Abstract
The title compound, systematic name 9-isopropylidene-2,6-dimethyl-11-oxatricyclo[6.2.1.01,5]undec-6-en-8-ol, C15H22O2, which crystallizes with two molecules of similar conformation in the asymmetric unit, features three fused rings, two of which are five-membered and the third six-membered. Of the two five-membered rings, the one with an O atom has a distinct envelope shape (with the O atom representing the flap). The six-membered ring is also envelope-shaped as it shares a common O atom with the five-membered ring. In the crystal, the two independent molecules are linked by a pair of O—H⋯O hydrogen bonds, generating a dimer.
Related literature
For the C2 modification isolated from Globba malaccensis Ridl, see: Muangsin et al. (2004 ▶).
Experimental
Crystal data
C15H22O2
M r = 234.33
Monoclinic,
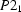
a = 9.3495 (7) Å
b = 12.535 (1) Å
c = 11.7727 (9) Å
β = 96.532 (1)°
V = 1370.76 (18) Å3
Z = 4
Mo Kα radiation
μ = 0.07 mm−1
T = 100 K
0.40 × 0.05 × 0.05 mm
Data collection
Bruker SMART APEX diffractometer
13257 measured reflections
3298 independent reflections
2882 reflections with I > 2σ(I)
R int = 0.046
Refinement
R[F 2 > 2σ(F 2)] = 0.037
wR(F 2) = 0.090
S = 1.03
3298 reflections
323 parameters
1 restraint
H atoms treated by a mixture of independent and constrained refinement
Δρmax = 0.21 e Å−3
Δρmin = −0.18 e Å−3
Data collection: APEX2 (Bruker, 2009 ▶); cell refinement: SAINT (Bruker, 2009 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: X-SEED (Barbour, 2001 ▶); software used to prepare material for publication: publCIF (Westrip, 2010 ▶).
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810040559/hb5676sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040559/hb5676Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| O1—H1⋯O4 | 0.89 (3) | 1.92 (3) | 2.799 (2) | 168 (3) |
| O3—H3⋯O2 | 0.86 (3) | 1.92 (3) | 2.771 (2) | 171 (3) |
Acknowledgments
We thank the University of Malaya for supporting this study.
supplementary crystallographic information
Comment
Zingerberaceae is a herbaceous plant found in tropical forests that comprises of 52 genera with 1500 species. Most species are found in the South East Asian region. Curcuma zedoaria, also known as white turmeric, is a species that is a rich source of terpenoids.
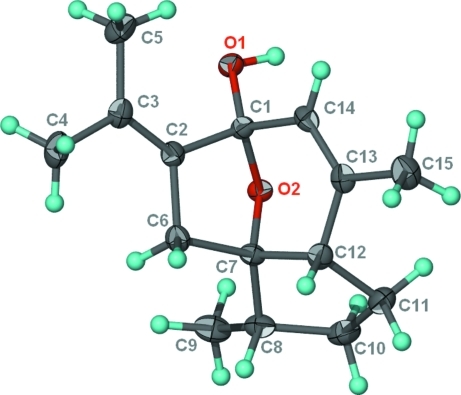
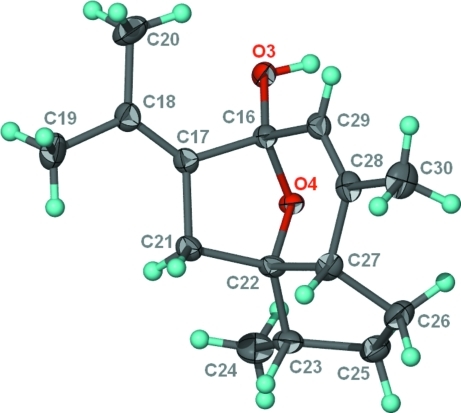
Curcumenol, isolated from Globba malaccensis Ridl, belongs to the monoclinic, space group C2, with a 16.8467 (4), b 7.6799 (2), c 11.8613 (10) Å and β 115.997 (1) °. This modification is less dense, as noted from its calculated density of 1.28 (Muangsin et al., 2004). The present modification (I), (Fig. 1) shows nearly identical bond dimensions in the two independent molecules. The two molecules form a dimer in the crystal, being linked by two O—H···O hydrogen bonds.
Experimental
The rhizome of Curcuma zedoaria was collected from Tawangmangu, Indonesia.
Dried rhizomes (1 kg) were powdered and extracted three times with n-hexane and after this, with dichloromethane, ethylacetate, and methanol. The extracts were concentrated under reduced pressure given several fractions.
The n-hexane crude extract (20 g) was subjected to column chromatography over silica gel 60(0.063–0.200 mm, 70–230 mesh ASTM) eluted with a mixture of n-hexane: ethyl acetate with increasing polarity. Separation by TLC gave 21 fractions.
