashita K., Yasuda H., Pines J., Yasumoto K., Nishitani H., Ohtsubo M., Hunter T., Sugimura T., Nishimoto T. Okadaic acid, a potent inhibitor of type 1 and type 2A protein phosphatases, activates cdc2/H1 kinase and transiently induces a premature mitosis-like state in BHK21 cells. EMBO J. 1990 Dec;9(13):4331–4338. doi: 10.1002/j.1460-2075.1990.tb07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuspa S. H., Hawley-Nelson P., Koehler B., Stanley J. R. A survey of transformation markers in differentiating epidermal cell lines in culture. Cancer Res. 1980 Dec;40(12):4694–4703. [PubMed] [Google Scholar]
- van Lohuizen M., Verbeek S., Krimpenfort P., Domen J., Saris C., Radaszkiewicz T., Berns A. Predisposition to lymphomagenesis in pim-1 transgenic mice: cooperation with c-myc and N-myc in murine leukemia virus-induced tumors. Cell. 1989 Feb 24;56(4):673–682. doi: 10.1016/0092-8674(89)90589-8. [DOI] [PubMed] [Google Scholar]







