Abstract
The title compound, C14H12N2, was synthesized by the reaction of 4-methyl-N-(2-nitrobenzyl)aniline with tin(II) chloride dihydrate in ethanol at 313 K. The indazole ring system is almost planar with a dihedral angle of 1.58 (10)° between the rings, whereas the plane of the attached p-tolyl substituent shows a dihedral angle of 46.26 (5)° with respect to the indazole core.
Related literature
For the pharmaceutical properties of indazole derivatives, see: Bistochi et al. (1981 ▶); Cerecetto et al. (2005 ▶); Corsi et al. (1976 ▶); Keppler & Hartmann (1994 ▶); Picciola et al. (1981 ▶); Rodgers et al. (1996 ▶); Sun et al. (1997 ▶); Ykeda et al. (1979 ▶). For synthetic procedures for indazoles, see: Stadlbauer (2002 ▶).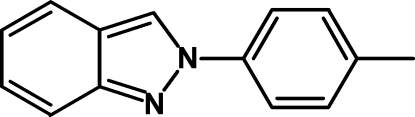
Experimental
Crystal data
C14H12N2
M r = 208.26
Monoclinic,

a = 12.539 (4) Å
b = 6.029 (2) Å
c = 14.401 (5) Å
β = 93.636 (5)°
V = 1086.4 (6) Å3
Z = 4
Mo Kα radiation
μ = 0.08 mm−1
T = 298 K
0.48 × 0.34 × 0.31 mm
Data collection
Bruker SMART CCD area-detector diffractometer
Absorption correction: multi-scan (SADABS; Sheldrick, 1996 ▶) T min = 0.969, T max = 0.980
5372 measured reflections
1915 independent reflections
1236 reflections with I > 2σ(I)
R int = 0.039
Refinement
R[F 2 > 2σ(F 2)] = 0.049
wR(F 2) = 0.140
S = 1.03
1911 reflections
145 parameters
H-atom parameters constrained
Δρmax = 0.27 e Å−3
Δρmin = −0.26 e Å−3
Data collection: SMART (Bruker, 1998 ▶); cell refinement: SAINT (Bruker, 1999 ▶); data reduction: SAINT; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810038948/im2233sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810038948/im2233Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Acknowledgments
This work was supported by the National Natural Science Foundation of China (30770602) and the Natural Science Foundation of Jiangsu Province, China (BK2010157).
supplementary crystallographic information
Comment
Indazole is well known as an aza analogue of indole, and a number of indazole derivatives have powerful pharmacological activities including anti-inflammatory (Bistochi et al., 1981; Picciola et al., 1981), antitumor (Keppler & Hartmann, 1994), anti-HIV (Sun et al., 1997; Rodgers et al., 1996), antidepressant (Ykeda et al., 1979), contraceptive activities (Corsi et al., 1976) as well as anti-aggregatory, and vasorelaxant activity by NO release (Cerecetto et al., 2005). Different approaches to the synthesis of 2-substituted indazoles have been reported (Stadlbauer, 2002). However, many of these still suffer from drawbacks as unsatisfactory yields, long reaction time and high temperature. Therefore, the development of more efficient methods for preparation of this kind of compounds is still an active research area.
We report here the crystal structure of the title compound, (I), which was synthesized by the reaction of 4-methyl-N-(2-nitrobenzyl)aniline with tin (II) chloride dihydrate using ethanol as solvent at 313 K.
In (I), the pyrazole ring (C1/C2/C7/N1/N2) is a new formed ring. The dihedral angle between the C1/C2/C7/N1/N2 plane and the C2/C3/C4/C5/C6/C7 plane is 1.58 (10)°, so the indazole ring shows an almost perfectly planar conformation. The dihedral angle between the C2/C3/C4/C5/C6/C7 plane and the C8/C9/C10/C11/C12/C13 of the p-tolyl substituent plane is 46.26 (5)°.
Experimental
The title compound, (I), was prepared by the reaction of 4-methyl-N-(2-nitrobenzyl)aniline (3 mmol) and tin (II) chloride dihydrate (6 mmol) in ethanol (20 ml) at 313 K (yield: 40%). Crystals of (I) suitable for X-ray diffraction were obtained by slow evaporation of an ethanolic solution. 1H NMR (DMSO-d6, δ): 2.39 (3H, s, CH3), 7.09–7.12 (1H, m, ArH), 7.29–7.33 (1H, m, ArH), 7.40 (2H, d, J = 8.4 Hz, ArH), 7.71 (1H, d, J = 8.8 Hz, ArH), 7.77 (1H, d, J = 8.8 Hz, ArH), 7.98 (2H, d, J = 8.4 Hz, ArH), 9.06 (1H, s, CH). 13C NMR (DMSO-d6, δ): 20.69, 117.56, 120.27, 121.01, 121.45, 122.13, 122.58, 126.77, 130.23, 137.53, 137.91, 148.98.
