Abstract
In the title compound, C17H14BrNO5, the dihedral angle between the 3-bromo-substituted benzene ring and the 4,5-dimethoxy-2-nitro-phenyl ring is 15.2 (1)°. The dihedral angles between the mean plane of the propenone group and the mean planes of the 3-bromo-substituted benzene and 4,5-dimethoxy-2-nitrophenyl rings are 6.9 (6) and 20.5 (5)°, respectively. Weak intermolecular C—H⋯O interactions contribute to crystal stability and π–π interactions [centroid–centroid distances = 3.7072 (18) and 3.6326 (18) Å] are also observed.
Related literature
For the biological activity of chalcones, see: Liu et al. (2003 ▶); Nielson et al. (1998 ▶); Rajas et al. (2002 ▶); Dinkova-Kostova et al. (1998 ▶). For their non-linear optical properties, see: Goto et al. (1991 ▶); Uchida et al. (1998 ▶);Tam et al. (1989 ▶); Indira et al. (2002 ▶); Sarojini et al. (2006 ▶). For the effect of bulky substituents on the spontaneous polarization of non-centrosymmetric crystals, see: Fichou et al. (1988 ▶). For the influence of the steric effect of the substituent on the molecular hyperpolarizability, see: Cho et al. (1996 ▶). For related structures, see: Butcher et al. (2007a
▶,b
▶,c
▶); Jasinski et al. (2010a
▶,b
▶,c
▶,d
▶,e
▶); Dutkiewicz et al. (2010 ▶); Kant et al. (2009 ▶); Yathirajan et al. (2007 ▶).
Experimental
Crystal data
C17H14BrNO5
M r = 392.20
Orthorhombic,
a = 6.8547 (2) Å
b = 8.3205 (2) Å
c = 27.1509 (6) Å
V = 1548.54 (7) Å3
Z = 4
Cu Kα radiation
μ = 3.88 mm−1
T = 123 K
0.55 × 0.12 × 0.06 mm
Data collection
Oxford Diffraction Xcalibur Diffractometer with Ruby Gemini detector
Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007 ▶) T min = 0.490, T max = 1.000
9914 measured reflections
3069 independent reflections
3011 reflections with I > 2σ(I)
R int = 0.040
Refinement
R[F 2 > 2σ(F 2)] = 0.032
wR(F 2) = 0.086
S = 1.07
3069 reflections
219 parameters
H-atom parameters constrained
Δρmax = 0.74 e Å−3
Δρmin = −0.42 e Å−3
Absolute structure: Flack (1983 ▶), 1228 Friedel pairs
Flack parameter: 0.08 (2)
Data collection: CrysAlis PRO (Oxford Diffraction, 2007 ▶); cell refinement: CrysAlis PRO; data reduction: CrysAlis RED; program(s) used to solve structure: SHELXS97 (Sheldrick, 2008 ▶); program(s) used to refine structure: SHELXL97 (Sheldrick, 2008 ▶); molecular graphics: SHELXTL (Sheldrick, 2008 ▶); software used to prepare material for publication: SHELXTL.
Supplementary Material
Crystal structure: contains datablocks I. DOI: 10.1107/S1600536810041292/lx2178sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041292/lx2178Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report
Table 1. Hydrogen-bond geometry (Å, °).
| D—H⋯A | D—H | H⋯A | D⋯A | D—H⋯A |
|---|---|---|---|---|
| C16—H16A⋯O5i | 0.98 | 2.46 | 3.383 (3) | 157 |
| C17—H17B⋯O3ii | 0.98 | 2.48 | 3.116 (4) | 123 |
Symmetry codes: (i) 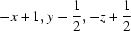 ; (ii)
; (ii) 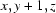 .
.