Fraction 10 (1.41 g) was chromatographed over silica gel (0.040–0.063 mm, mesh 230–400 ASTM) eluted with a gradient solvent system of n-hexane: ethyl aceate to give 5 fractions. The second fraction was further purified by high performance thin layerchromatography and using petroleum ether: ethyl acetate: acidified methanol in a 85:14:1 ratio as the developing solvent. Slow evaporation of the solvent gave (I) as colorless prisms.
Refinement
Carbon-bound H-atoms were placed in calculated positions (C—H 0.95 to 0.98 Å) and were included in the refinement in the riding model approximation, with U(H) set to 1.2 to 1.5U(C).
In the absence of heavy atoms, 2923 Friedel pairs were merged.
The hydroxy H-atoms were located in a difference Fourier map, and were refined without restraints; their Uiso values were freely refined.
The absolute configuration was assumed to be that of the C2 modification.
Figures
Fig. 1.
View of the first molecule of (I) at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Fig. 2.
View of the second molecule of (I) at the 70% probability level; hydrogen atoms are drawn as spheres of arbitrary radius.
Crystal data
| C15H22O2 | F(000) = 512 |
| Mr = 234.33 | Dx = 1.135 Mg m−3 |
| Monoclinic, P21 | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: P 2yb | Cell parameters from 3511 reflections |
| a = 9.3495 (7) Å | θ = 2.4–27.4° |
| b = 12.535 (1) Å | µ = 0.07 mm−1 |
| c = 11.7727 (9) Å | T = 100 K |
| β = 96.532 (1)° | Prism, colorless |
| V = 1370.76 (18) Å3 | 0.40 × 0.05 × 0.05 mm |
| Z = 4 |
Data collection
| Bruker SMART APEX diffractometer | 2882 reflections with I > 2σ(I) |
| Radiation source: fine-focus sealed tube | Rint = 0.046 |
| graphite | θmax = 27.5°, θmin = 2.2° |
| ω scans | h = −12→12 |
| 13257 measured reflections | k = −16→15 |
| 3298 independent reflections | l = −15→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.037 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.090 | H atoms treated by a mixture of independent and constrained refinement |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0535P)2 + 0.0302P] where P = (Fo2 + 2Fc2)/3 |
| 3298 reflections | (Δ/σ)max = 0.001 |
| 323 parameters | Δρmax = 0.21 e Å−3 |
| 1 restraint | Δρmin = −0.18 e Å−3 |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| O1 | 0.24626 (15) | 0.50024 (12) | 0.49296 (13) | 0.0165 (3) | |
| H1 | 0.268 (3) | 0.497 (2) | 0.569 (3) | 0.033 (7)* | |
| O2 | 0.06839 (14) | 0.62132 (11) | 0.52267 (11) | 0.0136 (3) | |
| O3 | 0.03989 (15) | 0.49267 (12) | 0.71017 (12) | 0.0160 (3) | |
| H3 | 0.045 (3) | 0.538 (3) | 0.656 (3) | 0.043 (9)* | |
| O4 | 0.28533 (14) | 0.46600 (12) | 0.72943 (11) | 0.0137 (3) | |
| C1 | 0.2006 (2) | 0.60463 (17) | 0.47181 (17) | 0.0141 (4) | |