Refinement
The C-bound H atoms were placed in calculated positions, with C—H = 0.93 or 0.96 Å, and included in the final cycles of refinement using a riding model, with Uiso(H) = 1.2–1.5(methyl) Ueq(C).
Figures
Fig. 1.
The molecular structure of (I), showing 40% probability displacement ellipsoids and the atom-numbering scheme.
Fig. 2.
The crystal packing of (I).
Crystal data
| C14H12N2 | F(000) = 440 |
| Mr = 208.26 | Dx = 1.273 Mg m−3 |
| Monoclinic, P21/n | Mo Kα radiation, λ = 0.71073 Å |
| Hall symbol: -P 2yn | Cell parameters from 1425 reflections |
| a = 12.539 (4) Å | θ = 2.8–24.4° |
| b = 6.029 (2) Å | µ = 0.08 mm−1 |
| c = 14.401 (5) Å | T = 298 K |
| β = 93.636 (5)° | Prism, colorless |
| V = 1086.4 (6) Å3 | 0.48 × 0.34 × 0.31 mm |
| Z = 4 |
Data collection
| Bruker SMART CCD area-detector diffractometer | 1915 independent reflections |
| Radiation source: fine-focus sealed tube | 1236 reflections with I > 2σ(I) |
| graphite | Rint = 0.039 |
| phi and ω scans | θmax = 25.0°, θmin = 2.2° |
| Absorption correction: multi-scan (SADABS; Sheldrick, 1996) | h = −12→14 |
| Tmin = 0.969, Tmax = 0.980 | k = −7→7 |
| 5372 measured reflections | l = −17→15 |
Refinement
| Refinement on F2 | Primary atom site location: structure-invariant direct methods |
| Least-squares matrix: full | Secondary atom site location: difference Fourier map |
| R[F2 > 2σ(F2)] = 0.049 | Hydrogen site location: inferred from neighbouring sites |
| wR(F2) = 0.140 | H-atom parameters constrained |
| S = 1.03 | w = 1/[σ2(Fo2) + (0.0692P)2 + 0.1862P] where P = (Fo2 + 2Fc2)/3 |
| 1911 reflections | (Δ/σ)max < 0.001 |
| 145 parameters | Δρmax = 0.27 e Å−3 |
| 0 restraints | Δρmin = −0.26 e Å−3 |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| N1 | 0.57580 (13) | 0.0040 (3) | 0.35143 (12) | 0.0442 (5) | |
| N2 | 0.55544 (13) | 0.2100 (3) | 0.38504 (11) | 0.0420 (5) | |
| C1 | 0.64349 (16) | 0.3229 (4) | 0.41472 (14) | 0.0457 (6) | |
| H1 | 0.6458 | 0.4648 | 0.4401 | 0.055* | |
| C2 | 0.72979 (16) | 0.1879 (4) | 0.40029 (13) | 0.0433 (5) | |
| C3 | 0.84218 (17) | 0.2057 (4) | 0.41335 (16) | 0.0566 (7) | |
| H3 | 0.8738 | 0.3327 | 0.4393 | 0.068* | |
| C4 | 0.90270 (19) | 0.0330 (5) | 0.38714 (17) | 0.0631 (7) | |
| H4 | 0.9767 | 0.0421 | 0.3958 | 0.076* | |
| C5 | 0.85600 (18) | −0.1603 (4) | 0.34703 (16) | 0.0573 (7) | |
| H5 | 0.9002 | −0.2748 | 0.3295 | 0.069* | |
| C6 | 0.74854 (17) | −0.1839 (4) | 0.33324 (15) | 0.0490 (6) | |
| H6 | 0.7188 | −0.3122 | 0.3067 | 0.059* | |
| C7 | 0.68372 (16) | −0.0084 (3) | 0.36036 (13) | 0.0409 (5) | |
| C8 | 0.44707 (16) | 0.2872 (3) | 0.38157 (13) | 0.0417 (5) | |
| C9 | 0.36778 (16) | 0.1513 (4) | 0.41005 (14) | 0.0473 (6) | |
| H9 | 0.3846 | 0.0112 | 0.4338 | 0.057* | |
| C10 | 0.26299 (17) | 0.2226 (4) | 0.40340 (15) | 0.0516 (6) | |