Acknowledgments
CSC thanks the University of Mysore for the research facilities and HSY thanks the University of Mysore for sabbatical leave. RJB acknowledges the NSF MRI program (grant No. CHE-0619278) for funds to purchase an X-ray diffractometer.
supplementary crystallographic information
Comment
Chalcones have displayed an impressive array of biological activities, among which antimalarial (Liu et al., 2003), antiprotozoal (Nielson et al., 1998), nitric oxide inhibition (Rajas et al., 2002) and anticancer activities (Dinkova-Kostova et al., 1998) have been cited in the literature. Among several organic compounds reported for non-linear optical (NLO) properties, chalcone derivatives are notable materials for their excellent blue-light transmittance and good crystallizability. They provide the necessary configuration to show NLO properties, with two planar rings connected through a conjugated double bond (Goto et al., 1991; Uchida et al., 1998; Tam et al., 1989; Indira et al., 2002, Sarojini et al., 2006). Substitution on either of the benzene rings greatly influences the non-centrosymmetric crystal packing. It is speculated that, in order to improve the activity, more bulky substituents should be introduced to increase the spontaneous polarization of non-centrosymmetric crystals (Fichou et al., 1988). The molecular hyperpolarizability is strongly influenced, not only by the electronic effect, but also by the steric effect of the substituent (Cho et al., 1996). The crystal structure studies of 2,3-dibromo-1-(2,4-dichlorophenyl)-3-(4,5-dimethoxy-2-nitrophenyl) propan-1-one (Yathirajan et al., 2007); (2E)-1-(4-methylphenyl)-3-(4-nitrophenyl)prop-2-en-1-one (Butcher et al., 2007a); (E)-3-(4-fluorophenyl)-1-(4-methylphenyl)prop-2-en-1-one (Butcher et al., 2007b); (2E)-3-(2-bromo-5-methoxyphenyl)-1-(2,4-dichlorophenyl) prop-2-en-1-one (Butcher et al., 2007c); (E)-3-(4-bromophenyl)-1-(3,4-dichlorophenyl)prop-2-en-1-one (Kant et al., 2009); (2E)-3-(4-bromophenyl)-1-(3-chlorophenyl) prop-2-en-1-one (Jasinski et al., 2010a); (2E)-1-(4-bromophenyl)-3-(4-fluorophenyl)prop-2-en-1-one (Dutkiewicz et al., 2010); (2E)-1-(2-bromophenyl)-3-(4-chlorophenyl) prop-2-en-1-one (Jasinski et al., 2010b); (2E)-1-(2-bromophenyl)-3-(4-methoxyphenyl)prop-2-en-1-one (Jasinski et al., 2010c); (2E)-1-(2-bromophenyl)-3- (3,4,5-trimethoxyphenyl)prop-2-en-1-one (Jasinski et al., 2010d) and (2E)-1-(2-bromophenyl)-3-(4-bromophenyl)prop-2-en-1-one (Jasinski et al., 2010e) have been reported. In continuation of our work on chalcones, the present paper reports the synthesis and crystal structure of a new chalcone, C17H14BrNO5.
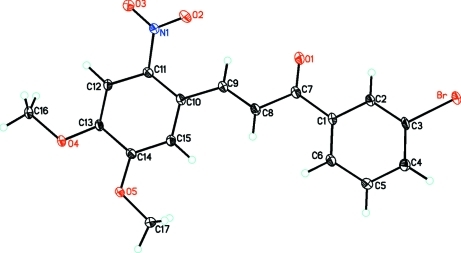
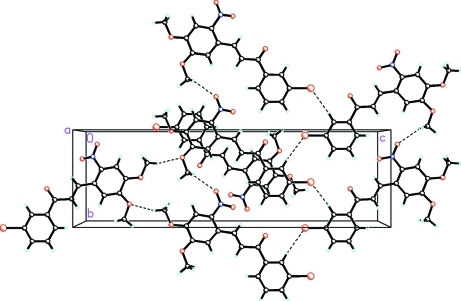
In the title compound the dihedral angle between the 3-bromo-substituted benzene ring and the 4,5-dimethoxy-2-nitro-phenyl ring is 15.2 (1)° (Fig. 2). The dihedral angles between the mean plane of the propenone group and the mean planes of the 3-bromo-substituted benzene and 4,5-dimethoxy-2-nitro-phenyl rings is 6.9 (6)° and 20.5 (5)°, respectively. While no classic hydrogen bonds are observed, weak intermolecular C—H···O (Table 1, Fig. 3) hydrogen bond interactions contribute to crystal stability.
