Abstract
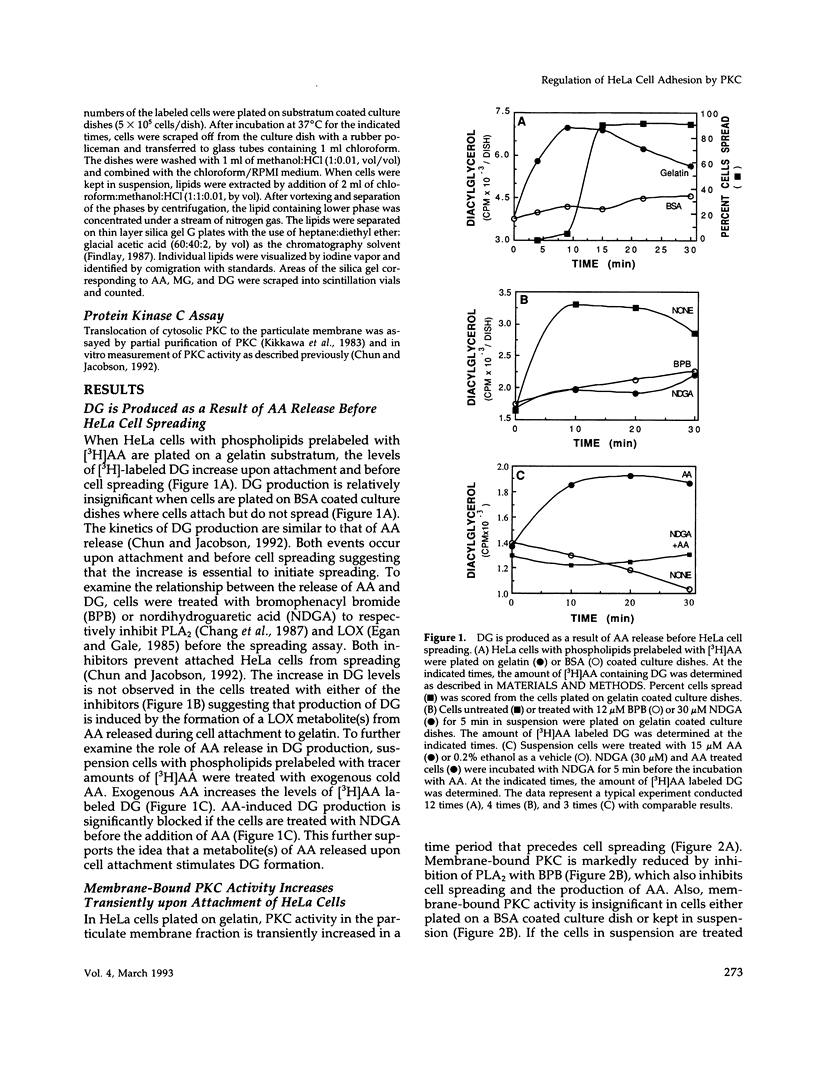
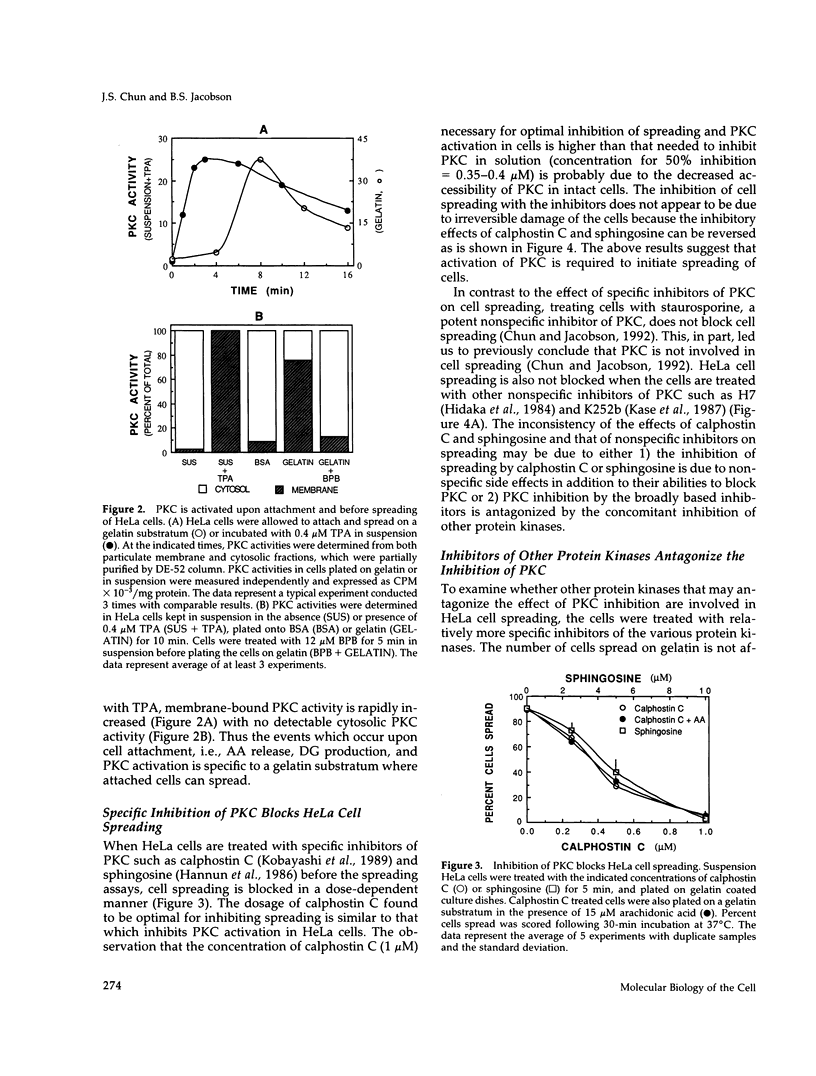
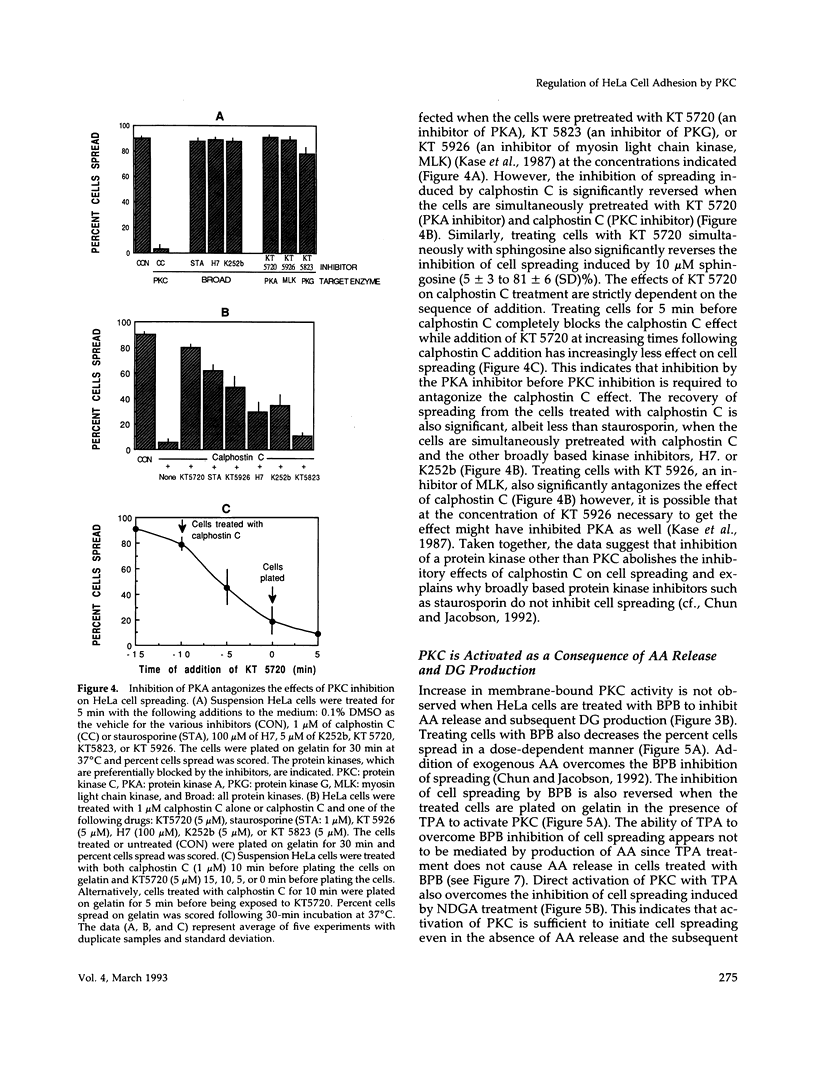
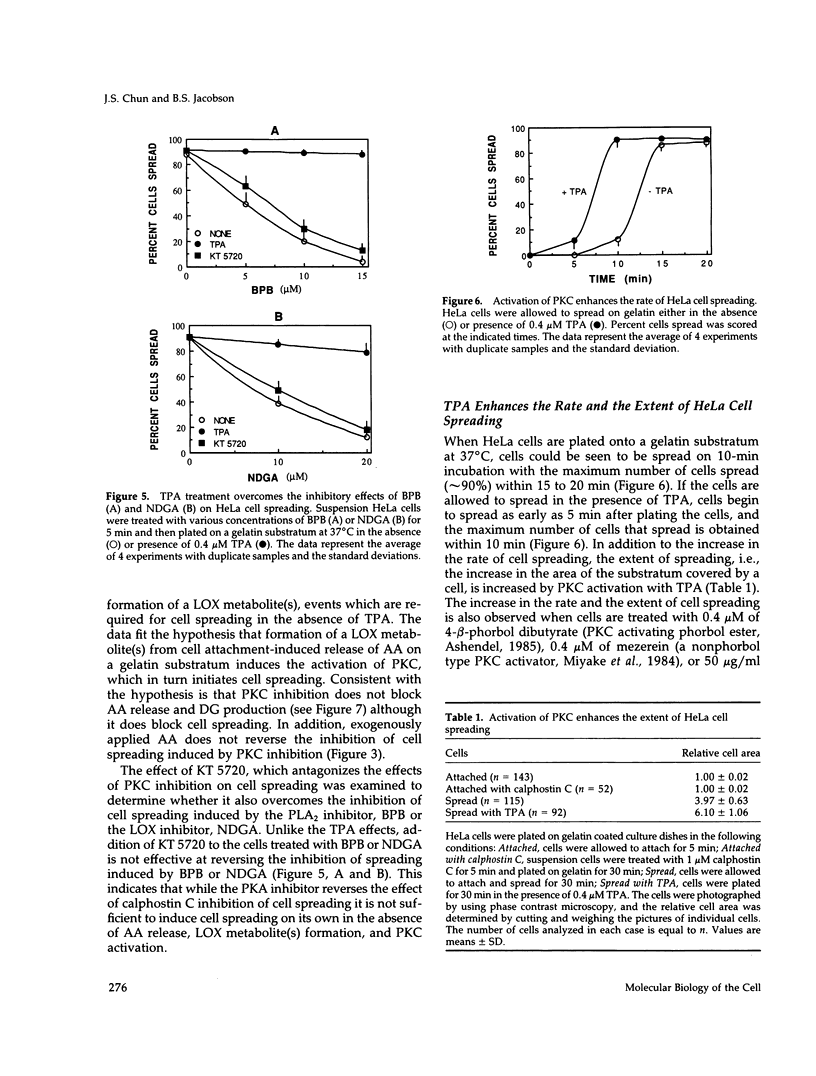
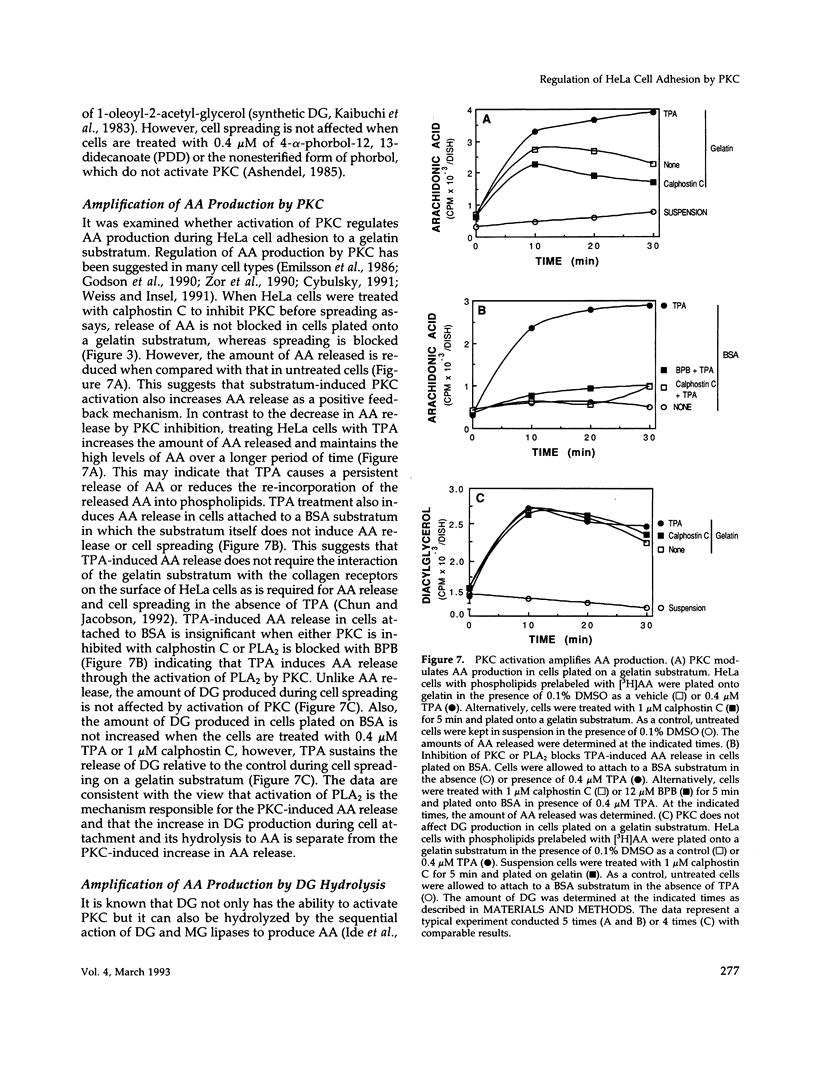
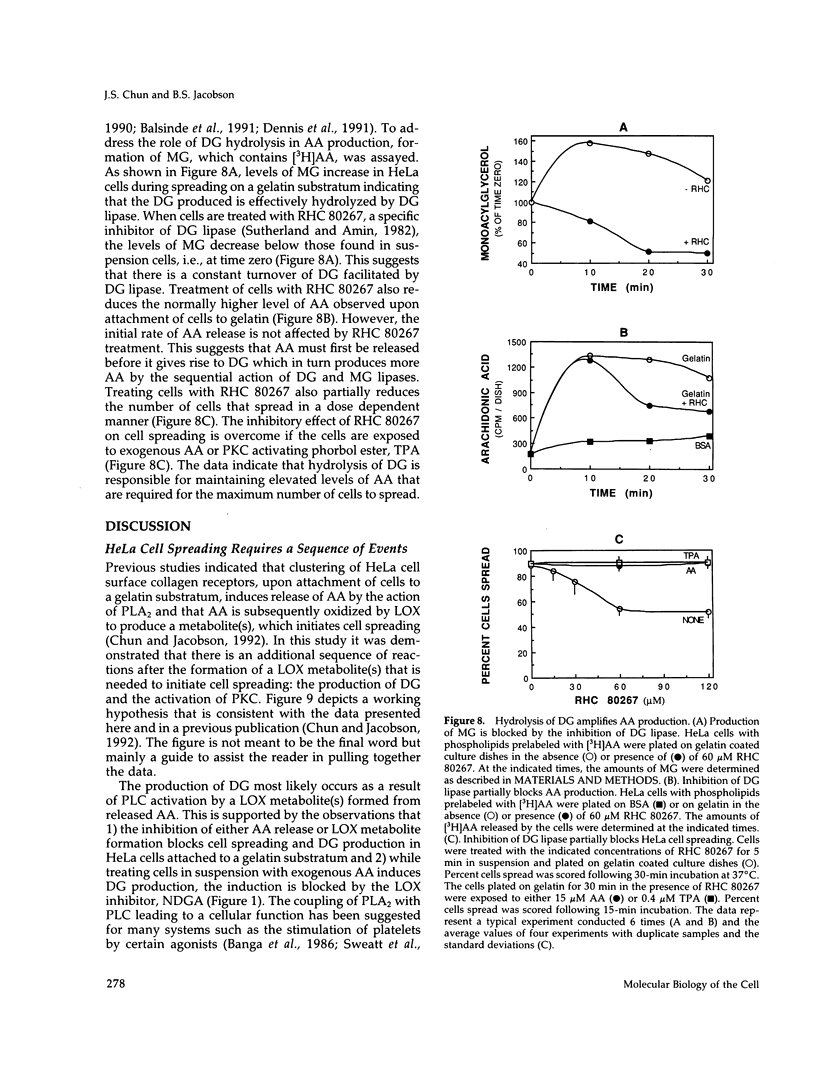
Release of arachidonic acid (AA) and subsequent formation of a lipoxygenase (LOX) metabolite(s) is an obligatory signal to induce spreading of HeLa cells on a gelatin substratum (Chun and Jacobson, 1992). This study characterizes signaling pathways that follow the LOX metabolite(s) formation. Levels of diacylglycerol (DG) increase upon attachment and before cell spreading on a gelatin substratum. DG production and cell spreading are insignificant when phospholipase A2 (PLA2) or LOX is blocked. In contrast, when cells in suspension where PLA2 activity is not stimulated are treated with exogenous AA, DG production is turned on, and inhibition of LOX turns it off. This indicates that the formation of a LOX metabolite(s) from AA released during cell attachment induces the production of DG. Consistent with the DG production is the activation of protein kinase C (PKC) which, as with AA and DG, occurs upon attachment and before cell spreading. Inhibition of AA release and subsequent DG production blocks both PKC activation and cell spreading. Cell spreading is also blocked by the inhibition of PKC with calphostin C or sphingosine. The inhibition of cell spreading induced by blocking AA release is reversed by the direct activation of PKC with phorbol ester. However, the inhibition of