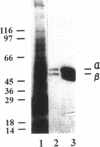
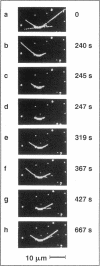
Abstract
Microtubules can adjust their length by the mechanism of dynamic instability, that is by switching between phases of growth and shrinkage. Thus far this phenomenon has been studied with microtubules that contain several components, that is, a mixture of tubulin isoforms, with or without a mixture of microtubule-associated proteins (MAPs), which can act as regulators of dynamic instability. Here we concentrate on the influence of the tubulin component. We have studied MAP-free microtubules from the marginal band of avian erythrocytes and compared them with mammalian brain microtubules. The erythrocyte system was selected because it represents a naturally stable aggregate of microtubules; second, the tubulin is largely homogeneous, in contrast to brain tubulin. Qualitatively, erythrocyte microtubules show similar features as brain microtubules, but they were found to be much less dynamic. The critical concentration of elongation, and the rates of association and dissociation of tubulin are all lower than with brain microtubules. Catastrophes are rare, rescues frequent, and shrinkage slow. This means that dynamic instability can be controlled by the tubulin isotype, independently of MAPs. Moreover, the extent of dynamic behavior is highly dependent on buffer conditions. In particular, dynamic instability is strongly enhanced in phosphate buffer, both for erythrocyte marginal band and brain microtubules. The lower stability in phosphate buffer argues against the hypothesis that a cap of tubulin.GDP.Pi subunits stabilizes microtubules. The difference in dynamics between tubulin isotypes and between the two ends of microtubules is preserved in the different buffer systems.