| C2 | 0.1560 (2) | 0.62872 (17) | 0.34601 (17) | 0.0153 (4) | |
| C3 | 0.2186 (2) | 0.59756 (17) | 0.25570 (17) | 0.0178 (4) | |
| C4 | 0.1634 (3) | 0.6348 (2) | 0.13744 (18) | 0.0266 (5) | |
| H4A | 0.0671 | 0.6656 | 0.1381 | 0.040* | |
| H4B | 0.1584 | 0.5741 | 0.0846 | 0.040* | |
| H4C | 0.2287 | 0.6890 | 0.1125 | 0.040* | |
| C5 | 0.3508 (2) | 0.52777 (19) | 0.2618 (2) | 0.0231 (5) | |
| H5A | 0.3878 | 0.5157 | 0.3421 | 0.035* | |
| H5B | 0.4248 | 0.5631 | 0.2226 | 0.035* | |
| H5C | 0.3257 | 0.4592 | 0.2248 | 0.035* | |
| C6 | 0.0287 (2) | 0.70366 (17) | 0.34497 (17) | 0.0171 (4) | |
| H6A | −0.0595 | 0.6710 | 0.3051 | 0.020* | |
| H6B | 0.0476 | 0.7719 | 0.3070 | 0.020* | |
| C7 | 0.0140 (2) | 0.72101 (17) | 0.47172 (17) | 0.0144 (4) | |
| C8 | −0.1354 (2) | 0.74213 (18) | 0.50599 (18) | 0.0188 (4) | |
| H8 | −0.1786 | 0.8026 | 0.4584 | 0.023* | |
| C9 | −0.2388 (2) | 0.6479 (2) | 0.4898 (2) | 0.0252 (5) | |
| H9A | −0.2524 | 0.6276 | 0.4089 | 0.038* | |
| H9B | −0.3317 | 0.6681 | 0.5145 | 0.038* | |
| H9C | −0.1987 | 0.5874 | 0.5356 | 0.038* | |
| C10 | −0.1057 (2) | 0.7814 (2) | 0.62979 (19) | 0.0231 (5) | |
| H10A | −0.0960 | 0.7204 | 0.6834 | 0.028* | |
| H10B | −0.1851 | 0.8277 | 0.6495 | 0.028* | |
| C11 | 0.0359 (2) | 0.84454 (19) | 0.63593 (19) | 0.0232 (5) | |
| H11A | 0.1000 | 0.8254 | 0.7057 | 0.028* | |
| H11B | 0.0168 | 0.9222 | 0.6371 | 0.028* | |
| C12 | 0.1062 (2) | 0.81353 (17) | 0.52712 (17) | 0.0169 (4) | |
| H12 | 0.0964 | 0.8756 | 0.4734 | 0.020* | |
| C13 | 0.2640 (2) | 0.78423 (17) | 0.54836 (17) | 0.0165 (4) | |
| C14 | 0.3074 (2) | 0.68708 (17) | 0.52234 (17) | 0.0153 (4) | |
| H14 | 0.4067 | 0.6693 | 0.5357 | 0.018* | |
| C15 | 0.3668 (2) | 0.86938 (19) | 0.5957 (2) | 0.0242 (5) | |
| H15A | 0.4647 | 0.8402 | 0.6069 | 0.036* | |
| H15B | 0.3395 | 0.8942 | 0.6692 | 0.036* | |
| H15C | 0.3634 | 0.9293 | 0.5420 | 0.036* | |
| C16 | 0.1638 (2) | 0.50276 (17) | 0.78584 (16) | 0.0139 (4) | |
| C17 | 0.1685 (2) | 0.42847 (17) | 0.88859 (17) | 0.0151 (4) | |
| C18 | 0.0631 (2) | 0.40510 (18) | 0.95131 (17) | 0.0185 (4) | |
| C19 | 0.0876 (2) | 0.3318 (2) | 1.0536 (2) | 0.0269 (5) | |
| H19A | 0.1859 | 0.3035 | 1.0596 | 0.040* | |
| H19B | 0.0187 | 0.2726 | 1.0443 | 0.040* | |
| H19C | 0.0740 | 0.3717 | 1.1232 | 0.040* | |
| C20 | −0.0861 (2) | 0.4506 (2) | 0.9308 (2) | 0.0283 (6) | |
| H20A | −0.0908 | 0.5024 | 0.8681 | 0.042* | |
| H20B | −0.1099 | 0.4861 | 1.0004 | 0.042* | |
| H20C | −0.1552 | 0.3930 | 0.9105 | 0.042* | |
| C21 | 0.3243 (2) | 0.39168 (18) | 0.91067 (17) | 0.0173 (4) | |
| H21A | 0.3322 | 0.3138 | 0.8986 | 0.021* | |
| H21B | 0.3659 | 0.4092 | 0.9895 | 0.021* | |
| C22 | 0.3990 (2) | 0.45412 (18) | 0.82278 (17) | 0.0154 (4) | |
