Overall, 61 (16%) of 388 participants had an appropriate change in surgical management based on MR findings—more than the 41 (11%) participants with an appropriate change based on positron emission mammography (PEM) findings (P = .003) and fewer than the 71 (18%) participants with an appropriate change based on combined PEM and MR findings (P = .004 for comparison with MR imaging alone); 25 (6.4%) women had excessive excisions on the basis of MR findings compared with 19 (4.9%) women who had them on the basis of PEM findings (P = .26) and 32 (8.2%) women who had them after undergoing combined PEM and MR imaging (P = .023 for comparison with MR imaging alone).
Abstract
Purpose:
To determine the performance of positron emission mammography (PEM), as compared with magnetic resonance (MR) imaging, including the effect on surgical management, in ipsilateral breasts with cancer.
Materials and Methods:
Four hundred seventy-two women with newly diagnosed breast cancer who were offered breast-conserving surgery consented from September 2006 to November 2008 to participate in a multicenter institutional review board–approved, HIPAA-compliant protocol. Participants underwent contrast material–enhanced MR imaging and fluorine 18 fluorodeoxyglucose PEM in randomized order; resultant images were interpreted independently. Added biopsies and changes in surgical procedure for the ipsilateral breast were correlated with histopathologic findings. Performance characteristics were compared by using the McNemar test and generalized estimating equations.
Results:
Three hundred eighty-eight women (median age, 58 years; age range, 26–93 years; median estimated tumor size, 1.5 cm) completed the study. Additional cancers were found in 82 (21%) women (82 ipsilateral breasts; median tumor size, 0.7 cm). Twenty-eight (34%) of the 82 breasts were identified with both PEM and MR imaging; 21 (26%) breasts, with MR imaging only; 14 (17%) breasts, with PEM only; and seven (8.5%) breasts, with mammography and ultrasonography. Twelve (15%) cases of additional cancer were missed at all imaging examinations. Integration of PEM and MR imaging increased cancer detection—to 61 (74%) of 82 breasts versus 49 (60%) of 82 breasts identified with MR imaging alone (P < .001). Of 306 breasts without additional cancer, 279 (91.2%) were correctly assessed with PEM compared with 264 (86.3%) that were correctly assessed with MR imaging (P = .03). The positive predictive value of biopsy prompted by PEM findings (47 [66%] of 71 cases) was higher than that of biopsy prompted by MR findings (61 [53%] of 116 cases) (P = .016). Of 116 additional cancers, 61 (53%) were depicted by MR imaging and 47 (41%) were depicted by PEM (P = .043). Fifty-six (14%) of the 388 women required mastectomy: 40 (71%) of these women were identified with MR imaging, and 20 (36%) were identified with PEM (P < .001). Eleven (2.8%) women underwent unnecessary mastectomy, which was prompted by only MR findings in five women, by only PEM findings in one, and by PEM and MR findings in five. Thirty-three (8.5%) women required wider excision: 24 (73%) of these women were identified with MR imaging, and 22 (67%) were identified with PEM.
Conclusion:
PEM and MR imaging had comparable breast-level sensitivity, although MR imaging had greater lesion-level sensitivity and more accurately depicted the need for mastectomy. PEM had greater specificity at the breast and lesion levels. Eighty-nine (23%) participants required more extensive surgery: 61 (69%) of these women were identified with MR imaging, and 41 (46%) were identified with PEM (P = .003). Fourteen (3.6%) women had tumors seen only at PEM.
© RSNA, 2010
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10100454/-/DC1
Introduction
When patients are appropriately selected, breast-conserving surgery (BCS) followed by radiation (ie, breast conservation therapy) is widely accepted as producing equivalent survival compared with mastectomy in women with newly diagnosed cancer (1). Breast conservation therapy is considered desirable when a cosmetically acceptable result can be achieved by means of complete removal of both the tumor and clear margins of excision. The size and extent of breast cancer are frequently underestimated on the basis of clinical examination and mammographic findings, with or without supplemental ultrasonography (US) (2–4). When the cancer size is underestimated and the patient has chosen breast conservation therapy, initial surgical margins may be positive for malignancy, requiring reexcision or mastectomy. When the focality of the tumor is underestimated, the margins may be clear, with residual tumor remaining in the breast. Such residual tumor may increase the risk of local recurrence, although this risk is markedly decreased when radiation therapy and/or systemic therapy is administered.