| H10 | 0.2097 | 0.1297 | 0.4231 | 0.062* | |
| C11 | 0.23572 (17) | 0.4304 (4) | 0.36780 (15) | 0.0486 (6) | |
| C12 | 0.31752 (18) | 0.5648 (4) | 0.34099 (16) | 0.0538 (6) | |
| H12 | 0.3012 | 0.7054 | 0.3177 | 0.065* | |
| C13 | 0.42283 (18) | 0.4963 (4) | 0.34777 (15) | 0.0508 (6) | |
| H13 | 0.4767 | 0.5901 | 0.3298 | 0.061* | |
| C14 | 0.12148 (18) | 0.5069 (5) | 0.35741 (19) | 0.0705 (8) | |
| H14A | 0.0757 | 0.3925 | 0.3788 | 0.106* | |
| H14B | 0.1025 | 0.5382 | 0.2931 | 0.106* | |
| H14C | 0.1133 | 0.6386 | 0.3937 | 0.106* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| N1 | 0.0509 (12) | 0.0386 (11) | 0.0432 (10) | −0.0059 (8) | 0.0047 (8) | −0.0025 (8) |
| N2 | 0.0471 (10) | 0.0377 (10) | 0.0415 (10) | −0.0048 (8) | 0.0052 (8) | −0.0015 (8) |
| C1 | 0.0542 (13) | 0.0396 (12) | 0.0435 (12) | −0.0105 (11) | 0.0031 (10) | −0.0045 (10) |
| C2 | 0.0475 (13) | 0.0466 (13) | 0.0358 (11) | −0.0073 (10) | 0.0036 (9) | 0.0012 (10) |
| C3 | 0.0516 (14) | 0.0626 (16) | 0.0550 (15) | −0.0127 (12) | −0.0006 (11) | −0.0055 (12) |
| C4 | 0.0455 (14) | 0.0786 (19) | 0.0649 (16) | −0.0015 (13) | 0.0008 (11) | 0.0010 (14) |
| C5 | 0.0574 (16) | 0.0582 (16) | 0.0569 (15) | 0.0101 (12) | 0.0085 (11) | 0.0021 (12) |
| C6 | 0.0571 (14) | 0.0443 (13) | 0.0462 (13) | −0.0015 (11) | 0.0089 (10) | 0.0016 (10) |
| C7 | 0.0474 (13) | 0.0419 (13) | 0.0338 (11) | −0.0041 (10) | 0.0061 (9) | 0.0037 (9) |
| C8 | 0.0481 (12) | 0.0409 (13) | 0.0360 (11) | −0.0024 (10) | 0.0034 (9) | −0.0015 (9) |
| C9 | 0.0538 (14) | 0.0410 (13) | 0.0471 (13) | −0.0047 (11) | 0.0024 (10) | 0.0072 (10) |
| C10 | 0.0494 (14) | 0.0526 (15) | 0.0530 (14) | −0.0080 (11) | 0.0044 (10) | 0.0026 (11) |
| C11 | 0.0513 (13) | 0.0533 (15) | 0.0408 (12) | 0.0021 (11) | −0.0006 (10) | −0.0063 (11) |
| C12 | 0.0639 (16) | 0.0453 (14) | 0.0516 (14) | 0.0050 (12) | −0.0002 (11) | 0.0034 (11) |
| C13 | 0.0569 (15) | 0.0437 (14) | 0.0523 (14) | −0.0078 (11) | 0.0064 (10) | 0.0051 (11) |
| C14 | 0.0573 (16) | 0.082 (2) | 0.0707 (17) | 0.0118 (14) | −0.0054 (12) | −0.0070 (15) |
Geometric parameters (Å, °)
| N1—C7 | 1.353 (2) | C6—H6 | 0.9300 |
| N1—N2 | 1.362 (2) | C8—C9 | 1.371 (3) |
| N2—C1 | 1.344 (2) | C8—C13 | 1.378 (3) |
| N2—C8 | 1.434 (3) | C9—C10 | 1.380 (3) |
| C1—C2 | 1.380 (3) | C9—H9 | 0.9300 |
| C1—H1 | 0.9300 | C10—C11 | 1.388 (3) |
| C2—C3 | 1.414 (3) | C10—H10 | 0.9300 |
| C2—C7 | 1.422 (3) | C11—C12 | 1.381 (3) |
| C3—C4 | 1.356 (3) | C11—C14 | 1.503 (3) |
| C3—H3 | 0.9300 | C12—C13 | 1.381 (3) |
| C4—C5 | 1.411 (3) | C12—H12 | 0.9300 |
| C4—H4 | 0.9300 | C13—H13 | 0.9300 |
| C5—C6 | 1.357 (3) | C14—H14A | 0.9600 |
| C5—H5 | 0.9300 | C14—H14B | 0.9600 |
| C6—C7 | 1.405 (3) | C14—H14C | 0.9600 |
| C7—N1—N2 | 103.03 (16) | C9—C8—C13 | 120.3 (2) |
| C1—N2—N1 | 113.95 (17) | C9—C8—N2 | 119.91 (19) |
