Abstract
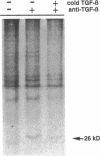
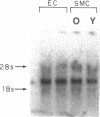
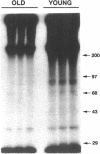
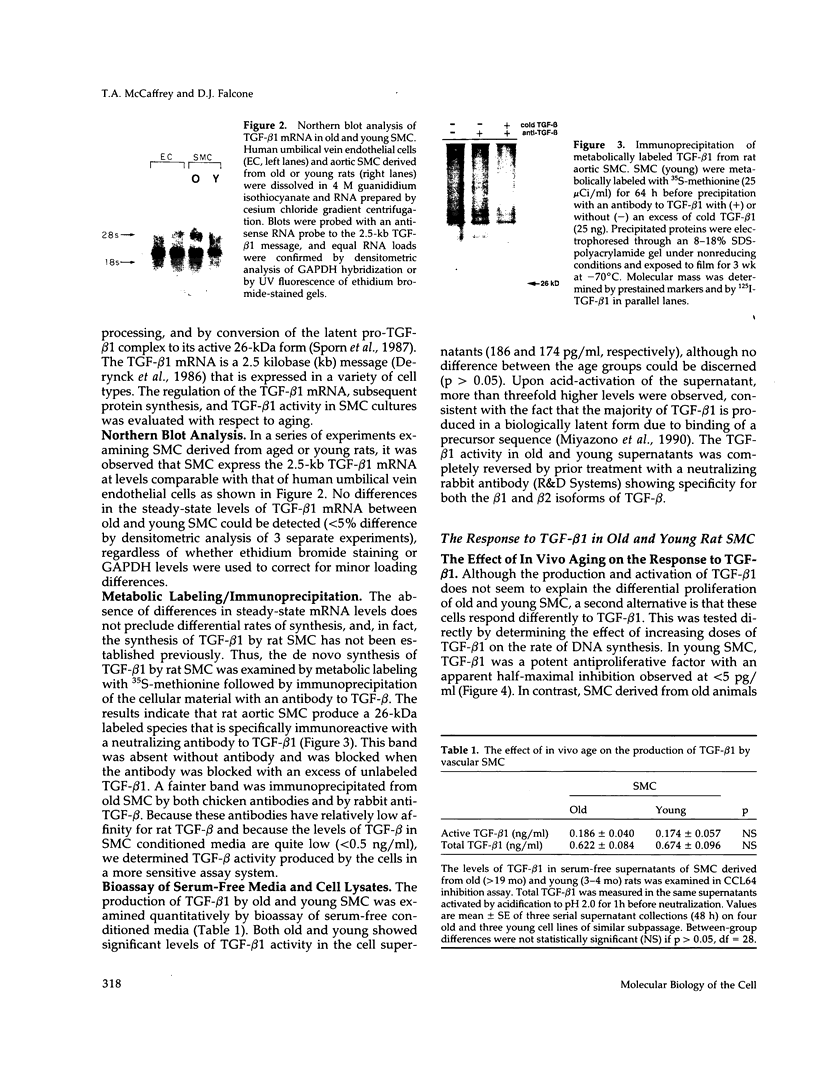
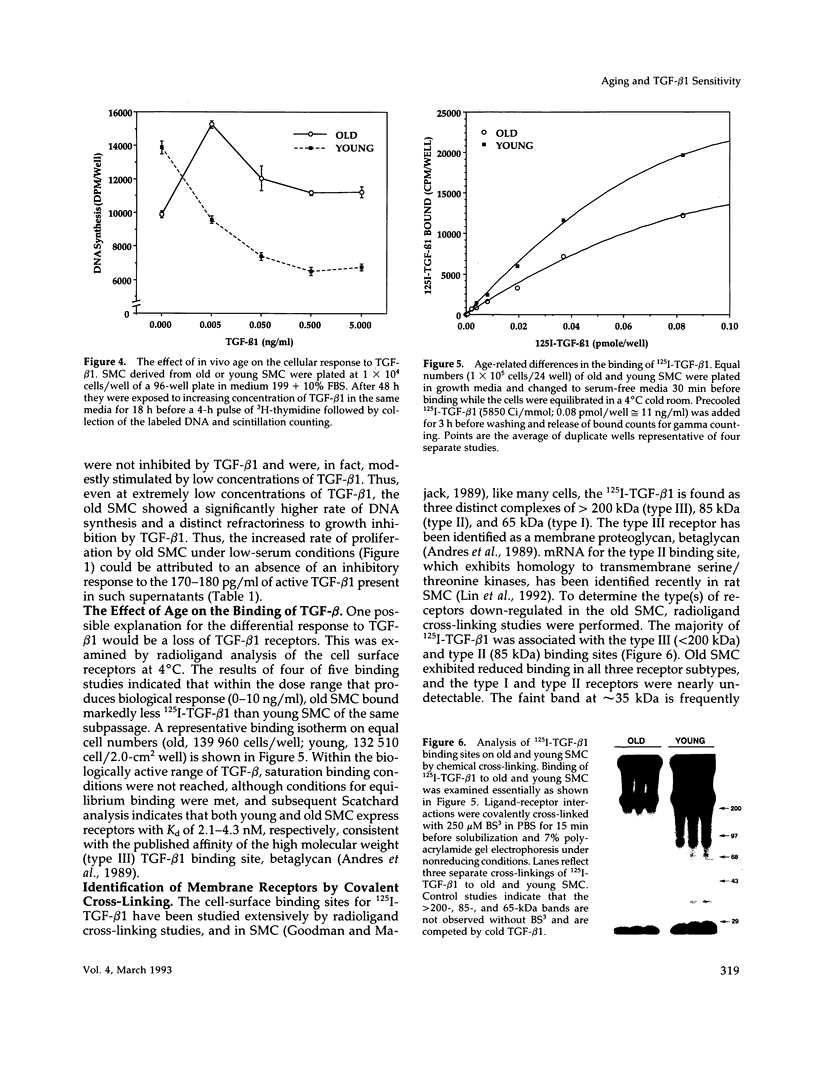
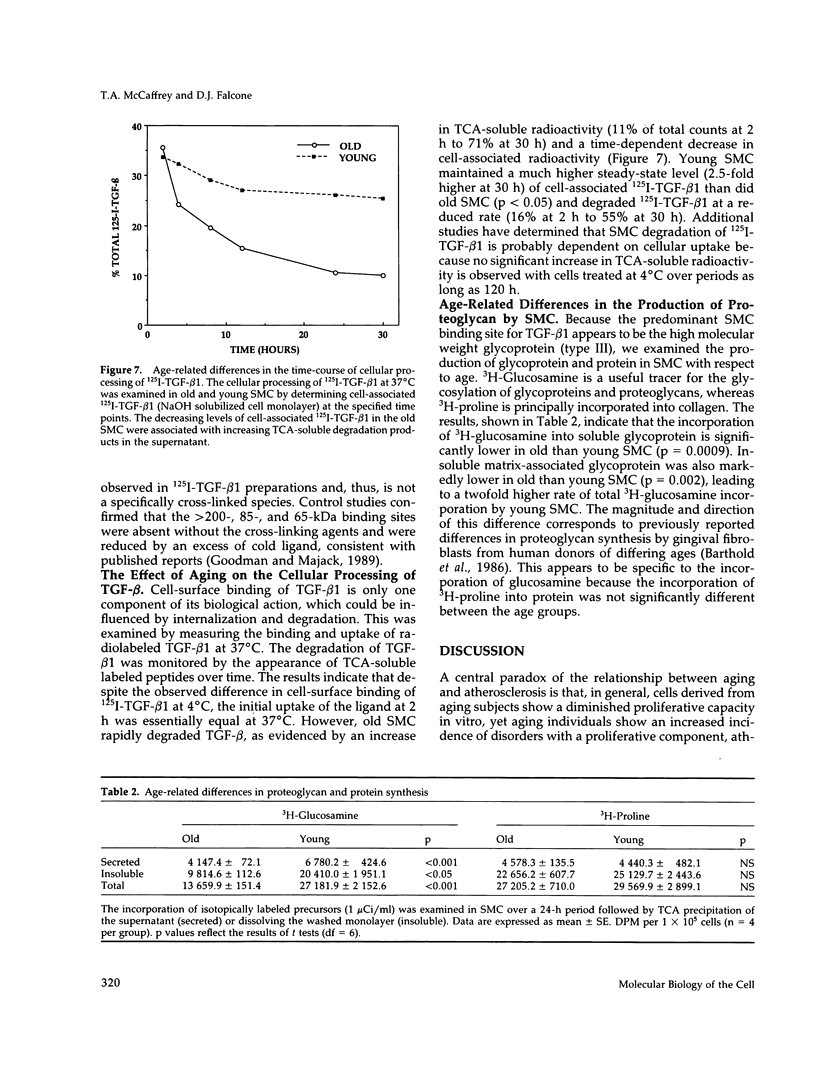
Previous studies have indicated that aged animals show an increased intimal hyperplasia after arterial injury. The present studies examined the hypothesis that the increased serum-free proliferation of aged smooth muscle cells (SMC), in vitro, was due to a loss of an antiproliferative signal, such as transforming growth factor-beta 1 (TGF-beta 1). Northern blot analysis of the mRNA derived from old (> 19 mo) or young (3-4 mo) rat aortic SMC indicated that both groups had an equivalent level of the 2.5 kB TGF-beta 1 message. Metabolic labeling with 35S-methionine and immunoprecipitation for TGF-beta 1 confirmed the de novo synthesis of TGF-beta 1 in rat SMC. Old and young SMC supernatants showed equal levels of active or latent (acid-activated) TGF-beta activity. Despite the similarities in the production of TGF-beta 1, old SMC were refractory to inhibition by TGF-beta 1, whereas young SMC were markedly inhibited (80%) by low levels of TGF-beta 1 (IC50 < 5 pg/ml). Binding studies at 4 degrees C indicated that old SMC exhibited reduced binding capacity for 125I-TGF-beta 1. Cross-linking studies confirmed that old SMC showed reduced binding of 125I-TGF-beta 1 to membrane sites corresponding to the high molecular weight type III receptor, as well as the 85-kDa type II and 65-kDa type I receptor. However, at 37 degrees C, old SMC degraded 125I-TGF-beta 1 more rapidly than young SMC. Combined, this data suggests that SMC derived from older animals are capable of normal production of TGF-beta 1 but fail to respond to the autocrine growth inhibitory effects of this agent, thereby leading to enhanced proliferation.