cell spreading induced by PKC inhibition is not reversed by exogenously applied AA. In addition, inhibition of PKC does not block AA release and DG production. The data indicate that there is a sequence of events triggered by HeLa cell attachment to a gelatin substratum that leads to the initiation of cell spreading: AA release, a LOX metabolite(s) formation, DG production, and PKC activation. The data also provide evidence indicating that HeLa cell spreading is a cyclic feedback amplification process centered on the production of AA, which is the first messenger produced in the sequence of messengers initiating cell spreading. Both DG and PKC activity that are increased during HeLa cell attachment to a gelatin substratum appear to be involved. DG not only activates PKC, which is essential for cell spreading, but is also hydrolyzed to AA. PKC, which is initially activated as consequence of AA production, also increases more AA production by activating PLA2.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashendel C. L. The phorbol ester receptor: a phospholipid-regulated protein kinase. Biochim Biophys Acta. 1985 Sep 9;822(2):219–242. doi: 10.1016/0304-4157(85)90009-7. [DOI] [PubMed] [Google Scholar]
- Balsinde J., Diez E., Mollinedo F. Arachidonic acid release from diacylglycerol in human neutrophils. Translocation of diacylglycerol-deacylating enzyme activities from an intracellular pool to plasma membrane upon cell activation. J Biol Chem. 1991 Aug 25;266(24):15638–15643. [PubMed] [Google Scholar]