| C23 | 0.5286 (2) | 0.40276 (19) | 0.77721 (18) | 0.0199 (5) | |
| H23 | 0.5990 | 0.3832 | 0.8444 | 0.024* | |
| C24 | 0.4981 (3) | 0.3034 (2) | 0.7059 (2) | 0.0280 (5) | |
| H24A | 0.4503 | 0.2504 | 0.7499 | 0.042* | |
| H24B | 0.5888 | 0.2738 | 0.6855 | 0.042* | |
| H24C | 0.4354 | 0.3213 | 0.6361 | 0.042* | |
| C25 | 0.5938 (2) | 0.4943 (2) | 0.71440 (19) | 0.0229 (5) | |
| H25A | 0.5450 | 0.5011 | 0.6356 | 0.027* | |
| H25B | 0.6977 | 0.4818 | 0.7105 | 0.027* | |
| C26 | 0.5709 (2) | 0.5955 (2) | 0.7840 (2) | 0.0247 (5) | |
| H26A | 0.5388 | 0.6555 | 0.7327 | 0.030* | |
| H26B | 0.6613 | 0.6162 | 0.8310 | 0.030* | |
| C27 | 0.4527 (2) | 0.56609 (18) | 0.86173 (17) | 0.0172 (4) | |
| H27 | 0.4996 | 0.5604 | 0.9422 | 0.021* | |
| C28 | 0.3310 (2) | 0.64559 (18) | 0.85997 (17) | 0.0176 (4) | |
| C29 | 0.1969 (2) | 0.61599 (17) | 0.82481 (17) | 0.0160 (4) | |
| H29 | 0.1210 | 0.6664 | 0.8241 | 0.019* | |
| C30 | 0.3704 (3) | 0.75649 (19) | 0.9014 (2) | 0.0253 (5) | |
| H30A | 0.2833 | 0.8004 | 0.8977 | 0.038* | |
| H30B | 0.4378 | 0.7880 | 0.8529 | 0.038* | |
| H30C | 0.4158 | 0.7532 | 0.9806 | 0.038* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| O1 | 0.0223 (7) | 0.0128 (7) | 0.0142 (8) | 0.0047 (6) | 0.0011 (6) | 0.0017 (6) |
| O2 | 0.0151 (6) | 0.0123 (7) | 0.0137 (7) | 0.0011 (5) | 0.0026 (5) | 0.0029 (6) |
| O3 | 0.0140 (7) | 0.0201 (8) | 0.0134 (7) | −0.0019 (6) | −0.0002 (5) | 0.0034 (6) |
| O4 | 0.0115 (6) | 0.0172 (8) | 0.0123 (7) | 0.0015 (6) | 0.0014 (5) | 0.0005 (6) |
| C1 | 0.0155 (9) | 0.0134 (10) | 0.0136 (9) | 0.0016 (8) | 0.0027 (7) | 0.0000 (8) |
| C2 | 0.0168 (9) | 0.0130 (10) | 0.0158 (10) | −0.0015 (8) | 0.0002 (7) | 0.0001 (8) |
| C3 | 0.0241 (10) | 0.0146 (10) | 0.0150 (10) | −0.0019 (9) | 0.0029 (8) | 0.0000 (8) |
| C4 | 0.0398 (13) | 0.0256 (12) | 0.0142 (10) | 0.0004 (11) | 0.0028 (9) | −0.0020 (9) |
| C5 | 0.0294 (12) | 0.0206 (12) | 0.0209 (11) | 0.0032 (9) | 0.0097 (9) | 0.0001 (9) |
| C6 | 0.0192 (10) | 0.0173 (11) | 0.0144 (10) | −0.0004 (8) | 0.0003 (8) | 0.0017 (8) |
| C7 | 0.0150 (9) | 0.0121 (10) | 0.0157 (10) | 0.0020 (8) | 0.0006 (7) | 0.0032 (8) |
| C8 | 0.0176 (10) | 0.0192 (12) | 0.0200 (10) | 0.0042 (8) | 0.0045 (8) | 0.0044 (9) |
| C9 | 0.0153 (10) | 0.0272 (13) | 0.0333 (13) | 0.0004 (9) | 0.0043 (9) | 0.0051 (10) |
| C10 | 0.0243 (11) | 0.0230 (12) | 0.0231 (11) | 0.0077 (9) | 0.0080 (9) | 0.0025 (9) |
| C11 | 0.0266 (11) | 0.0226 (13) | 0.0201 (11) | 0.0070 (10) | 0.0012 (9) | −0.0038 (9) |
| C12 | 0.0229 (10) | 0.0127 (10) | 0.0147 (10) | 0.0015 (8) | 0.0012 (8) | 0.0020 (8) |
| C13 | 0.0203 (10) | 0.0153 (11) | 0.0138 (10) | −0.0030 (8) | 0.0011 (8) | 0.0028 (8) |