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andres J. L., Stanley K., Cheifetz S., Massagué J. Membrane-anchored and soluble forms of betaglycan, a polymorphic proteoglycan that binds transforming growth factor-beta. J Cell Biol. 1989 Dec;109(6 Pt 1):3137–3145. doi: 10.1083/jcb.109.6.3137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartold P. M., Boyd R. R., Page R. C. Proteoglycans synthesized by gingival fibroblasts derived from human donors of different ages. J Cell Physiol. 1986 Jan;126(1):37–46. doi: 10.1002/jcp.1041260106. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Cheifetz S., Andres J., Laiho M., Massagué J. Transforming growth factor-beta receptors and binding proteoglycans. J Cell Sci Suppl. 1990;13:131–138. doi: 10.1242/jcs.1990.supplement_13.12. [DOI] [PubMed] [Google Scholar]
- Boyd F. T., Massagué J. Transforming growth factor-beta inhibition of epithelial cell proliferation linked to the expression of a 53-kDa membrane receptor. J Biol Chem. 1989 Feb 5;264(4):2272–2278. [PubMed] [Google Scholar]
- Cheifetz S., Andres J. L., Massagué J. The transforming growth factor-beta receptor type III is a membrane proteoglycan. Domain structure of the receptor. J Biol Chem. 1988 Nov 15;263(32):16984–16991. [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Dartsch P. C., Voisard R., Bauriedel G., Höfling B., Betz E. Growth characteristics and cytoskeletal organization of cultured smooth muscle cells from human primary stenosing and restenosing lesions. Arteriosclerosis. 1990 Jan-Feb;10(1):62–75. doi: 10.1161/01.atv.10.1.62. [DOI] [PubMed] [Google Scholar]
- Derynck R., Jarrett J. A., Chen E. Y., Goeddel D. V. The murine transforming growth factor-beta precursor. J Biol Chem. 1986 Apr 5;261(10):4377–4379. [PubMed] [Google Scholar]
- Ewton D. Z., Spizz G., Olson E. N., Florini J. R. Decrease in transforming growth factor-beta binding and action during differentiation in muscle cells. J Biol Chem. 1988 Mar 15;263(8):4029–4032. [PubMed] [Google Scholar]