- Banga H. S., Simons E. R., Brass L. F., Rittenhouse S. E. Activation of phospholipases A and C in human platelets exposed to epinephrine: role of glycoproteins IIb/IIIa and dual role of epinephrine. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9197–9201. doi: 10.1073/pnas.83.23.9197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhagyalakshmi A., Frangos J. A. Mechanism of shear-induced prostacyclin production in endothelial cells. Biochem Biophys Res Commun. 1989 Jan 16;158(1):31–37. doi: 10.1016/s0006-291x(89)80172-x. [DOI] [PubMed] [Google Scholar]
- Blackshear P. J., Nairn A. C., Kuo J. F. Protein kinases 1988: a current perspective. FASEB J. 1988 Nov;2(14):2957–2969. doi: 10.1096/fasebj.2.14.2972578. [DOI] [PubMed] [Google Scholar]
- Chang J., Musser J. H., McGregor H. Phospholipase A2: function and pharmacological regulation. Biochem Pharmacol. 1987 Aug 1;36(15):2429–2436. doi: 10.1016/0006-2952(87)90512-0. [DOI] [PubMed] [Google Scholar]
- Chun J. S., Jacobson B. S. Spreading of HeLa cells on a collagen substratum requires a second messenger formed by the lipoxygenase metabolism of arachidonic acid released by collagen receptor clustering. Mol Biol Cell. 1992 May;3(5):481–492. doi: 10.1091/mbc.3.5.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cybulsky A. V. Release of arachidonic acid by complement C5b-9 complex in glomerular epithelial cells. Am J Physiol. 1991 Sep;261(3 Pt 2):F427–F436. doi: 10.1152/ajprenal.1991.261.3.F427. [DOI] [PubMed] [Google Scholar]