Full text
PDF

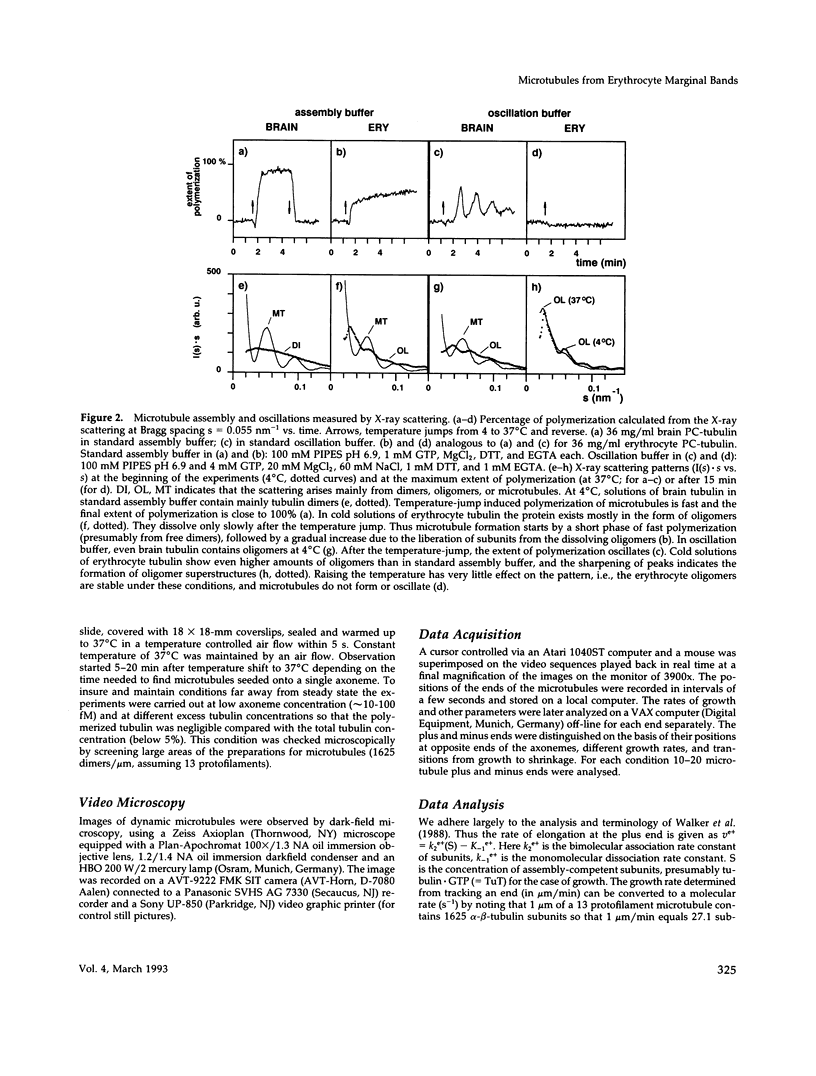


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayley P. M., Schilstra M. J., Martin S. R. Microtubule dynamic instability: numerical simulation of microtubule transition properties using a Lateral Cap model. J Cell Sci. 1990 Jan;95(Pt 1):33–48. doi: 10.1242/jcs.95.1.33. [DOI] [PubMed] [Google Scholar]
- Bell C. W., Fraser C., Sale W. S., Tang W. J., Gibbons I. R. Preparation and purification of dynein. Methods Cell Biol. 1982;24:373–397. doi: 10.1016/s0091-679x(08)60666-4. [DOI] [PubMed] [Google Scholar]
- Belmont L. D., Hyman A. A., Sawin K. E., Mitchison T. J. Real-time visualization of cell cycle-dependent changes in microtubule dynamics in cytoplasmic extracts. Cell. 1990 Aug 10;62(3):579–589. doi: 10.1016/0092-8674(90)90022-7. [DOI] [PubMed] [Google Scholar]
- Bergen L. G., Borisy G. G. Head-to-tail polymerization of microtubules in vitro. Electron microscope analysis of seeded assembly. J Cell Biol. 1980 Jan;84(1):141–150. doi: 10.1083/jcb.84.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulinski J. C., Gundersen G. G. Stabilization of post-translational modification of microtubules during cellular morphogenesis. Bioessays. 1991 Jun;13(6):285–293. doi: 10.1002/bies.950130605. [DOI] [PubMed] [Google Scholar]
- Caplow M. Microtubule dynamics. Curr Opin Cell Biol. 1992 Feb;4(1):58–65. doi: 10.1016/0955-0674(92)90059-l. [DOI] [PubMed] [Google Scholar]