| C14 | 0.0139 (9) | 0.0190 (11) | 0.0128 (10) | −0.0013 (8) | 0.0011 (7) | 0.0036 (8) |
| C15 | 0.0260 (11) | 0.0192 (12) | 0.0263 (12) | −0.0064 (9) | −0.0014 (9) | 0.0006 (10) |
| C16 | 0.0130 (9) | 0.0163 (10) | 0.0122 (9) | 0.0007 (8) | 0.0016 (7) | 0.0002 (8) |
| C17 | 0.0159 (9) | 0.0152 (10) | 0.0134 (9) | −0.0011 (8) | −0.0015 (8) | 0.0002 (8) |
| C18 | 0.0192 (10) | 0.0214 (12) | 0.0144 (10) | −0.0023 (9) | −0.0003 (8) | 0.0024 (9) |
| C19 | 0.0257 (12) | 0.0342 (14) | 0.0205 (11) | −0.0088 (10) | 0.0016 (9) | 0.0083 (10) |
| C20 | 0.0165 (11) | 0.0448 (16) | 0.0241 (12) | −0.0011 (11) | 0.0049 (9) | 0.0047 (11) |
| C21 | 0.0172 (10) | 0.0199 (11) | 0.0144 (10) | 0.0026 (9) | 0.0010 (8) | 0.0033 (8) |
| C22 | 0.0123 (9) | 0.0213 (11) | 0.0119 (10) | 0.0018 (8) | −0.0023 (7) | 0.0017 (8) |
| C23 | 0.0155 (10) | 0.0271 (13) | 0.0173 (10) | 0.0053 (9) | 0.0021 (8) | 0.0033 (9) |
| C24 | 0.0269 (11) | 0.0262 (14) | 0.0322 (13) | 0.0075 (10) | 0.0096 (10) | −0.0035 (10) |
| C25 | 0.0163 (10) | 0.0322 (13) | 0.0206 (11) | 0.0011 (9) | 0.0040 (8) | 0.0044 (10) |
| C26 | 0.0169 (10) | 0.0300 (13) | 0.0272 (12) | −0.0034 (9) | 0.0034 (9) | 0.0039 (10) |
| C27 | 0.0158 (10) | 0.0238 (12) | 0.0114 (10) | −0.0031 (8) | −0.0008 (8) | −0.0002 (8) |
| C28 | 0.0228 (10) | 0.0179 (11) | 0.0128 (10) | −0.0004 (9) | 0.0041 (8) | −0.0005 (8) |
| C29 | 0.0183 (9) | 0.0151 (11) | 0.0150 (10) | 0.0018 (8) | 0.0044 (8) | 0.0014 (8) |
| C30 | 0.0295 (12) | 0.0221 (12) | 0.0240 (12) | −0.0065 (10) | 0.0023 (9) | −0.0060 (10) |
Geometric parameters (Å, °)
| O1—C1 | 1.390 (3) | C14—H14 | 0.9500 |
| O1—H1 | 0.89 (3) | C15—H15A | 0.9800 |
| O2—C1 | 1.449 (2) | C15—H15B | 0.9800 |
| O2—C7 | 1.453 (2) | C15—H15C | 0.9800 |
| O3—C16 | 1.385 (2) | C16—C29 | 1.513 (3) |
| O3—H3 | 0.86 (3) | C16—C17 | 1.523 (3) |
| O4—C22 | 1.447 (2) | C17—C18 | 1.329 (3) |
| O4—C16 | 1.455 (2) | C17—C21 | 1.522 (3) |
| C1—C14 | 1.511 (3) | C18—C20 | 1.501 (3) |
| C1—C2 | 1.523 (3) | C18—C19 | 1.512 (3) |
| C2—C3 | 1.329 (3) | C19—H19A | 0.9800 |
| C2—C6 | 1.515 (3) | C19—H19B | 0.9800 |
| C3—C4 | 1.503 (3) | C19—H19C | 0.9800 |
| C3—C5 | 1.510 (3) | C20—H20A | 0.9800 |
| C4—H4A | 0.9800 | C20—H20B | 0.9800 |
| C4—H4B | 0.9800 | C20—H20C | 0.9800 |
| C4—H4C | 0.9800 | C21—C22 | 1.529 (3) |
| C5—H5A | 0.9800 | C21—H21A | 0.9900 |
| C5—H5B | 0.9800 | C21—H21B | 0.9900 |
| C5—H5C | 0.9800 | C22—C23 | 1.522 (3) |
| C6—C7 | 1.530 (3) | C22—C27 | 1.542 (3) |
| C6—H6A | 0.9900 | C23—C24 | 1.511 (3) |
| C6—H6B | 0.9900 | C23—C25 | 1.529 (3) |
| C7—C8 | 1.521 (3) | C23—H23 | 1.0000 |
| C7—C12 | 1.545 (3) | C24—H24A | 0.9800 |
| C8—C9 | 1.524 (3) | C24—H24B | 0.9800 |
| C8—C10 | 1.534 (3) | C24—H24C | 0.9800 |
| C8—H8 | 1.0000 | C25—C26 | 1.539 (3) |