- Danilov Y. N., Juliano R. L. Phorbol ester modulation of integrin-mediated cell adhesion: a postreceptor event. J Cell Biol. 1989 May;108(5):1925–1933. doi: 10.1083/jcb.108.5.1925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis E. A., Rhee S. G., Billah M. M., Hannun Y. A. Role of phospholipase in generating lipid second messengers in signal transduction. FASEB J. 1991 Apr;5(7):2068–2077. doi: 10.1096/fasebj.5.7.1901288. [DOI] [PubMed] [Google Scholar]
- Emilsson A., Wijkander J., Sundler R. Diacylglycerol induces deacylation of phosphatidylinositol and mobilization of arachidonic acid in mouse macrophages. Comparison with induction by phorbol diester. Biochem J. 1986 Nov 1;239(3):685–690. doi: 10.1042/bj2390685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godson C., Weiss B. A., Insel P. A. Differential activation of protein kinase C alpha is associated with arachidonate release in Madin-Darby canine kidney cells. J Biol Chem. 1990 May 25;265(15):8369–8372. [PubMed] [Google Scholar]
- Grillone L. R., Clark M. A., Godfrey R. W., Stassen F., Crooke S. T. Vasopressin induces V1 receptors to activate phosphatidylinositol- and phosphatidylcholine-specific phospholipase C and stimulates the release of arachidonic acid by at least two pathways in the smooth muscle cell line, A-10. J Biol Chem. 1988 Feb 25;263(6):2658–2663. [PubMed] [Google Scholar]
- Grossi I. M., Fitzgerald L. A., Umbarger L. A., Nelson K. K., Diglio C. A., Taylor J. D., Honn K. V. Bidirectional control of membrane expression and/or activation of the tumor cell IRGpIIb/IIIa receptor and tumor cell adhesion by lipoxygenase products of arachidonic acid and linoleic acid. Cancer Res. 1989 Feb 15;49(4):1029–1037. [PubMed] [Google Scholar]