- Goodman L. V., Majack R. A. Vascular smooth muscle cells express distinct transforming growth factor-beta receptor phenotypes as a function of cell density in culture. J Biol Chem. 1989 Mar 25;264(9):5241–5244. [PubMed] [Google Scholar]
- Gown A. M. Cell type and cell state specific antibodies in the analysis of early lesions of human atherosclerosis. Am J Hypertens. 1992 Jun;5(6 Pt 2):114S–117S. doi: 10.1093/ajh/5.6.114s. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Hariri R. J., Alonso D. R., Hajjar D. P., Coletti D., Weksler M. E. Aging and arteriosclerosis. I. Development of myointimal hyperplasia after endothelial injury. J Exp Med. 1986 Oct 1;164(4):1171–1178. doi: 10.1084/jem.164.4.1171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hariri R. J., Hajjar D. P., Coletti D., Alonso D. R., Weksler M. E., Rabellino E. Aging and arteriosclerosis. Cell cycle kinetics of young and old arterial smooth muscle cells. Am J Pathol. 1988 Apr;131(1):132–136. [PMC free article] [PubMed] [Google Scholar]
- Holt P. R., Yeh K. Y. Colonic proliferation is increased in senescent rats. Gastroenterology. 1988 Dec;95(6):1556–1563. doi: 10.1016/s0016-5085(88)80077-5. [DOI] [PubMed] [Google Scholar]
- Howe P. H., Cunningham M. R., Leof E. B. Inhibition of mink lung epithelial cell proliferation by transforming growth factor-beta is coupled through a pertussis-toxin-sensitive substrate. Biochem J. 1990 Mar 1;266(2):537–543. doi: 10.1042/bj2660537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimchi A., Wang X. F., Weinberg R. A., Cheifetz S., Massagué J. Absence of TGF-beta receptors and growth inhibitory responses in retinoblastoma cells. Science. 1988 Apr 8;240(4849):196–199. doi: 10.1126/science.2895499. [DOI] [PubMed] [Google Scholar]
- Laiho M., Weis M. B., Massagué J. Concomitant loss of transforming growth factor (TGF)-beta receptor types I and II in TGF-beta-resistant cell mutants implicates both receptor types in signal transduction. J Biol Chem. 1990 Oct 25;265(30):18518–18524. [PubMed] [Google Scholar]