Experimental
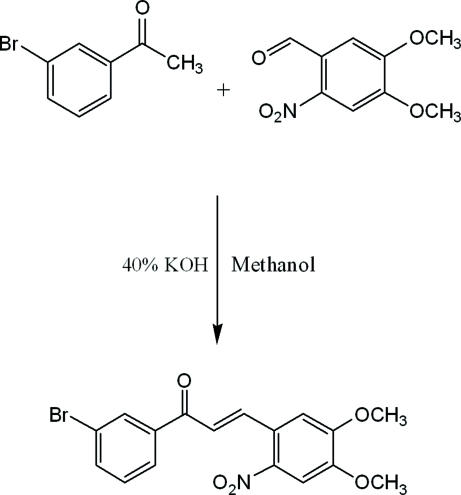
1-(3-Bromophenyl)ethanone (1.99 g, 0.01 mol) was mixed with 4,5-dimethoxy-2-nitrobenzaldehyde (2.11 g, 0.01 mol) and dissolved in methanol (30 ml). To this, 3 ml of KOH (40%) was added and the reaction mixture was stirred for 6 h (Fig. 1). The resulting crude solid was filtered, washed successively with distilled water and finally recrystallized from ethanol (95%) to give the pure chalcone. Pale yellow, small needle shaped crystals suitable for X-ray diffraction studies were grown by the slow evaporation of the dimethylformamide solution at room temperature (m.p.: 409–411 K).
Refinement
The parameters of all the H atoms have been constrained within the riding atom approximation. C—H bond lengths were constrained to 0.95 or 0.98 Å for aryl or methyl H atoms, Uiso(H) = 1.18–1.22Ueq(Caryl); Uiso(H) = 1.59–1.51Ueq(Cmethyl).
Figures
Fig. 1.
Reaction scheme for the title compound.
Fig. 2.
Molecular structure of the title compound showing the atom labeling scheme and 50% probability displacement ellipsoids.
Fig. 3.
Packing diagram of the title compound viewed down the a axis. Dashed lines indicate weak intermolecular C—H···O hydrogen bond interactions.
Crystal data
| C17H14BrNO5 | F(000) = 792 |
| Mr = 392.20 | Dx = 1.682 Mg m−3 |
| Orthorhombic, P212121 | Cu Kα radiation, λ = 1.54178 Å |
| Hall symbol: P 2ac 2ab | Cell parameters from 8339 reflections |
| a = 6.8547 (2) Å | θ = 4.9–74.0° |
| b = 8.3205 (2) Å | µ = 3.88 mm−1 |
| c = 27.1509 (6) Å | T = 123 K |
| V = 1548.54 (7) Å3 | Needle, colorless |
| Z = 4 | 0.55 × 0.12 × 0.06 mm |
Data collection
| Oxford Diffraction Xcalibur Diffractometer with Ruby Gemini detector | 3069 independent reflections |
| Radiation source: Enhance (Cu) X-ray Source | 3011 reflections with I > 2σ(I) |
| graphite | Rint = 0.040 |
| Detector resolution: 10.5081 pixels mm-1 | θmax = 74.1°, θmin = 5.6° |
| ω scans | h = −8→5 |
| Absorption correction: multi-scan (CrysAlis RED; Oxford Diffraction, 2007) | k = −9→10 |
| Tmin = 0.490, Tmax = 1.000 | l = −32→33 |
| 9914 measured reflections |
Refinement
| Refinement on F2 | Secondary atom site location: difference Fourier map |
| Least-squares matrix: full | Hydrogen site location: inferred from neighbouring sites |
| R[F2 > 2σ(F2)] = 0.032 | H-atom parameters constrained |
| wR(F2) = 0.086 | w = 1/[σ2(Fo2) + (0.0497P)2 + 1.5041P] where P = (Fo2 + 2Fc2)/3 |
| S = 1.07 | (Δ/σ)max = 0.003 |
| 3069 reflections | Δρmax = 0.74 e Å−3 |
| 219 parameters | Δρmin = −0.42 e Å−3 |