- Hannun Y. A., Loomis C. R., Merrill A. H., Jr, Bell R. M. Sphingosine inhibition of protein kinase C activity and of phorbol dibutyrate binding in vitro and in human platelets. J Biol Chem. 1986 Sep 25;261(27):12604–12609. [PubMed] [Google Scholar]
- Hidaka H., Inagaki M., Kawamoto S., Sasaki Y. Isoquinolinesulfonamides, novel and potent inhibitors of cyclic nucleotide dependent protein kinase and protein kinase C. Biochemistry. 1984 Oct 9;23(21):5036–5041. doi: 10.1021/bi00316a032. [DOI] [PubMed] [Google Scholar]
- Huang K. P. The mechanism of protein kinase C activation. Trends Neurosci. 1989 Nov;12(11):425–432. doi: 10.1016/0166-2236(89)90091-x. [DOI] [PubMed] [Google Scholar]
- Ide H., Koyama S., Nakazawa Y. Diacylglycerol generated in the phospholipid vesicles by phospholipase C is effectively utilized by diacylglycerol lipase in rat liver cytosol. Biochim Biophys Acta. 1990 May 22;1044(2):179–186. doi: 10.1016/0005-2760(90)90301-d. [DOI] [PubMed] [Google Scholar]
- Kaibuchi K., Takai Y., Sawamura M., Hoshijima M., Fujikura T., Nishizuka Y. Synergistic functions of protein phosphorylation and calcium mobilization in platelet activation. J Biol Chem. 1983 Jun 10;258(11):6701–6704. [PubMed] [Google Scholar]
- Kanoh H., Yamada K., Sakane F. Diacylglycerol kinase: a key modulator of signal transduction? Trends Biochem Sci. 1990 Feb;15(2):47–50. doi: 10.1016/0968-0004(90)90172-8. [DOI] [PubMed] [Google Scholar]