It is desirable to accurately map the extent of the tumor before administering treatment to facilitate optimal decision making for the patient and the treating physicians. This may be especially important in young women, in women with dense breasts, in women with invasive lobular histologic findings, or when an extensive intraductal component is present, as residual mammographically occult tumor is more common in these situations (2,5,6). Assessing neoadjuvant treatment response also depends on accurate measurements of tumor size prior to and following treatment; magnetic resonance (MR) imaging has been shown to be more effective than mammography or clinical examination in this setting (7,8), and tumor size is more accurately depicted by MR imaging than by US (9). In a meta-analysis, preoperative contrast material–enhanced MR imaging was shown to depict additional ipsilateral disease in 16% of women with breast cancer (5). It is not clear, however, whether this examination results in improved patient outcomes. Approximately 1% of women undergo unnecessary mastectomy as a result of preoperative MR findings (5), although MR imaging–prompted biopsy has high positive predictive value (PPV), with a median PPV of 0.69 across 19 series (5). In a single-institution series, MR imaging was believed to have delayed definitive surgery for an average of 22 days owing to additional prompted biopsies (10).
Dedicated high-spatial-resolution positron emission mammography (PEM—ie, positron emission tomography with detectors specialized for imaging the breast) with fluorine 18 fluorodeoxyglucose (FDG) has been shown to have a high PPV of 0.88 and to depict breast malignancies not seen on mammograms and/or US images with overall sensitivity of 90% (11). As such, PEM appears to be more specific and possibly more accurate than MR imaging, although to our knowledge, a direct comparison has not been performed previously. The purpose of this study was to determine the performance characteristics of PEM, as compared with MR imaging, including the effect on surgical management, in ipsilateral breasts with cancer.
Materials and Methods
This study was funded in part by Naviscan (San Diego, Calif), the manufacturer of the PEM device described herein. All PEM examinations were provided free of charge to participants. One author (W.A.B.) is a consultant for Naviscan, with compensation based on the fair-market value time spent and not tied to outcomes. This author received a laptop computer from Medipattern (Toronto, Ontario, Canada) and has consulted as a reader for SuperSonic, Imagine (Aix, Provence, France). One author (D.N.) is a previous employee of and holds stock in Naviscan. Another author (J.E.K.) is a current employee of and holds stock options in Naviscan. An author (E.D.P.) who is neither an employee of nor consultant for Naviscan had control of the inclusion of any data and information that might have represented a conflict of interest for those authors who are or were employees of or consultants for Naviscan. The laboratory of this author receives research support from GE Medical Systems (Waukesha, Wis), Sectra NA (Shelton, Conn), Konica Minolta Medical Imaging USA (Wayne, NJ), and Hologic (Bedford, Mass) and is negotiating a research contract with Naviscan. Two authors (K.S.M., J.P.M.) are employees of Certus International (St Louis, Mo), which served as the central research organization and was paid by Naviscan. The participating sites met the technical and experience requirements, and obtained institutional review board approval, to participate in this Health Insurance Portability and Accountability Act–compliant study. Details regarding the study investigators, inclusion and exclusion criteria, imaging methods, data collection, and statistical analyses are given in Appendix E1 (online) and summarized here.
Participants
Women 25 years of age or older with newly diagnosed invasive and/or intraductal breast cancer detected at core-needle or vacuum-assisted biopsy (ie, an index cancer) were recruited from six sites and provided written informed consent. To be eligible, the women had to be candidates for BCS on the basis of the recommendation of a breast surgeon and the following criteria: The tumor was confined to one quadrant (<4 cm in largest overall extent at initial review of prestudy images) and was not believed to involve the skin or chest wall. In women with large breasts, a tumor size of up to 5 cm was allowed.
Imaging
PEM and MR imaging examinations were performed after study enrollment in randomized order, within 5 business days of each other, without regard to timing in the menstrual cycle. Enrolled participants had to agree to undergo any recommended biopsies and/or follow-up imaging, even if they were considering mastectomy. Additional biopsies could not be performed during the interval between MR imaging and PEM.