| 0 restraints | Absolute structure: Flack (1983), 1228 Friedel pairs |
| Primary atom site location: structure-invariant direct methods | Flack parameter: 0.08 (2) |
Special details
| Geometry. All e.s.d.'s (except the e.s.d. in the dihedral angle between two l.s. planes) are estimated using the full covariance matrix. The cell e.s.d.'s are taken into account individually in the estimation of e.s.d.'s in distances, angles and torsion angles; correlations between e.s.d.'s in cell parameters are only used when they are defined by crystal symmetry. An approximate (isotropic) treatment of cell e.s.d.'s is used for estimating e.s.d.'s involving l.s. planes. |
| Refinement. Refinement of F2 against ALL reflections. The weighted R-factor wR and goodness of fit S are based on F2, conventional R-factors R are based on F, with F set to zero for negative F2. The threshold expression of F2 > σ(F2) is used only for calculating R-factors(gt) etc. and is not relevant to the choice of reflections for refinement. R-factors based on F2 are statistically about twice as large as those based on F, and R- factors based on ALL data will be even larger. |
Fractional atomic coordinates and isotropic or equivalent isotropic displacement parameters (Å2)
| x | y | z | Uiso*/Ueq | ||
| Br | 0.59396 (5) | 0.54840 (4) | 0.757218 (10) | 0.02935 (11) | |
| O1 | 0.5372 (4) | 0.1224 (3) | 0.60992 (8) | 0.0329 (6) | |
| O2 | 0.7701 (4) | −0.1958 (3) | 0.50347 (8) | 0.0314 (5) | |
| O3 | 0.6537 (4) | −0.3581 (3) | 0.44871 (9) | 0.0332 (6) | |
| O4 | 0.5820 (4) | 0.0031 (2) | 0.30190 (7) | 0.0240 (4) | |
| O5 | 0.5346 (4) | 0.2869 (3) | 0.33757 (7) | 0.0258 (5) | |
| N1 | 0.6855 (4) | −0.2223 (3) | 0.46423 (10) | 0.0227 (5) | |
| C1 | 0.5862 (5) | 0.4042 (3) | 0.61065 (10) | 0.0200 (5) | |
| C2 | 0.5815 (5) | 0.4043 (3) | 0.66260 (10) | 0.0227 (6) | |
| H2A | 0.5685 | 0.3064 | 0.6803 | 0.027* | |
| C3 | 0.5960 (4) | 0.5491 (4) | 0.68724 (9) | 0.0229 (5) | |
| C4 | 0.6120 (5) | 0.6943 (4) | 0.66260 (11) | 0.0247 (6) | |
| H4A | 0.6189 | 0.7925 | 0.6804 | 0.030* | |
| C5 | 0.6179 (5) | 0.6939 (4) | 0.61128 (11) | 0.0246 (6) | |
| H5A | 0.6296 | 0.7925 | 0.5938 | 0.030* | |
| C6 | 0.6065 (4) | 0.5491 (4) | 0.58544 (10) | 0.0230 (5) | |
| H6A | 0.6126 | 0.5494 | 0.5505 | 0.028* | |
| C7 | 0.5701 (5) | 0.2433 (4) | 0.58575 (11) | 0.0233 (6) | |
| C8 | 0.5993 (5) | 0.2348 (4) | 0.53138 (10) | 0.0224 (6) | |
| H8A | 0.6323 | 0.3286 | 0.5132 | 0.027* | |
| C9 | 0.5783 (5) | 0.0934 (3) | 0.50851 (10) | 0.0213 (5) | |
| H9A | 0.5488 | 0.0027 | 0.5284 | 0.026* | |