| C9—H9A | 0.9800 | C25—H25A | 0.9900 |
| C9—H9B | 0.9800 | C25—H25B | 0.9900 |
| C9—H9C | 0.9800 | C26—C27 | 1.557 (3) |
| C10—C11 | 1.537 (3) | C26—H26A | 0.9900 |
| C10—H10A | 0.9900 | C26—H26B | 0.9900 |
| C10—H10B | 0.9900 | C27—C28 | 1.511 (3) |
| C11—C12 | 1.554 (3) | C27—H27 | 1.0000 |
| C11—H11A | 0.9900 | C28—C29 | 1.328 (3) |
| C11—H11B | 0.9900 | C28—C30 | 1.505 (3) |
| C12—C13 | 1.514 (3) | C29—H29 | 0.9500 |
| C12—H12 | 1.0000 | C30—H30A | 0.9800 |
| C13—C14 | 1.330 (3) | C30—H30B | 0.9800 |
| C13—C15 | 1.500 (3) | C30—H30C | 0.9800 |
| C1—O1—H1 | 105 (2) | H15A—C15—H15C | 109.5 |
| C1—O2—C7 | 103.23 (13) | H15B—C15—H15C | 109.5 |
| C16—O3—H3 | 108 (2) | O3—C16—O4 | 108.49 (15) |
| C22—O4—C16 | 103.31 (13) | O3—C16—C29 | 114.09 (17) |
| O1—C1—O2 | 108.73 (15) | O4—C16—C29 | 106.99 (15) |
| O1—C1—C14 | 113.42 (17) | O3—C16—C17 | 113.64 (17) |
| O2—C1—C14 | 107.18 (16) | O4—C16—C17 | 102.46 (15) |
| O1—C1—C2 | 113.92 (17) | C29—C16—C17 | 110.27 (16) |
| O2—C1—C2 | 102.77 (15) | C18—C17—C21 | 126.42 (19) |
| C14—C1—C2 | 110.05 (16) | C18—C17—C16 | 128.31 (19) |
| C3—C2—C6 | 126.34 (18) | C21—C17—C16 | 105.13 (16) |
| C3—C2—C1 | 128.53 (19) | C17—C18—C20 | 124.1 (2) |
| C6—C2—C1 | 105.00 (16) | C17—C18—C19 | 121.5 (2) |
| C2—C3—C4 | 120.92 (19) | C20—C18—C19 | 114.39 (18) |
| C2—C3—C5 | 124.38 (19) | C18—C19—H19A | 109.5 |
| C4—C3—C5 | 114.66 (17) | C18—C19—H19B | 109.5 |
| C3—C4—H4A | 109.5 | H19A—C19—H19B | 109.5 |
| C3—C4—H4B | 109.5 | C18—C19—H19C | 109.5 |
| H4A—C4—H4B | 109.5 | H19A—C19—H19C | 109.5 |
| C3—C4—H4C | 109.5 | H19B—C19—H19C | 109.5 |
| H4A—C4—H4C | 109.5 | C18—C20—H20A | 109.5 |
| H4B—C4—H4C | 109.5 | C18—C20—H20B | 109.5 |
| C3—C5—H5A | 109.5 | H20A—C20—H20B | 109.5 |
| C3—C5—H5B | 109.5 | C18—C20—H20C | 109.5 |
| H5A—C5—H5B | 109.5 | H20A—C20—H20C | 109.5 |
| C3—C5—H5C | 109.5 | H20B—C20—H20C | 109.5 |
| H5A—C5—H5C | 109.5 | C17—C21—C22 | 103.41 (16) |
| H5B—C5—H5C | 109.5 | C17—C21—H21A | 111.1 |
| C2—C6—C7 | 103.77 (16) | C22—C21—H21A | 111.1 |
| C2—C6—H6A | 111.0 | C17—C21—H21B | 111.1 |
| C7—C6—H6A | 111.0 | C22—C21—H21B | 111.1 |
| C2—C6—H6B | 111.0 | H21A—C21—H21B | 109.0 |
| C7—C6—H6B | 111.0 | O4—C22—C23 | 108.73 (16) |
| H6A—C6—H6B | 109.0 | O4—C22—C21 | 102.34 (15) |
| O2—C7—C8 | 109.23 (15) | C23—C22—C21 | 117.75 (18) |
| O2—C7—C6 | 102.38 (16) | O4—C22—C27 | 108.54 (16) |
| C8—C7—C6 | 118.09 (17) | C23—C22—C27 | 104.17 (16) |
| O2—C7—C12 | 108.61 (15) | C21—C22—C27 | 115.02 (16) |
| C8—C7—C12 | 104.05 (16) | C24—C23—C22 | 115.91 (18) |
| C6—C7—C12 | 114.25 (16) | C24—C23—C25 | 114.24 (18) |
| C7—C8—C9 | 114.86 (18) | C22—C23—C25 | 103.31 (18) |
| C7—C8—C10 | 103.63 (17) | C24—C23—H23 | 107.7 |
| C9—C8—C10 | 114.29 (18) | C22—C23—H23 | 107.7 |