- Caplow M., Ruhlen R., Shanks J., Walker R. A., Salmon E. D. Stabilization of microtubules by tubulin-GDP-Pi subunits. Biochemistry. 1989 Oct 3;28(20):8136–8141. doi: 10.1021/bi00446a026. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Didry D., Melki R., Chabre M., Pantaloni D. Stabilization of microtubules by inorganic phosphate and its structural analogues, the fluoride complexes of aluminum and beryllium. Biochemistry. 1988 May 17;27(10):3555–3559. doi: 10.1021/bi00410a005. [DOI] [PubMed] [Google Scholar]
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Melki R., Pantaloni D., Hill T. L., Chen Y. Synchronous oscillations in microtubule polymerization. Proc Natl Acad Sci U S A. 1987 Aug;84(15):5257–5261. doi: 10.1073/pnas.84.15.5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drechsel D. N., Hyman A. A., Cobb M. H., Kirschner M. W. Modulation of the dynamic instability of tubulin assembly by the microtubule-associated protein tau. Mol Biol Cell. 1992 Oct;3(10):1141–1154. doi: 10.1091/mbc.3.10.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dye R. B., Flicker P. F., Lien D. Y., Williams R. C., Jr End-stabilized microtubules observed in vitro: stability, subunit, interchange, and breakage. Cell Motil Cytoskeleton. 1992;21(3):171–186. doi: 10.1002/cm.970210302. [DOI] [PubMed] [Google Scholar]
- Erickson H. P., O'Brien E. T. Microtubule dynamic instability and GTP hydrolysis. Annu Rev Biophys Biomol Struct. 1992;21:145–166. doi: 10.1146/annurev.bb.21.060192.001045. [DOI] [PubMed] [Google Scholar]