| C10 | 0.5971 (4) | 0.0665 (3) | 0.45512 (9) | 0.0191 (5) | |
| C11 | 0.6283 (4) | −0.0843 (3) | 0.43402 (10) | 0.0199 (6) | |
| C12 | 0.6209 (4) | −0.1121 (3) | 0.38335 (10) | 0.0206 (6) | |
| H12A | 0.6378 | −0.2178 | 0.3708 | 0.025* | |
| C13 | 0.5890 (5) | 0.0144 (3) | 0.35161 (9) | 0.0198 (5) | |
| C14 | 0.5639 (5) | 0.1705 (3) | 0.37128 (10) | 0.0208 (6) | |
| C15 | 0.5659 (4) | 0.1943 (3) | 0.42198 (10) | 0.0203 (6) | |
| H15A | 0.5457 | 0.2995 | 0.4346 | 0.024* | |
| C16 | 0.5963 (5) | −0.1555 (4) | 0.28128 (10) | 0.0255 (6) | |
| H16A | 0.5866 | −0.1490 | 0.2453 | 0.038* | |
| H16B | 0.4900 | −0.2223 | 0.2941 | 0.038* | |
| H16C | 0.7219 | −0.2032 | 0.2904 | 0.038* | |
| C17 | 0.5316 (6) | 0.4499 (4) | 0.35512 (11) | 0.0317 (7) | |
| H17A | 0.5242 | 0.5236 | 0.3270 | 0.048* | |
| H17B | 0.6509 | 0.4714 | 0.3739 | 0.048* | |
| H17C | 0.4177 | 0.4657 | 0.3764 | 0.048* |
Atomic displacement parameters (Å2)
| U11 | U22 | U33 | U12 | U13 | U23 | |
| Br | 0.04312 (19) | 0.03241 (16) | 0.01252 (15) | 0.00224 (15) | −0.00131 (12) | −0.00345 (11) |
| O1 | 0.0530 (16) | 0.0300 (11) | 0.0157 (10) | −0.0028 (11) | 0.0034 (10) | −0.0018 (9) |
| O2 | 0.0428 (14) | 0.0333 (12) | 0.0180 (11) | 0.0038 (11) | −0.0085 (10) | 0.0032 (9) |
| O3 | 0.0540 (16) | 0.0227 (11) | 0.0228 (11) | 0.0026 (10) | 0.0012 (10) | 0.0005 (9) |
| O4 | 0.0377 (12) | 0.0219 (9) | 0.0123 (8) | −0.0006 (9) | −0.0012 (9) | −0.0026 (7) |
| O5 | 0.0456 (14) | 0.0191 (10) | 0.0125 (9) | −0.0002 (9) | −0.0034 (9) | 0.0008 (8) |
| N1 | 0.0303 (13) | 0.0223 (12) | 0.0154 (11) | 0.0032 (10) | 0.0026 (10) | 0.0010 (10) |
| C1 | 0.0200 (13) | 0.0264 (13) | 0.0135 (12) | 0.0000 (12) | −0.0016 (12) | −0.0037 (10) |
| C2 | 0.0286 (15) | 0.0251 (13) | 0.0145 (13) | 0.0015 (13) | 0.0007 (13) | −0.0009 (10) |
| C3 | 0.0288 (14) | 0.0309 (14) | 0.0089 (11) | 0.0029 (16) | −0.0014 (11) | −0.0035 (11) |
| C4 | 0.0282 (16) | 0.0251 (13) | 0.0207 (14) | 0.0019 (13) | 0.0019 (14) | −0.0027 (11) |
| C5 | 0.0298 (16) | 0.0241 (14) | 0.0200 (14) | 0.0004 (13) | −0.0003 (13) | 0.0022 (11) |
| C6 | 0.0255 (14) | 0.0285 (14) | 0.0150 (12) | 0.0026 (15) | −0.0007 (11) | −0.0004 (11) |
| C7 | 0.0263 (15) | 0.0280 (14) | 0.0158 (13) | 0.0005 (13) | −0.0018 (12) | −0.0008 (11) |
| C8 | 0.0258 (14) | 0.0269 (13) | 0.0146 (13) | −0.0019 (14) | 0.0013 (12) | 0.0002 (11) |
| C9 | 0.0252 (14) | 0.0243 (13) | 0.0144 (12) | 0.0000 (12) | 0.0004 (12) | 0.0002 (10) |
| C10 | 0.0207 (12) | 0.0234 (13) | 0.0133 (12) | −0.0023 (13) | 0.0001 (11) | −0.0006 (10) |