Data Collection
The findings of conventional imaging (mammography and often targeted US) performed prior to study entry were reviewed with the histopathology results. Independent interpretations of MR and PEM images were performed by different investigators who were blinded to the results of the other examination. There were exceptions to this blinding: In 20 cases, the same investigator interpreted both PEM and MR imaging examinations because clinical care would have otherwise been delayed (with randomization order followed for interpretation); in another nine cases, the interpreting investigator referred to the MR image results while interpreting the PEM study; and in two cases in which the interpreting investigator referred to the PEM study while interpreting the MR images. We included these 31 cases in which the interpretation was not blinded to the other imaging results, but we also calculated results with these cases excluded and found no substantive differences in the conclusions. The investigators had full knowledge of the conventional imaging and prestudy biopsy results. In reviewing each image set (conventional, MR, and PEM), the investigator described the suspected overall extent and focality of the tumor and ascertained whether or not the participant was likely to be a candidate for BCS. Integrated interpretation across all modalities was then performed at the sites by a study investigator to determine the overall extent of suspected disease.
Each presurgical and surgical procedure was documented, and the details of the histopathologic correlations are described in Appendix E1 (online). After all surgeries, the site investigators documented whether mastectomy had been performed and whether the mastectomy had been appropriate or inappropriate (ie, lumpectomy would have sufficed) on the basis of the true disease extent. If the mastectomy was inappropriate, the investigators detailed whether the procedure was prompted by imaging findings—and, if so, by which imaging study—or by the patient’s preference. Investigators were asked whether the surgical management planned after conventional imaging had been modified (to wider local excision, mastectomy, or excision of a high-risk lesion) owing to PEM or MR findings and whether or not this had proved to be appropriate. Finally, estimates of disease extent on each PEM and MR imaging study were rated as accurate, underestimated, or overestimated (by 2 cm or more in size and/or additional foci). For this analysis, a suspicious lesion that underwent biopsy after study entry and was found to be benign preoperatively was included as an overestimation.
Analysis
Only those lesions that did not undergo biopsy prior to study entry were considered in the analysis, with a separate review of index cancer detection. Lesions assigned a Breast Imaging Reporting and Data System (BI-RADS) score of 4a or higher or 3 with a recommendation for biopsy were considered to be positive for cancer at imaging (ie, imaging positive). Since PEM and MR imaging were performed as diagnostic examinations, lesions assigned a BI-RADS assessment 3 or lower with recommendation for additional imaging or for short-term or routine follow-up were considered imaging negative. Positive truth was defined as a diagnosis of malignancy within 1 year on the basis of the most severe histopathologic result for that lesion. High-risk lesions detected at core-needle biopsy (atypical ductal hyperplasia, atypical lobular hyperplasia, lobular carcinoma in situ, columnar cell change with atypia, radial sclerosing lesion, atypical papilloma) were excised, as were lesions showing benign papilloma in the core-needle biopsy specimen. Negative truth was defined as a diagnosis of benign lesion or high-risk lesion on the basis of the most severe finding at histopathologic analysis, or a probably benign lesion that decreased in size or resolved at any follow-up. For lesions initially considered probably benign, the absence of cancer after biopsy or the absence of a suspicious change after a minimum of 6 months of imaging follow-up (median follow-up, 10.5 months; range, 6.1–19.2 months) was considered negative truth. Similarly, an integrated interpretation of BI-RADS 2 across all modalities or BI-RADS 1 or 2 after additional imaging was considered negative truth.
At the breast level, if any additional malignancy was present and was true-positive at imaging, the breast was considered to have true-positive findings, even if another lesion in that breast was false-negative or false-positive. A breast with both false-positive and false-negative lesions was classified as a false-negative case at the breast level. An additional description of the statistical analyses is given in Appendix E1 (online). Diagnostic performance characteristics, including sensitivity, specificity, accuracy, PPV, and area under the receiver operating characteristic curve, were estimated and compared for each imaging modality and for integrated imaging assessments. P < .05 was considered to indicate statistical significance.
Results
A total of 388 eligible women (mean age, 57.8 years; median age, 58 years; age range, 26–93 years), each of whom had one breast with newly diagnosed cancer (Fig E1 [online]), completed the study protocol. The median estimated invasive tumor size at study entry was 1.5 cm (range, 0.4–6.9 cm). The median time from routine-view mammography to the first study imaging examination was 30 days (standard deviation, 21 days; range, 0–121 days). Of the 388 participants, 271 (70%) underwent digital mammography and 117 (30%) underwent screen-film mammography prior to study entry. The mean injected dose of FDG was 10.9 mCi (403.3 MBq) ± 0.8 (standard deviation) (range, 7.6–12.8 mCi [281.2–473.6 MBq]); the mean blood glucose level, 91 mg/dL ± 15 (range, 51–148 mg/dL); and the mean circulation time, 69 minutes ± 14 (range, 44–155 minutes). The demographics of the subjects who completed the protocol (ie, participants) did not differ markedly from those of the women who did not complete the protocol, with the exceptions that the study participants were less likely to have known metastatic axillary nodes, had a smaller median invasive tumor size, were less likely to have multiple known malignancies, and were more likely to have recently taken hormone replacement medication (Table E1 [online]).