- Gildersleeve R. F., Cross A. R., Cullen K. E., Fagen A. P., Williams R. C., Jr Microtubules grow and shorten at intrinsically variable rates. J Biol Chem. 1992 Apr 25;267(12):7995–8006. [PubMed] [Google Scholar]
- Goldstein D. A. Calculation of the concentrations of free cations and cation-ligand complexes in solutions containing multiple divalent cations and ligands. Biophys J. 1979 May;26(2):235–242. doi: 10.1016/S0006-3495(79)85247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- Herzog W., Weber K. In vitro assembly of pure tubulin into microtubules in the absence of microtubule-associated proteins and glycerol. Proc Natl Acad Sci U S A. 1977 May;74(5):1860–1864. doi: 10.1073/pnas.74.5.1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Hotani H., Horio T. Dynamics of microtubules visualized by darkfield microscopy: treadmilling and dynamic instability. Cell Motil Cytoskeleton. 1988;10(1-2):229–236. doi: 10.1002/cm.970100127. [DOI] [PubMed] [Google Scholar]
- Hyman A. A., Salser S., Drechsel D. N., Unwin N., Mitchison T. J. Role of GTP hydrolysis in microtubule dynamics: information from a slowly hydrolyzable analogue, GMPCPP. Mol Biol Cell. 1992 Oct;3(10):1155–1167. doi: 10.1091/mbc.3.10.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson K. A., Borisy G. G. Thermodynamic analysis of microtubule self-assembly in vitro. J Mol Biol. 1979 Sep 15;133(2):199–216. doi: 10.1016/0022-2836(79)90530-8. [DOI] [PubMed] [Google Scholar]
- Joshi H. C., Cleveland D. W. Diversity among tubulin subunits: toward what functional end? Cell Motil Cytoskeleton. 1990;16(3):159–163. doi: 10.1002/cm.970160302. [DOI] [PubMed] [Google Scholar]