- Lin H. Y., Wang X. F., Ng-Eaton E., Weinberg R. A., Lodish H. F. Expression cloning of the TGF-beta type II receptor, a functional transmembrane serine/threonine kinase. Cell. 1992 Feb 21;68(4):775–785. doi: 10.1016/0092-8674(92)90152-3. [DOI] [PubMed] [Google Scholar]
- López-Casillas F., Cheifetz S., Doody J., Andres J. L., Lane W. S., Massagué J. Structure and expression of the membrane proteoglycan betaglycan, a component of the TGF-beta receptor system. Cell. 1991 Nov 15;67(4):785–795. doi: 10.1016/0092-8674(91)90073-8. [DOI] [PubMed] [Google Scholar]
- Majack R. A., Majesky M. W., Goodman L. V. Role of PDGF-A expression in the control of vascular smooth muscle cell growth by transforming growth factor-beta. J Cell Biol. 1990 Jul;111(1):239–247. doi: 10.1083/jcb.111.1.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters D. B., Griggs C. T., Berde C. B. High sensitivity quantification of RNA from gels and autoradiograms with affordable optical scanning. Biotechniques. 1992 Jun;12(6):902-6, 908-11. [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Borth W., Brayton C. F., Weksler B. B. Fucoidan is a non-anticoagulant inhibitor of intimal hyperplasia. Biochem Biophys Res Commun. 1992 Apr 30;184(2):773–781. doi: 10.1016/0006-291x(92)90657-7. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Brayton C. F., Agarwal L. A., Welt F. G., Weksler B. B. Transforming growth factor-beta activity is potentiated by heparin via dissociation of the transforming growth factor-beta/alpha 2-macroglobulin inactive complex. J Cell Biol. 1989 Jul;109(1):441–448. doi: 10.1083/jcb.109.1.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaffrey T. A., Falcone D. J., Du B. Transforming growth factor-beta 1 is a heparin-binding protein: identification of putative heparin-binding regions and isolation of heparins with varying affinity for TGF-beta 1. J Cell Physiol. 1992 Aug;152(2):430–440. doi: 10.1002/jcp.1041520226. [DOI] [PubMed] [Google Scholar]