With the most severe diagnosis at study entry per participant considered the index malignancy, 84 (21.6%) of the 388 index cancers were ductal carcinoma in situ (DCIS), 302 (77.8%) were invasive cancers, one was Paget disease of the nipple, and one was pleomorphic lobular carcinoma in situ being treated as a malignancy. Of the 388 participants, 372 (95.9%) had known solitary malignancy at study entry; 14 (3.6%), known multifocal tumor; and two (0.5%), known multicentric tumor (believed to be candidates for double lumpectomy), yielding a total of 404 ipsilateral index cancers. No participants were known to have bilateral cancer.
After all treatment surgeries had been performed, 283 (72.9%) of the 388 ipsilateral breasts had a solitary tumor; 66 (17.0%), multifocal disease; 21 (5.4%), multicentric disease; 11 (2.8%), both multifocal and multicentric tumors; three (0.8%), multiple separate cancers that could not be further classified; and four (1.0%), diffuse tumor throughout the breast. Forty-five (12%) breasts had an extensive intraductal component. Seventy-five (19%) breasts had pure DCIS, and 311 had invasive tumor, with an overall median invasive tumor size of 1.5 cm (range, 0.1–8.0 cm). The median time to initial surgery for the participants with no additional suspicious findings at imaging was 15 days (range, 2–100 days) compared with 22 days (range, 5–76 days) for those with suspicious findings.
Performance in Detection of Known Malignancies
Among the 404 index malignancies in 388 breasts, 386 index lesion sites in 370 breasts were confirmed with surgery. PEM tended to better depict cancer when it was present, depicting 357 (92.5%) of the 386 foci versus 344 (89.1%) foci depicted with MR imaging (P = .079, nonsignificant difference). Biopsy sites were more readily identified with MR imaging (Table 1). The absence of visible tumor or biopsy site changes on MR and PEM images in seven and 19 breasts, respectively, did not accurately predict the absence of residual tumor at surgery: All of these breasts were found to have residual tumor at surgery. The documented reasons that the known malignancy was not seen on the PEM images in 19 breasts were as follows: There was no increase in FDG uptake in 11 breasts, the malignancy was not included in the field of view in six breasts, and unknown for two breasts.
Table 1.
Results for 388 Breasts with 404 Sites of Known Malignancy

Note.—Sensitivity data are based on 386 index lesion sites with residual malignancy confirmed at surgery. Investigators documented that the known (index) malignancy was seen at imaging, that only the biopsy site changes were seen, or that nothing was seen at the site of known tumor. Results were calculated by considering only the tumor to be seen as positive for cancer as well as by considering the tumor or biopsy site to be seen as positive. Specificity data are based on the 18 sites found to have no residual tumor at surgery. Accuracy data are based on the 404 original lesion sites (386 with residual cancer plus 18 without residual cancer at surgery). Numbers in parentheses are percentages.
NA = not applicable.
Breast-Level Performance in Detection of Additional Tumor
Of 388 ipsilateral breasts, 82 (21%) were found to have additional foci of tumor after study entry. Of these 82 breasts, 49 (60%; 95% confidence interval [CI]: 48%, 70%) were identified with MR imaging; 42 (51%; 95% CI: 40%, 62%), with PEM (P = .24); and 22 (27%; 95% CI: 18%, 38%), with conventional imaging review (Table 2). An important result was the detection of additional disease with only MR imaging in 21 (26%) of the 82 breasts with additional tumor, with only PEM in 14 (17%) (P = .31) breasts, and with only conventional imaging review in seven (8.5%). The addition of conventional imaging review to either MR imaging or PEM significantly improved sensitivity compared with the sensitivity of MR imaging or PEM alone (Table 2). Integrating the PEM and MR findings significantly improved the detection of additional cancer: to 61 (74%) of 82 breasts versus 49 (60%) of 82 breasts with MR imaging alone (P < .001). Twelve women (3.1% of all 388 participants, 15% of 82 participants with additional tumor) had additional tumor foci in the ipsilateral breast that was not identified with any imaging modality: Eight breasts had 10 invasive lesions with a median size of 0.3 cm (range, 0.1–1.0 cm), and four breasts had DCIS. At evaluation of the 306 breasts without additional cancer, PEM was significantly more specific than MR imaging (91.2% versus 86.3%, P = .032) and similar in performance to conventional imaging (Table 2). Accuracy at the breast level was similar across modalities at 80.7%–82.7%. The area under the receiver operating characteristic curve was 0.76 for MR imaging (95% CI: 0.70, 0.82) and 0.72 for PEM (95% CI: 0.66, 0.78) (P = .37).