| C1—N2—C8 | 127.16 (19) | C13—C8—N2 | 119.78 (19) |
| N1—N2—C8 | 118.82 (16) | C8—C9—C10 | 119.9 (2) |
| N2—C1—C2 | 106.85 (19) | C8—C9—H9 | 120.1 |
| N2—C1—H1 | 126.6 | C10—C9—H9 | 120.1 |
| C2—C1—H1 | 126.6 | C9—C10—C11 | 121.2 (2) |
| C1—C2—C3 | 136.0 (2) | C9—C10—H10 | 119.4 |
| C1—C2—C7 | 104.43 (17) | C11—C10—H10 | 119.4 |
| C3—C2—C7 | 119.5 (2) | C12—C11—C10 | 117.6 (2) |
| C4—C3—C2 | 118.4 (2) | C12—C11—C14 | 120.8 (2) |
| C4—C3—H3 | 120.8 | C10—C11—C14 | 121.6 (2) |
| C2—C3—H3 | 120.8 | C11—C12—C13 | 121.9 (2) |
| C3—C4—C5 | 121.5 (2) | C11—C12—H12 | 119.0 |
| C3—C4—H4 | 119.2 | C13—C12—H12 | 119.0 |
| C5—C4—H4 | 119.2 | C8—C13—C12 | 119.1 (2) |
| C6—C5—C4 | 121.9 (2) | C8—C13—H13 | 120.4 |
| C6—C5—H5 | 119.0 | C12—C13—H13 | 120.4 |
| C4—C5—H5 | 119.0 | C11—C14—H14A | 109.5 |
| C5—C6—C7 | 117.8 (2) | C11—C14—H14B | 109.5 |
| C5—C6—H6 | 121.1 | H14A—C14—H14B | 109.5 |
| C7—C6—H6 | 121.1 | C11—C14—H14C | 109.5 |
| N1—C7—C6 | 127.45 (19) | H14A—C14—H14C | 109.5 |
| N1—C7—C2 | 111.74 (18) | H14B—C14—H14C | 109.5 |
| C6—C7—C2 | 120.80 (19) |
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: IM2233).
References
- Bistochi, G. A., De Meo, G., Pedini, M., Ricci, A., Brouilhet, H., Bucherie, S., Rabaud, M. & Jacquignon, P. (1981). Farm. Ed. Sci.36, 315–333. [PubMed]
- Bruker (1998). SMART Bruker AXS Inc., Madison, Wisconsin, USA.
- Bruker (1999). SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
- Cerecetto, H., Gerpe, A., Gonzalez, M., Aran, V. J. & de Ocarizi, C. O. (2005). Mini Rev. Med. Chem.5, 869–878. [DOI] [PubMed]
- Corsi, G., Palazzo, G., Germani, C., Barcellona, P. S. & Silvestrini, B. (1976). J. Med. Chem.19, 778–783.
- Keppler, B. K. & Hartmann, M. (1994). Met. Based Drugs, 1, 145–149. [DOI] [PMC free article] [PubMed]
- Picciola, G., Ravenna, F., Carenimi, G., Gentili, P. & Riva, M. (1981). Farm. Ed. Sci.36, 1037–1056. [PubMed]
- Rodgers, J. D., Johnson, B. L., Wang, H., Greenberg, R. A., Erickson, V. S., Klabe, R. M., Cordova, B. C., Rayner, M. M., Lam, G. N. & Chang, C. H. (1996). Bioorg. Med. Chem. Lett.6, 2919–2924.
- Sheldrick, G. M. (1996). SADABS University of Göttingen, Germany.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Stadlbauer, W. (2002). Science of Synthesis, Vol. 12. Stuttgart: Thieme.
- Sun, J. H., Teleha, C. A., Yan, J. S., Rodgers, J. D. & Nugiel, D. A. (1997). J. Org. Chem.62, 5627–5629.
- Ykeda, Y., Takano, N., Matsushita, H., Shiraki, Y., Koide, T., Nagashima, R., Fujimura, Y., Shindo, M., Suzuki, S. & Iwasaki, T. (1979). Arzneim. Forsch.29, 511–520. [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks global, I. DOI: 10.1107/S1600536810038948/im2233sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810038948/im2233Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report