- Kase H., Iwahashi K., Nakanishi S., Matsuda Y., Yamada K., Takahashi M., Murakata C., Sato A., Kaneko M. K-252 compounds, novel and potent inhibitors of protein kinase C and cyclic nucleotide-dependent protein kinases. Biochem Biophys Res Commun. 1987 Jan 30;142(2):436–440. doi: 10.1016/0006-291x(87)90293-2. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Minakuchi R., Takai Y., Nishizuka Y. Calcium-activated, phospholipid-dependent protein kinase (protein kinase C) from rat brain. Methods Enzymol. 1983;99:288–298. doi: 10.1016/0076-6879(83)99064-x. [DOI] [PubMed] [Google Scholar]
- Kikkawa U., Nishizuka Y. The role of protein kinase C in transmembrane signalling. Annu Rev Cell Biol. 1986;2:149–178. doi: 10.1146/annurev.cb.02.110186.001053. [DOI] [PubMed] [Google Scholar]
- Kobayashi E., Nakano H., Morimoto M., Tamaoki T. Calphostin C (UCN-1028C), a novel microbial compound, is a highly potent and specific inhibitor of protein kinase C. Biochem Biophys Res Commun. 1989 Mar 15;159(2):548–553. doi: 10.1016/0006-291x(89)90028-4. [DOI] [PubMed] [Google Scholar]
- Mercurio A. M., Shaw L. M. Macrophage interactions with laminin: PMA selectively induces the adherence and spreading of mouse macrophages on a laminin substratum. J Cell Biol. 1988 Nov;107(5):1873–1880. doi: 10.1083/jcb.107.5.1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mire-Sluis A. R., Cox C. A., Hoffbrand A. V., Wickremasinghe R. G. Inhibitors of arachidonic acid lipoxygenase impair the stimulation of inositol phospholipid hydrolysis by the T lymphocyte mitogen phytohaemagglutinin. FEBS Lett. 1989 Nov 20;258(1):84–88. doi: 10.1016/0014-5793(89)81621-7. [DOI] [PubMed] [Google Scholar]