| C7—C8—H8 | 107.9 | C25—C23—H23 | 107.7 |
| C9—C8—H8 | 107.9 | C23—C24—H24A | 109.5 |
| C10—C8—H8 | 107.9 | C23—C24—H24B | 109.5 |
| C8—C9—H9A | 109.5 | H24A—C24—H24B | 109.5 |
| C8—C9—H9B | 109.5 | C23—C24—H24C | 109.5 |
| H9A—C9—H9B | 109.5 | H24A—C24—H24C | 109.5 |
| C8—C9—H9C | 109.5 | H24B—C24—H24C | 109.5 |
| H9A—C9—H9C | 109.5 | C23—C25—C26 | 105.89 (17) |
| H9B—C9—H9C | 109.5 | C23—C25—H25A | 110.6 |
| C8—C10—C11 | 105.77 (17) | C26—C25—H25A | 110.6 |
| C8—C10—H10A | 110.6 | C23—C25—H25B | 110.6 |
| C11—C10—H10A | 110.6 | C26—C25—H25B | 110.6 |
| C8—C10—H10B | 110.6 | H25A—C25—H25B | 108.7 |
| C11—C10—H10B | 110.6 | C25—C26—C27 | 105.59 (19) |
| H10A—C10—H10B | 108.7 | C25—C26—H26A | 110.6 |
| C10—C11—C12 | 105.99 (18) | C27—C26—H26A | 110.6 |
| C10—C11—H11A | 110.5 | C25—C26—H26B | 110.6 |
| C12—C11—H11A | 110.5 | C27—C26—H26B | 110.6 |
| C10—C11—H11B | 110.5 | H26A—C26—H26B | 108.8 |
| C12—C11—H11B | 110.5 | C28—C27—C22 | 112.18 (17) |
| H11A—C11—H11B | 108.7 | C28—C27—C26 | 114.80 (18) |
| C13—C12—C7 | 111.99 (17) | C22—C27—C26 | 105.62 (17) |
| C13—C12—C11 | 114.85 (17) | C28—C27—H27 | 108.0 |
| C7—C12—C11 | 105.52 (17) | C22—C27—H27 | 108.0 |
| C13—C12—H12 | 108.1 | C26—C27—H27 | 108.0 |
| C7—C12—H12 | 108.1 | C29—C28—C30 | 123.2 (2) |
| C11—C12—H12 | 108.1 | C29—C28—C27 | 120.2 (2) |
| C14—C13—C15 | 122.5 (2) | C30—C28—C27 | 116.66 (18) |
| C14—C13—C12 | 120.07 (19) | C28—C29—C16 | 120.68 (18) |
| C15—C13—C12 | 117.38 (19) | C28—C29—H29 | 119.7 |
| C13—C14—C1 | 120.76 (19) | C16—C29—H29 | 119.7 |
| C13—C14—H14 | 119.6 | C28—C30—H30A | 109.5 |
| C1—C14—H14 | 119.6 | C28—C30—H30B | 109.5 |
| C13—C15—H15A | 109.5 | H30A—C30—H30B | 109.5 |
| C13—C15—H15B | 109.5 | C28—C30—H30C | 109.5 |
| H15A—C15—H15B | 109.5 | H30A—C30—H30C | 109.5 |
| C13—C15—H15C | 109.5 | H30B—C30—H30C | 109.5 |
| C7—O2—C1—O1 | −166.41 (15) | C22—O4—C16—O3 | −165.83 (16) |
| C7—O2—C1—C14 | 70.62 (18) | C22—O4—C16—C29 | 70.65 (18) |
| C7—O2—C1—C2 | −45.36 (18) | C22—O4—C16—C17 | −45.36 (18) |
| O1—C1—C2—C3 | −41.0 (3) | O3—C16—C17—C18 | −42.5 (3) |
| O2—C1—C2—C3 | −158.4 (2) | O4—C16—C17—C18 | −159.3 (2) |
| C14—C1—C2—C3 | 87.7 (3) | C29—C16—C17—C18 | 87.1 (3) |
| O1—C1—C2—C6 | 142.99 (17) | O3—C16—C17—C21 | 141.52 (17) |
| O2—C1—C2—C6 | 25.6 (2) | O4—C16—C17—C21 | 24.7 (2) |
| C14—C1—C2—C6 | −88.34 (19) | C29—C16—C17—C21 | −88.93 (19) |
| C6—C2—C3—C4 | −1.1 (3) | C21—C17—C18—C20 | 175.0 (2) |
| C1—C2—C3—C4 | −176.4 (2) | C16—C17—C18—C20 | −0.2 (4) |
| C6—C2—C3—C5 | 176.2 (2) | C21—C17—C18—C19 | −2.8 (4) |
| C1—C2—C3—C5 | 1.0 (4) | C16—C17—C18—C19 | −178.0 (2) |
| C3—C2—C6—C7 | −173.7 (2) | C18—C17—C21—C22 | −172.6 (2) |
| C1—C2—C6—C7 | 2.5 (2) | C16—C17—C21—C22 | 3.6 (2) |