- Lange G., Mandelkow E. M., Jagla A., Mandelkow E. Tubulin oligomers and microtubule oscillations. Antagonistic role of microtubule stabilizers and destabilizers. Eur J Biochem. 1988 Dec 1;178(1):61–69. doi: 10.1111/j.1432-1033.1988.tb14429.x. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. The reconstitution of microtubules from purified calf brain tubulin. Biochemistry. 1975 Nov 18;14(23):5183–5187. doi: 10.1021/bi00694a025. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Herrmann M., Rühl U. Tubulin domains probed by limited proteolysis and subunit-specific antibodies. J Mol Biol. 1985 Sep 20;185(2):311–327. doi: 10.1016/0022-2836(85)90406-1. [DOI] [PubMed] [Google Scholar]
- Mandelkow E. M., Lange G., Jagla A., Spann U., Mandelkow E. Dynamics of the microtubule oscillator: role of nucleotides and tubulin-MAP interactions. EMBO J. 1988 Feb;7(2):357–365. doi: 10.1002/j.1460-2075.1988.tb02821.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelkow E. M., Mandelkow E., Milligan R. A. Microtubule dynamics and microtubule caps: a time-resolved cryo-electron microscopy study. J Cell Biol. 1991 Sep;114(5):977–991. doi: 10.1083/jcb.114.5.977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Melki R., Carlier M. F., Pantaloni D., Timasheff S. N. Cold depolymerization of microtubules to double rings: geometric stabilization of assemblies. Biochemistry. 1989 Nov 14;28(23):9143–9152. doi: 10.1021/bi00449a028. [DOI] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Murphy D. B. Purification of tubulin and tau from chicken erythrocytes: tubulin isotypes and mechanisms of microtubule assembly. Methods Enzymol. 1991;196:235–246. doi: 10.1016/0076-6879(91)96022-j. [DOI] [PubMed] [Google Scholar]