| C11 | 0.0227 (15) | 0.0214 (13) | 0.0155 (13) | −0.0002 (11) | 0.0002 (11) | 0.0025 (10) |
| C12 | 0.0267 (16) | 0.0199 (12) | 0.0150 (13) | −0.0012 (12) | 0.0015 (12) | −0.0037 (10) |
| C13 | 0.0250 (14) | 0.0229 (13) | 0.0115 (11) | −0.0017 (12) | 0.0004 (11) | −0.0024 (10) |
| C14 | 0.0253 (15) | 0.0213 (13) | 0.0159 (13) | −0.0019 (12) | 0.0010 (11) | 0.0023 (11) |
| C15 | 0.0232 (15) | 0.0213 (13) | 0.0163 (13) | −0.0007 (11) | −0.0001 (11) | −0.0022 (10) |
| C16 | 0.0371 (16) | 0.0259 (14) | 0.0135 (12) | 0.0011 (14) | −0.0005 (14) | −0.0053 (10) |
| C17 | 0.056 (2) | 0.0186 (14) | 0.0210 (14) | −0.0006 (15) | 0.0011 (13) | 0.0006 (13) |
Geometric parameters (Å, °)
| Br—C3 | 1.900 (3) | C7—C8 | 1.491 (4) |
| O1—C7 | 1.222 (4) | C8—C9 | 1.338 (4) |
| O2—N1 | 1.233 (4) | C8—H8A | 0.9500 |
| O3—N1 | 1.225 (4) | C9—C10 | 1.472 (4) |
| O4—C13 | 1.354 (3) | C9—H9A | 0.9500 |
| O4—C16 | 1.436 (3) | C10—C11 | 1.395 (4) |
| O5—C14 | 1.348 (4) | C10—C15 | 1.410 (4) |
| O5—C17 | 1.437 (4) | C11—C12 | 1.396 (4) |
| N1—C11 | 1.465 (4) | C12—C13 | 1.378 (4) |
| C1—C6 | 1.393 (4) | C12—H12A | 0.9500 |
| C1—C2 | 1.411 (4) | C13—C14 | 1.415 (4) |
| C1—C7 | 1.504 (4) | C14—C15 | 1.391 (4) |
| C2—C3 | 1.382 (4) | C15—H15A | 0.9500 |
| C2—H2A | 0.9500 | C16—H16A | 0.9800 |
| C3—C4 | 1.386 (4) | C16—H16B | 0.9800 |
| C4—C5 | 1.394 (4) | C16—H16C | 0.9800 |
| C4—H4A | 0.9500 | C17—H17A | 0.9800 |
| C5—C6 | 1.397 (4) | C17—H17B | 0.9800 |
| C5—H5A | 0.9500 | C17—H17C | 0.9800 |
| C6—H6A | 0.9500 | ||
| C13—O4—C16 | 116.8 (2) | C10—C9—H9A | 117.2 |
| C14—O5—C17 | 117.1 (2) | C11—C10—C15 | 116.1 (2) |
| O3—N1—O2 | 123.1 (3) | C11—C10—C9 | 123.7 (3) |
| O3—N1—C11 | 118.9 (3) | C15—C10—C9 | 120.0 (2) |
| O2—N1—C11 | 118.0 (2) | C10—C11—C12 | 123.3 (3) |
| C6—C1—C2 | 119.5 (2) | C10—C11—N1 | 121.1 (2) |
| C6—C1—C7 | 123.8 (2) | C12—C11—N1 | 115.5 (2) |
| C2—C1—C7 | 116.6 (2) | C13—C12—C11 | 119.7 (3) |
| C3—C2—C1 | 118.9 (3) | C13—C12—H12A | 120.2 |
| C3—C2—H2A | 120.6 | C11—C12—H12A | 120.2 |
| C1—C2—H2A | 120.6 | O4—C13—C12 | 125.1 (2) |
| C2—C3—C4 | 122.2 (2) | O4—C13—C14 | 115.9 (2) |
| C2—C3—Br | 118.8 (2) | C12—C13—C14 | 119.0 (2) |
| C4—C3—Br | 119.1 (2) | O5—C14—C15 | 124.8 (3) |
| C3—C4—C5 | 118.9 (3) | O5—C14—C13 | 114.9 (2) |
| C3—C4—H4A | 120.6 | C15—C14—C13 | 120.2 (3) |
| C5—C4—H4A | 120.6 | C14—C15—C10 | 121.7 (3) |
| C4—C5—C6 | 120.2 (3) | C14—C15—H15A | 119.1 |
| C4—C5—H5A | 119.9 | C10—C15—H15A | 119.1 |
| C6—C5—H5A | 119.9 | O4—C16—H16A | 109.5 |
| C1—C6—C5 | 120.4 (2) | O4—C16—H16B | 109.5 |