| C1—O2—C7—C8 | 172.96 (16) | C16—O4—C22—C23 | 173.18 (17) |
| C1—O2—C7—C6 | 47.00 (17) | C16—O4—C22—C21 | 47.91 (18) |
| C1—O2—C7—C12 | −74.17 (17) | C16—O4—C22—C27 | −74.09 (17) |
| C2—C6—C7—O2 | −29.52 (19) | C17—C21—C22—O4 | −30.8 (2) |
| C2—C6—C7—C8 | −149.48 (18) | C17—C21—C22—C23 | −149.86 (18) |
| C2—C6—C7—C12 | 87.7 (2) | C17—C21—C22—C27 | 86.7 (2) |
| O2—C7—C8—C9 | −48.6 (2) | O4—C22—C23—C24 | −49.5 (2) |
| C6—C7—C8—C9 | 67.7 (2) | C21—C22—C23—C24 | 66.2 (3) |
| C12—C7—C8—C9 | −164.47 (18) | C27—C22—C23—C24 | −165.10 (18) |
| O2—C7—C8—C10 | 76.7 (2) | O4—C22—C23—C25 | 76.2 (2) |
| C6—C7—C8—C10 | −166.94 (19) | C21—C22—C23—C25 | −168.10 (18) |
| C12—C7—C8—C10 | −39.1 (2) | C27—C22—C23—C25 | −39.4 (2) |
| C7—C8—C10—C11 | 34.1 (2) | C24—C23—C25—C26 | 162.08 (18) |
| C9—C8—C10—C11 | 159.84 (18) | C22—C23—C25—C26 | 35.3 (2) |
| C8—C10—C11—C12 | −15.7 (2) | C23—C25—C26—C27 | −17.4 (2) |
| O2—C7—C12—C13 | 38.7 (2) | O4—C22—C27—C28 | 38.7 (2) |
| C8—C7—C12—C13 | 155.00 (16) | C23—C22—C27—C28 | 154.41 (17) |
| C6—C7—C12—C13 | −74.8 (2) | C21—C22—C27—C28 | −75.2 (2) |
| O2—C7—C12—C11 | −86.90 (18) | O4—C22—C27—C26 | −87.07 (18) |
| C8—C7—C12—C11 | 29.4 (2) | C23—C22—C27—C26 | 28.7 (2) |
| C6—C7—C12—C11 | 159.54 (17) | C21—C22—C27—C26 | 159.03 (17) |
| C10—C11—C12—C13 | −132.17 (19) | C25—C26—C27—C28 | −131.0 (2) |
| C10—C11—C12—C7 | −8.3 (2) | C25—C26—C27—C22 | −6.9 (2) |
| C7—C12—C13—C14 | −1.7 (3) | C22—C27—C28—C29 | −1.6 (3) |
| C11—C12—C13—C14 | 118.7 (2) | C26—C27—C28—C29 | 119.0 (2) |
| C7—C12—C13—C15 | 176.55 (17) | C22—C27—C28—C30 | 177.41 (17) |
| C11—C12—C13—C15 | −63.1 (2) | C26—C27—C28—C30 | −62.0 (2) |
| C15—C13—C14—C1 | −178.33 (18) | C30—C28—C29—C16 | −179.05 (19) |
| C12—C13—C14—C1 | −0.2 (3) | C27—C28—C29—C16 | −0.1 (3) |
| O1—C1—C14—C13 | −154.67 (18) | O3—C16—C29—C28 | −154.60 (18) |
| O2—C1—C14—C13 | −34.7 (2) | O4—C16—C29—C28 | −34.6 (2) |
| C2—C1—C14—C13 | 76.4 (2) | C17—C16—C29—C28 | 76.1 (2) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| O1—H1···O4 | 0.89 (3) | 1.92 (3) | 2.799 (2) | 168 (3) |
| O3—H3···O2 | 0.86 (3) | 1.92 (3) | 2.771 (2) | 171 (3) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: HB5676).
References
- Barbour, L. J. (2001). J. Supramol. Chem.1, 189–191.
- Bruker (2009). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Muangsin, N., Ngamrojnavanich, N., Onanong, S., Chaichit, N., Roengsumran, S. & Petsom, A. (2004). J. Struct. Chem.45, 293–297.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Westrip, S. P. (2010). J. Appl. Cryst.43, 920–925.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810040559/hb5676sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810040559/hb5676Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report