Table 2.
Breast-Level Performance of Conventional Imaging, PEM, MR Imaging, and Combined Modalities
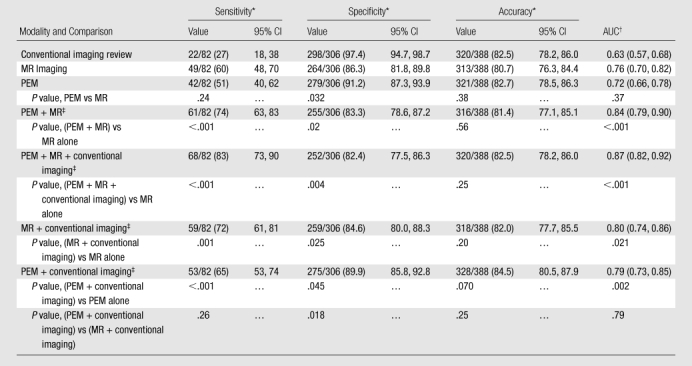
Note.—Data are values of breast-level performance for conventional Imaging, PEM, MR imaging, and combined imaging modalities for 82 breasts with additional ipsilateral malignancies among 388 participants with newly diagnosed breast cancer.
Values are numbers of ipsilateral breasts, with percentages in parentheses. Ninety-five percent CIs for percentages are also given.
AUC = area under receiver operating characteristic curve. Numbers in parentheses are 95% CIs.
Integrated interpretation across modalities. Integrated interpretations could be upgraded or downgraded compared with individual modality interpretations.
Lesion-Level Performance in Detection of Additional Tumor
Fifty (13%) of the 388 women underwent 59 ipsilateral presurgical percutaneous core-needle biopsies after study entry, with 26 (44%) of these procedures yielding malignancies (Table 3). A total of 305 discrete nonindex ipsilateral lesions were identified: 211 of these lesions underwent biopsy or direct excision, including 116 malignancies, 14 high-risk lesions (five at core-needle biopsy, all excised with no upgrades), and 81 benign lesions. Follow-up imaging findings showed the remaining 94 lesions to be benign. Of the 116 additional malignant foci, 56 (48%) were DCIS, 37 (32%) were invasive ductal carcinoma (IDC) with or without DCIS, three (3%) were combined IDC–invasive lobular carcinoma with or without DCIS, 19 (16%) were invasive lobular carcinoma with or without DCIS, and one (1%) was a metastatic intramammary node. These additional tumor foci had a median size of 0.7 cm (standard deviation, 1.7; range, 0.1–7.3 cm); the nearly purely invasive tumor foci (with <10% DCIS) had a median size of 0.6 cm (standard deviation, 1.8; range, 0.1–7.3 cm).
Table 3.
PPVs of Additional Ipsilateral Biopsies among 388 Women with Newly Diagnosed Breast Cancer

Note.—Data are numbers of malignant lesions found at biopsy/total number of lesions biopsy. Numbers in parentheses are percentages. In Total row, numbers in brackets are 95% CIs for percentages.
Lesions considered suspicious (ie, imaging positive, BI-RADS 4a or higher, or BI-RADS 3 with recommendation for biopsy) at review of conventional images only, MR images only, PEM images only, or both MR and PEM images. The lesions suspicious on both conventional and MR images are included among the MR lesions, and the lesions suspicious on both conventional and PEM images are included among the PEM lesions.
Biopsy may have been recommended at additional imaging performed after the breast lesion was assigned a score of BI-RADS 3 or lower, a lesion assigned a score of BI-RADS 3 or lower was included in the excised tissue, or the lesion was not identified until surgery.
Included vacuum-assisted biopsy.
Core-needle procedures included two fine-needle aspiration biopsies that yielded benign findings: one prompted by MR findings and one performed only after additional imaging.