| C1—C6—H6A | 119.8 | H16A—C16—H16B | 109.5 |
| C5—C6—H6A | 119.8 | O4—C16—H16C | 109.5 |
| O1—C7—C8 | 121.2 (3) | H16A—C16—H16C | 109.5 |
| O1—C7—C1 | 120.3 (3) | H16B—C16—H16C | 109.5 |
| C8—C7—C1 | 118.5 (3) | O5—C17—H17A | 109.5 |
| C9—C8—C7 | 119.1 (3) | O5—C17—H17B | 109.5 |
| C9—C8—H8A | 120.5 | H17A—C17—H17B | 109.5 |
| C7—C8—H8A | 120.5 | O5—C17—H17C | 109.5 |
| C8—C9—C10 | 125.5 (3) | H17A—C17—H17C | 109.5 |
| C8—C9—H9A | 117.2 | H17B—C17—H17C | 109.5 |
| C6—C1—C2—C3 | 0.4 (5) | C9—C10—C11—N1 | −13.0 (5) |
| C7—C1—C2—C3 | 179.9 (3) | O3—N1—C11—C10 | 157.6 (3) |
| C1—C2—C3—C4 | 1.0 (5) | O2—N1—C11—C10 | −25.0 (4) |
| C1—C2—C3—Br | −178.9 (3) | O3—N1—C11—C12 | −26.6 (4) |
| C2—C3—C4—C5 | −1.4 (5) | O2—N1—C11—C12 | 150.9 (3) |
| Br—C3—C4—C5 | 178.6 (3) | C10—C11—C12—C13 | 2.7 (5) |
| C3—C4—C5—C6 | 0.4 (5) | N1—C11—C12—C13 | −173.1 (3) |
| C2—C1—C6—C5 | −1.4 (5) | C16—O4—C13—C12 | 4.4 (5) |
| C7—C1—C6—C5 | 179.1 (3) | C16—O4—C13—C14 | −176.6 (3) |
| C4—C5—C6—C1 | 1.0 (5) | C11—C12—C13—O4 | 178.8 (3) |
| C6—C1—C7—O1 | −174.2 (3) | C11—C12—C13—C14 | −0.2 (5) |
| C2—C1—C7—O1 | 6.3 (5) | C17—O5—C14—C15 | 8.8 (5) |
| C6—C1—C7—C8 | 7.0 (5) | C17—O5—C14—C13 | −172.7 (3) |
| C2—C1—C7—C8 | −172.5 (3) | O4—C13—C14—O5 | 0.6 (4) |
| O1—C7—C8—C9 | 3.7 (5) | C12—C13—C14—O5 | 179.7 (3) |
| C1—C7—C8—C9 | −177.6 (3) | O4—C13—C14—C15 | 179.1 (3) |
| C7—C8—C9—C10 | 178.3 (3) | C12—C13—C14—C15 | −1.8 (5) |
| C8—C9—C10—C11 | 161.5 (3) | O5—C14—C15—C10 | 179.9 (3) |
| C8—C9—C10—C15 | −24.4 (5) | C13—C14—C15—C10 | 1.5 (5) |
| C15—C10—C11—C12 | −2.9 (4) | C11—C10—C15—C14 | 0.8 (4) |
| C9—C10—C11—C12 | 171.4 (3) | C9—C10—C15—C14 | −173.8 (3) |
| C15—C10—C11—N1 | 172.6 (3) |
Hydrogen-bond geometry (Å, °)
| D—H···A | D—H | H···A | D···A | D—H···A |
| C16—H16A···O5i | 0.98 | 2.46 | 3.383 (3) | 157 |
| C17—H17B···O3ii | 0.98 | 2.48 | 3.116 (4) | 123 |
Symmetry codes: (i) −x+1, y−1/2, −z+1/2; (ii) x, y+1, z.
Table 2 π-π hydrogen-bond geometry (Å)
Cg1 and Cg2 are the centroids of the C1–C6 and C10–C15 rings, respectively.
| Cg···Cg | D···A |
| Cg1···Cg2i | 3.7072 (18) |
| Cg1···Cg2ii | 3.6326 (18) |
Symmetry codes: (i) -1/2+x, 1/2-y, 1-z; (ii) 1/2+x, 1/2-y, 1-z.
Footnotes
Supplementary data and figures for this paper are available from the IUCr electronic archives (Reference: LX2178).
References
- Butcher, R. J., Jasinski, J. P., Yathirajan, H. S., Narayana, B. & Mayekar, A. N. (2007c). Acta Cryst. E63, o4253–o4254.
- Butcher, R. J., Jasinski, J. P., Yathirajan, H. S., Narayana, B. & Veena, K. (2007b). Acta Cryst. E63, o3833.