- Miyake R., Tanaka Y., Tsuda T., Kaibuchi K., Kikkawa U., Nishizuka Y. Activation of protein kinase C by non-phorbol tumor promoter, mezerein. Biochem Biophys Res Commun. 1984 Jun 15;121(2):649–656. doi: 10.1016/0006-291x(84)90231-6. [DOI] [PubMed] [Google Scholar]
- Needleman P., Turk J., Jakschik B. A., Morrison A. R., Lefkowith J. B. Arachidonic acid metabolism. Annu Rev Biochem. 1986;55:69–102. doi: 10.1146/annurev.bi.55.070186.000441. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Pandol S. J., Hsu Y. L., Kondratenko N. F., Schoeffield-Payne M. S., Steinbach J. H. Dual pathways for agonist-stimulated arachidonic acid release in pancreatic acini: roles in secretion. Am J Physiol. 1991 Mar;260(3 Pt 1):G423–G433. doi: 10.1152/ajpgi.1991.260.3.G423. [DOI] [PubMed] [Google Scholar]
- Rapuano B. E., Bockman R. S. Tumor necrosis factor-alpha stimulates phosphatidylinositol breakdown by phospholipase C to coordinately increase the levels of diacylglycerol, free arachidonic acid and prostaglandins in an osteoblast (MC3T3-E1) cell line. Biochim Biophys Acta. 1991 Feb 19;1091(3):374–384. doi: 10.1016/0167-4889(91)90203-a. [DOI] [PubMed] [Google Scholar]