Of the 116 malignant lesions that were not known at study entry, 61 (53%; 95% CI: 43%, 62%) were reported as suspicious at MR imaging; this was more than the 47 (41%; 95% CI: 32%, 50%) malignant lesions reported as suspicious at PEM (P = .04) and much more than the 24 (21%; 95% CI: 14%, 29%) lesions reported as suspicious at conventional imaging review (P < .001). PEM was also much more sensitive than conventional imaging (P < .001) (Table 4). For characterization of the 189 benign lesions, PEM was more specific than MR imaging, with 151 (79.9%) of these lesions imaging negative on PEM images compared with 124 (65.6%) imaging negative on MR images (P = .002), but it was less specific than conventional imaging, where 178 (94.2%) of these lesions were imaging negative (P < .001). Results at the lesion level were not significantly different when the eight probably benign lesions that were not followed up were excluded or when they were considered malignant (Table E2 [online]). Lesion-level accuracy was similar across the modalities: 60.7% (185 of 305 lesions) for MR imaging, 64.9% (198 of 305 lesions) for PEM (P = .25), and 66.2% (202 of 305 lesions) for conventional imaging (P = .14 for comparison with MR imaging). The PPVs of biopsies prompted by PEM findings (66% [47 of 71 lesions]) (P = .016) or by conventional imaging review (69% [24 of 35 lesions]) (P = .041) were higher than the PPV of biopsies prompted by MR findings (53% [61 of 116 lesions]). The PPV of biopsy was higher with combined PEM and conventional imaging (65% [60 of 92 lesions]) than with combined MR and conventional imaging (54% [73 of 136 lesions]) (P = .009) (Table 4). Lesions that were suspicious on both PEM and MR images (30/40 [75%]) were more likely to be malignant than were lesions that were suspicious on MR images only (31/76 [41%]) (P ≤ .001).
Table 4.
Lesion-Level Performance of Conventional Imaging, PEM, MR Imaging, and Combined Modalities

Note.—Data are values of lesion-level performance for conventional Imaging, PEM, MR imaging, and combined imaging modalities for 116 additional ipsilateral malignancies among 388 participants with newly diagnosed breast cancer, with eight probably benign lesions that did not have follow up considered benign.
Values are numbers of lesions, with percentages in parentheses. Ninety-five percent CIs for percentages are also given.
AUC = area under receiver operating characteristic curve. Numbers in parentheses are 95% CIs.
Integrated interpretation across modalities. Integrated interpretations could be upgraded or downgraded compared with individual modality interpretations.
MR imaging was less sensitive for detection of DCIS foci (39% [22/56]) than for detection of any invasive cancer (64% [38/59]) (P = .007) (Table 5), although no difference based on tumor type was seen with PEM. The addition of PEM to MR imaging significantly improved the detection of DCIS, from 22 (39%) of 56 lesions with MR imaging alone to 32 (57%) lesions (P = .001), and another seven DCIS foci were seen only at conventional imaging review, for an overall sensitivity of combined conventional imaging, PEM, and MR imaging of 70% for the detection of DCIS. MR imaging was more sensitive for detection of invasive cancer than was PEM: Of 37 IDC lesions with or without DCIS, 21 (57%) were seen at MR imaging compared with 12 (32%) that were seen at PEM (P = .02). The addition of PEM to MR imaging improved the detection of invasive cancer, from 38 (64%) of 59 lesions depicted with MR imaging alone to 43 (73%) lesions (P = .025). Two high-grade IDC foci with or without DCIS were seen only at conventional image review.
Table 5.
MR, PEM, and Combined PEM-MR Detection Rates as Functions of Histopathologic Results for 211 Lesions Undergoing Biopsy after Study Entry

Note.—Data are imaging detection rates as functions of histopathologic analysis for 211 lesions that underwent biopsy after study entry. NA = not applicable because there were too few entries for P value comparison.
ILC = invasive lobular carcinoma.
Data are numbers of lesions, with percentages in parentheses.
Integrated assessment of PEM and MR findings, with exclusion of lesions seen only on conventional images.
High-risk lesions were seven atypical ductal hyperplasia lesions, four lobular carcinoma in situ lesions, two atypical lobular hyperplasia lesions, and one radial scar–complex sclerosing lesion.
Benign lesions found to be positive at any imaging modality were 19 fibrocystic changes, eight fibroadenomas, five lymph nodes, one papilloma, and one ruptured cyst. Details on 13 benign lesions were not available.