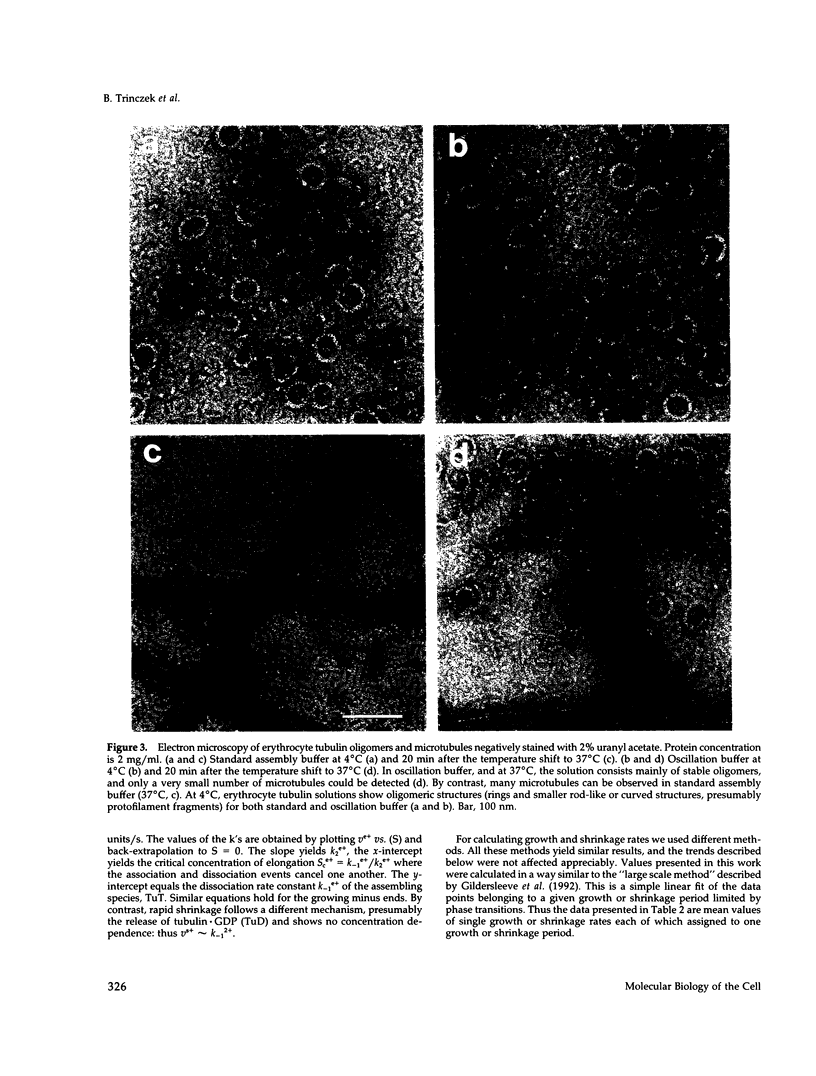
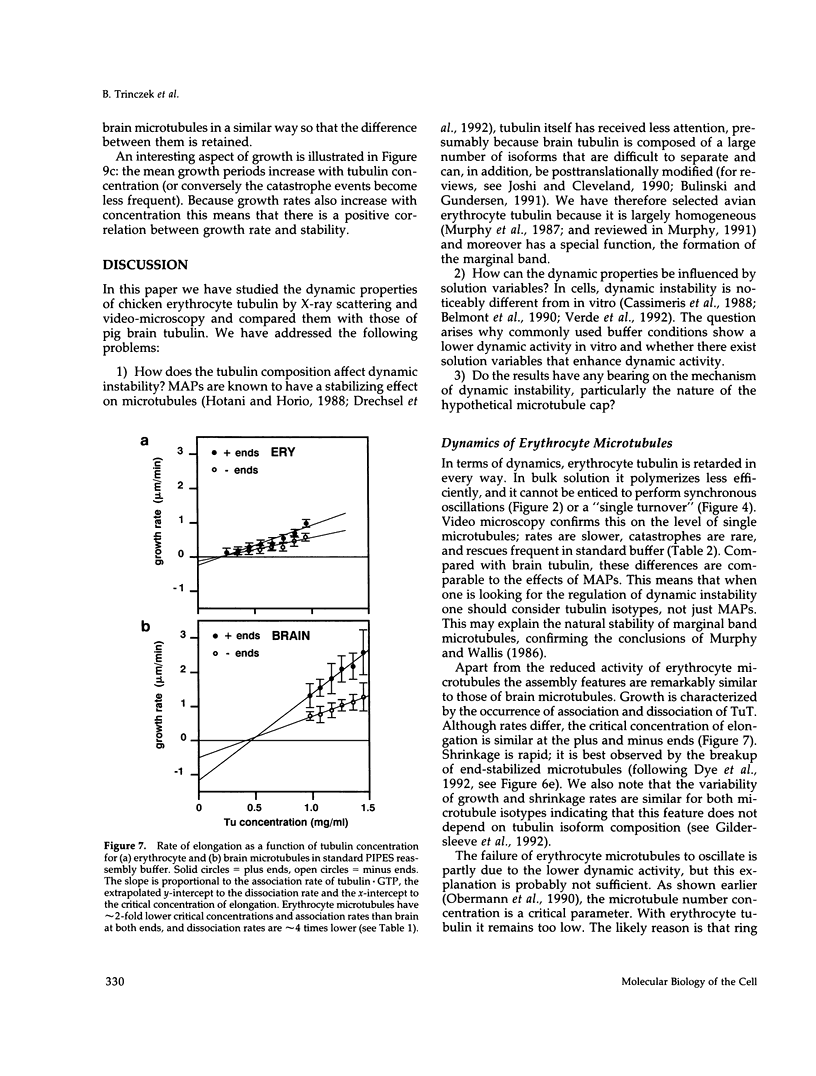
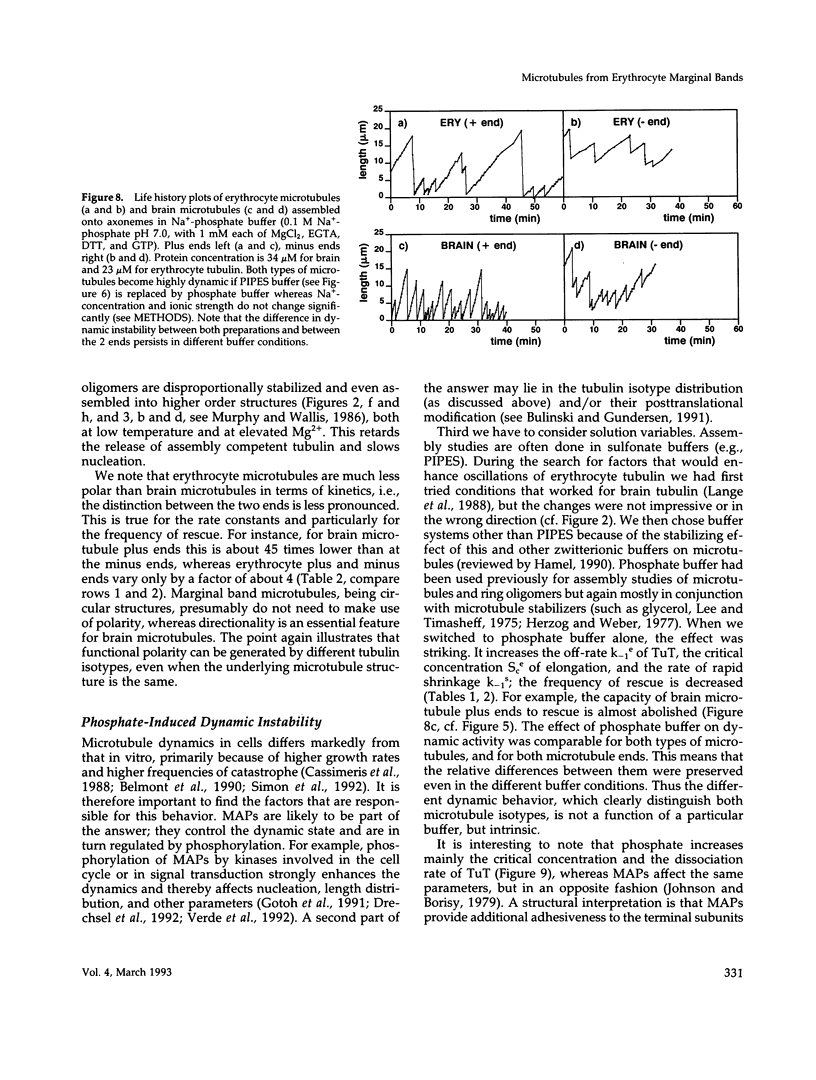
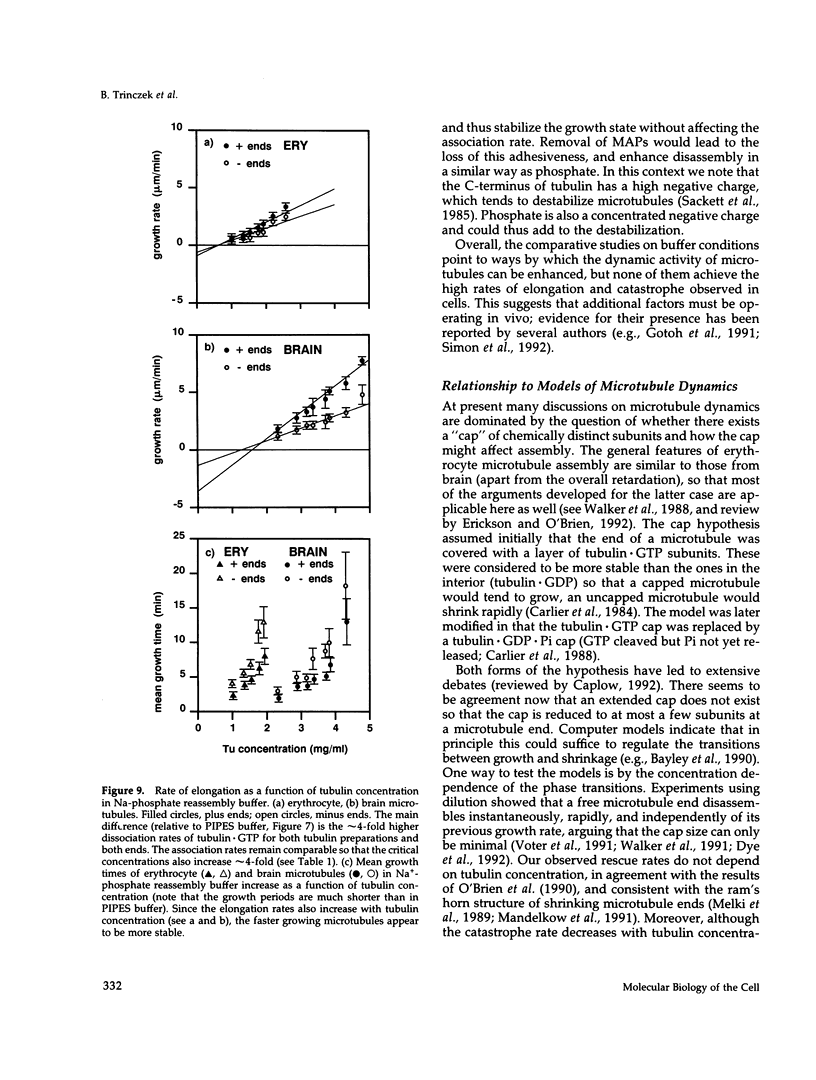
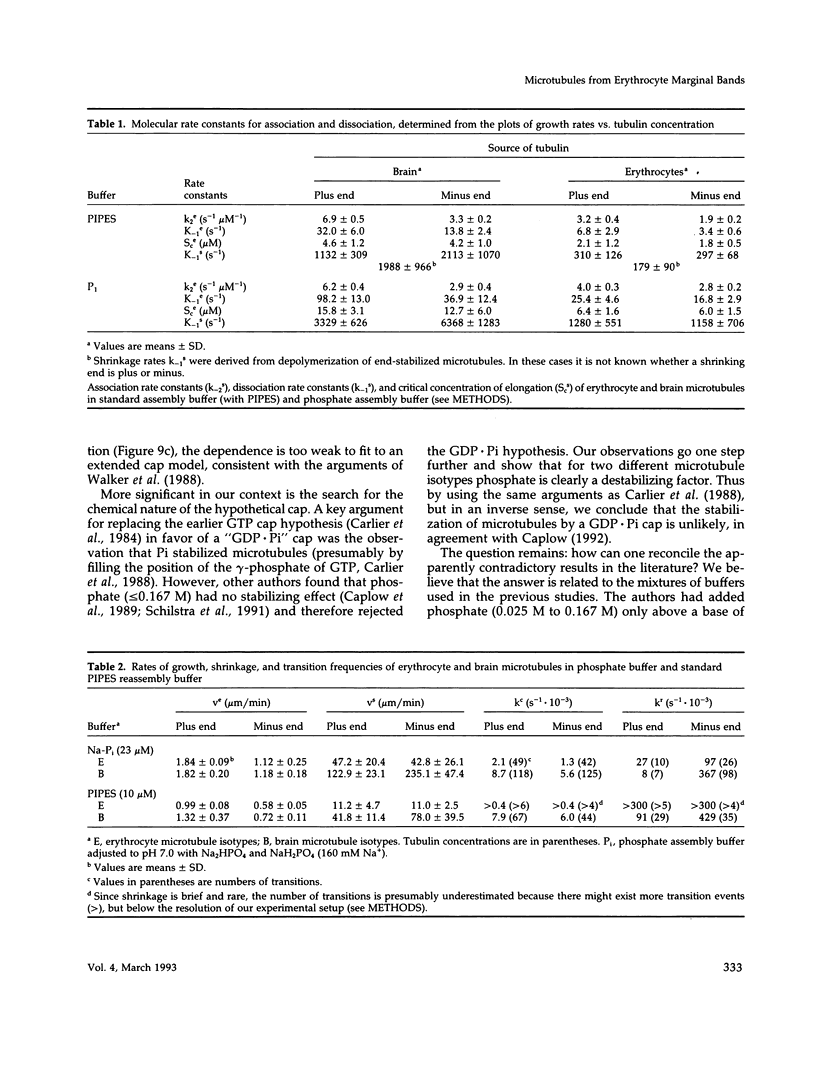
- Murphy D. B., Wallis K. T. Erythrocyte microtubule assembly in vitro. Determination of the effects of erythrocyte tau, tubulin isoforms, and tubulin oligomers on erythrocyte tubulin assembly, and comparison with brain microtubule assembly. J Biol Chem. 1985 Oct 5;260(22):12293–12301. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Erythrocyte microtubule assembly in vitro. Tubulin oligomers limit the rate of microtubule self-assembly. J Biol Chem. 1986 Feb 15;261(5):2319–2324. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T. Isolation of microtubule protein from chicken erythrocytes and determination of the critical concentration for tubulin polymerization in vitro and in vivo. J Biol Chem. 1983 Jul 10;258(13):8357–8364. [PubMed] [Google Scholar]
- Murphy D. B., Wallis K. T., Machlin P. S., Ratrie H., 3rd, Cleveland D. W. The sequence and expression of the divergent beta-tubulin in chicken erythrocytes. J Biol Chem. 1987 Oct 15;262(29):14305–14312. [PubMed] [Google Scholar]
- O'Brien E. T., Salmon E. D., Walker R. A., Erickson H. P. Effects of magnesium on the dynamic instability of individual microtubules. Biochemistry. 1990 Jul 17;29(28):6648–6656. doi: 10.1021/bi00480a014. [DOI] [PubMed] [Google Scholar]
- Obermann H., Mandelkow E. M., Lange G., Mandelkow E. Microtubule oscillations. Role of nucleation and microtubule number concentration. J Biol Chem. 1990 Mar 15;265(8):4382–4388. [PubMed] [Google Scholar]