- McCaffrey T. A., Nicholson A. C., Szabo P. E., Weksler M. E., Weksler B. B. Aging and arteriosclerosis. The increased proliferation of arterial smooth muscle cells isolated from old rats is associated with increased platelet-derived growth factor-like activity. J Exp Med. 1988 Jan 1;167(1):163–174. doi: 10.1084/jem.167.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazono K., Yuki K., Takaku F., Wernstedt C., Kanzaki T., Olofsson A., Hellman U., Heldin C. H. Latent forms of TGF-beta: structure and biology. Ann N Y Acad Sci. 1990;593:51–58. doi: 10.1111/j.1749-6632.1990.tb16099.x. [DOI] [PubMed] [Google Scholar]
- Nikol S., Isner J. M., Pickering J. G., Kearney M., Leclerc G., Weir L. Expression of transforming growth factor-beta 1 is increased in human vascular restenosis lesions. J Clin Invest. 1992 Oct;90(4):1582–1592. doi: 10.1172/JCI116027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spagnoli L. G., Orlandi A., Mauriello A., Santeusanio G., de Angelis C., Lucreziotti R., Ramacci M. T. Aging and atherosclerosis in the rabbit. 1. Distribution, prevalence and morphology of atherosclerotic lesions. Atherosclerosis. 1991 Jul;89(1):11–24. doi: 10.1016/0021-9150(91)90003-l. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stemerman M. B., Weinstein R., Rowe J. W., Maciag T., Fuhro R., Gardner R. Vascular smooth muscle cell growth kinetics in vivo in aged rats. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3863–3866. doi: 10.1073/pnas.79.12.3863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakefield L. M., Thompson N. L., Flanders K. C., O'Connor-McCourt M. D., Sporn M. B. Transforming growth factor-beta: multifunctional regulator of cell growth and phenotype. Ann N Y Acad Sci. 1988;551:290–298. doi: 10.1111/j.1749-6632.1988.tb22355.x. [DOI] [PubMed] [Google Scholar]