There was no significant effect of investigative site, hormone use, or menopausal status on the sensitivity or specificity of PEM or MR imaging. MR imaging tended to be more sensitive than PEM in small breasts (brassiere cup size A or B), depicting 23 (58%) of 40 tumor foci compared with 14 (35%) depicted by PEM (P = .066, not significant). MR imaging was more sensitive than PEM in dense breasts (sensitivity, 57% [34/60] vs 37% [22/60] with PEM; P = .031). The rate of additional cancer detection was similar for all breast density categories, with the exception that no additional cancers were found in fatty breasts. The sensitivities of MR imaging and PEM were also analyzed according to invasive tumor size for the 40 additional nearly purely invasive lesions: Of 16 1–5-mm T1a lesions, five (31%) were depicted by MR imaging and four (25%) were depicted by PEM (P >.99). Of 13 6–10-mm T1b lesions, 12 (92%) were depicted by MR imaging and six (46%) were depicted by PEM (P = .041). All five (100%) 11–20-mm T1c lesions were depicted by MR imaging, and three (60%) were depicted by PEM (P = .48). The sensitivity of PEM improved with increasing lesion size (P = .021). The sensitivity of MR imaging tended to improve with increasing lesion size (P = .058)
Surgical Management
Of 388 study participants, 56 (14%) ultimately required mastectomy on the basis of the extent of disease (Figure, Fig E2 [online]). Of these 56 women in whom mastectomy was necessary, 40 (71%) were correctly identified with MR imaging (22 of whom were identified only by MR imaging); 20 (36%) were correctly identified with PEM (Table 6) (P < .001), 18 were correctly identified with both MR imaging and PEM, and two were correctly identified with PEM only. The disease extent was underestimated on all imaging in 14 participants who eventually underwent appropriate mastectomy, eight (57%) of whom had an extensive intraductal component. Eleven (2.8% of all participants) women underwent inappropriate mastectomy that was prompted by imaging: five cases prompted by MR findings only, one prompted by PEM findings only, and five prompted by both.
Figure a:

Images obtained in 68-year-old woman who previously underwent right mastectomy for cancer and was noted to have lump in left breast. (a) Lump was shown to correspond to 2.8-cm indistinctly marginated mass (triangular marker) on craniocaudal mammogram. US-guided core-needle biopsy revealed grade III IDC. (b) Craniocaudal and (c) mediolateral oblique PEM images (5.6-mm section thickness) obtained beginning 70 minutes after intravenous injection of 10.4 mCi (384.8 MBq) of FDG show intense rim uptake in known cancer (curved arrows). Approximately 5 cm medial and inferior to this region, intense FDG uptake was noted in second, 1-cm mass (straight arrow). (d) Axial maximum intensity projection of left breast subtraction of precontrast images from three-dimensional spoiled gradient-echo T1-weighted MR images obtained 90 seconds after intravenous injection of 0.1 mmol of gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo) per kilogram of body weight also shows rim enhancement in known malignancy (curved arrows) and intense enhancement in second, 1-cm mass (long straight arrow) in lower inner region of breast. Metastatic axillary nodes were suspected at MR imaging. The patient opted for direct mastectomy; histopathologic analysis confirmed multicentric grade III IDC, the largest of which was 3.0 cm. The second mass was confirmed to be 1.2-cm grade III IDC with a less than 5% DCIS component. A third 0.7-cm grade III IDC (straight short arrow) noted in the immediate retroareolar region was difficult to distinguish from normal nipple enhancement but visible on both PEM and MR images. Two of five sentinel nodes showed metastatic disease. Both PEM and MR imaging correctly depicted multicentric disease that was not seen at mammography.
Table 6.
Accuracy of Surgical Management Based on PEM or MR Findings in 388 Breasts with Newly Diagnosed Cancer

Note.—All data except P values are numbers of lesions, with percentages in parentheses. Conventional imaging refers to mammography and US.
Disease extent underestimated by at least 2 cm or additional foci.
Disease extent overestimated by at least 2 cm, or additional foci.
P values for comparison of MR and PEM data.
Twenty-six women chose initial mastectomy when lumpectomy would have sufficed. Five more women who chose mastectomy after initial attempt at BCS when lumpectomy would have sufficed are included in patient preference data, for a total of 31 cases of mastectomy prompted by patient preference. A total of 23 mastectomies were performed after BCS attempt; 18 of these procedures were required on the basis of the disease extent.
Both PEM and MR imaging depicted a high-risk lesion (lobular carcinoma in situ and atypical ductal hyperplasia) that required excision.