- Pirollet F., Job D., Margolis R. L., Garel J. R. An oscillatory mode for microtubule assembly. EMBO J. 1987 Nov;6(11):3247–3252. doi: 10.1002/j.1460-2075.1987.tb02642.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt L. F., Cleveland D. W. A survey of the alpha-tubulin gene family in chicken: unexpected sequence heterogeneity in the polypeptides encoded by five expressed genes. EMBO J. 1988 Apr;7(4):931–940. doi: 10.1002/j.1460-2075.1988.tb02898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell S. W., Grasser W. A., Murphy D. B. Direct observation of microtubule treadmilling by electron microscopy. J Cell Biol. 1985 Nov;101(5 Pt 1):1637–1642. doi: 10.1083/jcb.101.5.1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sackett D. L., Bhattacharyya B., Wolff J. Tubulin subunit carboxyl termini determine polymerization efficiency. J Biol Chem. 1985 Jan 10;260(1):43–45. [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Schilstra M. J., Bayley P. M., Martin S. R. The effect of solution composition on microtubule dynamic instability. Biochem J. 1991 Aug 1;277(Pt 3):839–847. doi: 10.1042/bj2770839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. New features of microtubule behaviour observed in vivo. Nature. 1988 Jul 28;334(6180):356–359. doi: 10.1038/334356a0. [DOI] [PubMed] [Google Scholar]