- Shinomura T., Asaoka Y., Oka M., Yoshida K., Nishizuka Y. Synergistic action of diacylglycerol and unsaturated fatty acid for protein kinase C activation: its possible implications. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5149–5153. doi: 10.1073/pnas.88.12.5149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland C. A., Amin D. Relative activities of rat and dog platelet phospholipase A2 and diglyceride lipase. Selective inhibition of diglyceride lipase by RHC 80267. J Biol Chem. 1982 Dec 10;257(23):14006–14010. [PubMed] [Google Scholar]
- Sweatt J. D., Blair I. A., Cragoe E. J., Limbird L. E. Inhibitors of Na+/H+ exchange block epinephrine- and ADP-induced stimulation of human platelet phospholipase C by blockade of arachidonic acid release at a prior step. J Biol Chem. 1986 Jul 5;261(19):8660–8666. [PubMed] [Google Scholar]
- Takenaga K., Takahashi K. Effects of 12-O-tetradecanoylphorbol-13-acetate on adhesiveness and lung-colonizing ability of Lewis lung carcinoma cells. Cancer Res. 1986 Jan;46(1):375–380. [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Weiss B. A., Insel P. A. Intracellular Ca2+ and protein kinase C interact to regulate alpha 1-adrenergic- and bradykinin receptor-stimulated phospholipase A2 activation in Madin-Darby canine kidney cells. J Biol Chem. 1991 Feb 5;266(4):2126–2133. [PubMed] [Google Scholar]
- Zor U., Her E., Harell T., Fischer G., Naor Z., Braquet P., Ferber E., Reiss N. Arachidonic acid release by basophilic leukemia cells and macrophages stimulated by Ca2+ ionophores, antigen and diacylglycerol: essential role for protein kinase C and prevention by glucocorticosteroids. Biochim Biophys Acta. 1991 Feb 19;1091(3):385–392. doi: 10.1016/0167-4889(91)90204-b. [DOI] [PubMed] [Google Scholar]