- Butcher, R. J., Jasinski, J. P., Yathirajan, H. S., Veena, K. & Narayana, B. (2007a). Acta Cryst. E63, o3680.
- Cho, B. R., Je, J. T., Kim, H. S., Jean, S. J., Song, O. K. & Wang, C. H. (1996). Bull. Korean Chem. Soc.17, 693–695.
- Dinkova-Kostova, A. T., Abey-Gunawardana, C. & Talalay, P. (1998). J. Med. Chem.41, 5287–5296. [DOI] [PubMed]
- Dutkiewicz, G., Veena, K., Narayana, B., Yathirajan, H. S. & Kubicki, M. (2010). Acta Cryst. E66, o1243–o1244. [DOI] [PMC free article] [PubMed]
- Fichou, D., Watanabe, T., Takeda, T., Miyata, S., Goto, Y. & Nakayama, M. (1988). Jpn J. Appl. Phys.27, 429–430.
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
- Goto, Y., Hayashi, A., Kimura, Y. & Nakayama, M. (1991). J. Cryst. Growth, 108, 688–698.
- Indira, J., Karat, P. P. & Sarojini, B. K. (2002). J. Cryst. Growth, 242, 209–214.
- Jasinski, J. P., Butcher, R. J., Narayana, B., Veena, K. & Yathirajan, H. S. (2010a). Acta Cryst. E66, o158. [DOI] [PMC free article] [PubMed]
- Jasinski, J. P., Butcher, R. J., Veena, K., Narayana, B. & Yathirajan, H. S. (2010b). Acta Cryst. E66, o1638. [DOI] [PMC free article] [PubMed]
- Jasinski, J. P., Butcher, R. J., Veena, K., Narayana, B. & Yathirajan, H. S. (2010c). Acta Cryst. E66, o1661. [DOI] [PMC free article] [PubMed]
- Jasinski, J. P., Butcher, R. J., Veena, K., Narayana, B. & Yathirajan, H. S. (2010d). Acta Cryst. E66, o1676. [DOI] [PMC free article] [PubMed]
- Jasinski, J. P., Butcher, R. J., Veena, K., Narayana, B. & Yathirajan, H. S. (2010e). Acta Cryst. E<66, o1701. [DOI] [PMC free article] [PubMed]
- Kant, R., Kamni,, Narayana, B., Veena, K. & Yathirajan, H. S. (2009). Acta Cryst. E65, o836. [DOI] [PMC free article] [PubMed]
- Liu, M., Wilairat, P., Croft, S. L., Tan, A. L. C. & Go, M. I. (2003). Bioorg. Med. Chem.11, 2729–2738. [DOI] [PubMed]
- Nielson, S. F., Christensen, S. B., Cruciani, G., Kharazmi, A. & Liljefors, T. (1998). J. Med. Chem.41, 4819–4832. [DOI] [PubMed]
- Oxford Diffraction (2007). CrysAlis PRO and CrysAlis RED Oxford Diffraction Ltd, Abingdon, England.
- Rajas, J., Paya, M., Domingues, J. N. & Ferrandiz, M. L. (2002). Bioorg. Med. Chem. Lett.12, 1951–1954. [DOI] [PubMed]
- Sarojini, B. K., Narayana, B., Ashalatha, B. V., Indira, J. & Lobo, K. J. (2006). J. Cryst. Growth, 295, 54–59.
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. [DOI] [PubMed]
- Tam, W., Guerin, B., Calabrese, J. C. & Stevenson, S. H. (1989). Chem. Phys. Lett.154, 93–96.
- Uchida, T., Kozawa, K., Sakai, T., Aoki, M., Yoguchi, H., Abduryim, A. & Watanabe, Y. (1998). Mol. Cryst. Liq. Cryst.315, 135–140.
- Yathirajan, H. S., Mayekar, A. N., Narayana, B., Sarojini, B. K. & Bolte, M. (2007). Acta Cryst. E63, o2196–o2197.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Crystal structure: contains datablocks I. DOI: 10.1107/S1600536810041292/lx2178sup1.cif
Structure factors: contains datablocks I. DOI: 10.1107/S1600536810041292/lx2178Isup2.hkl
Additional supplementary materials: crystallographic information; 3D view; checkCIF report