- Simon J. R., Parsons S. F., Salmon E. D. Buffer conditions and non-tubulin factors critically affect the microtubule dynamic instability of sea urchin egg tubulin. Cell Motil Cytoskeleton. 1992;21(1):1–14. doi: 10.1002/cm.970210102. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Spann U., Renner W., Mandelkow E. M., Bordas J., Mandelkow E. Tubulin oligomers and microtubule assembly studied by time-resolved X-ray scattering: separation of prenucleation and nucleation events. Biochemistry. 1987 Feb 24;26(4):1123–1132. doi: 10.1021/bi00378a021. [DOI] [PubMed] [Google Scholar]
- Summers K., Kirschner M. W. Characteristics of the polar assembly and disassembly of microtubules observed in vitro by darkfield light microscopy. J Cell Biol. 1979 Oct;83(1):205–217. doi: 10.1083/jcb.83.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Dogterom M., Stelzer E., Karsenti E., Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992 Sep;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., O'Brien E. T., Erickson H. P. Dilution-induced disassembly of microtubules: relation to dynamic instability and the GTP cap. Cell Motil Cytoskeleton. 1991;18(1):55–62. doi: 10.1002/cm.970180106. [DOI] [PubMed] [Google Scholar]
- Walker R. A., O'Brien E. T., Pryer N. K., Soboeiro M. F., Voter W. A., Erickson H. P., Salmon E. D. Dynamic instability of individual microtubules analyzed by video light microscopy: rate constants and transition frequencies. J Cell Biol. 1988 Oct;107(4):1437–1448. doi: 10.1083/jcb.107.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker R. A., Pryer N. K., Salmon E. D. Dilution of individual microtubules observed in real time in vitro: evidence that cap size is small and independent of elongation rate. J Cell Biol. 1991 Jul;114(1):73–81. doi: 10.1083/jcb.114.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]