Figure b:
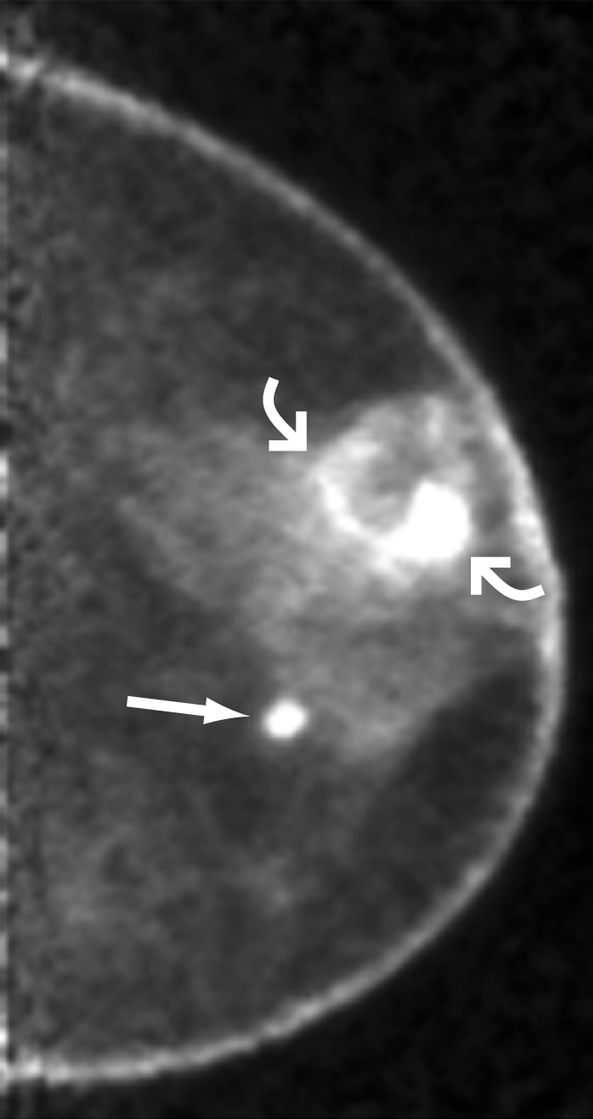
Images obtained in 68-year-old woman who previously underwent right mastectomy for cancer and was noted to have lump in left breast. (a) Lump was shown to correspond to 2.8-cm indistinctly marginated mass (triangular marker) on craniocaudal mammogram. US-guided core-needle biopsy revealed grade III IDC. (b) Craniocaudal and (c) mediolateral oblique PEM images (5.6-mm section thickness) obtained beginning 70 minutes after intravenous injection of 10.4 mCi (384.8 MBq) of FDG show intense rim uptake in known cancer (curved arrows). Approximately 5 cm medial and inferior to this region, intense FDG uptake was noted in second, 1-cm mass (straight arrow). (d) Axial maximum intensity projection of left breast subtraction of precontrast images from three-dimensional spoiled gradient-echo T1-weighted MR images obtained 90 seconds after intravenous injection of 0.1 mmol of gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo) per kilogram of body weight also shows rim enhancement in known malignancy (curved arrows) and intense enhancement in second, 1-cm mass (long straight arrow) in lower inner region of breast. Metastatic axillary nodes were suspected at MR imaging. The patient opted for direct mastectomy; histopathologic analysis confirmed multicentric grade III IDC, the largest of which was 3.0 cm. The second mass was confirmed to be 1.2-cm grade III IDC with a less than 5% DCIS component. A third 0.7-cm grade III IDC (straight short arrow) noted in the immediate retroareolar region was difficult to distinguish from normal nipple enhancement but visible on both PEM and MR images. Two of five sentinel nodes showed metastatic disease. Both PEM and MR imaging correctly depicted multicentric disease that was not seen at mammography.
Figure c:

Images obtained in 68-year-old woman who previously underwent right mastectomy for cancer and was noted to have lump in left breast. (a) Lump was shown to correspond to 2.8-cm indistinctly marginated mass (triangular marker) on craniocaudal mammogram. US-guided core-needle biopsy revealed grade III IDC. (b) Craniocaudal and (c) mediolateral oblique PEM images (5.6-mm section thickness) obtained beginning 70 minutes after intravenous injection of 10.4 mCi (384.8 MBq) of FDG show intense rim uptake in known cancer (curved arrows). Approximately 5 cm medial and inferior to this region, intense FDG uptake was noted in second, 1-cm mass (straight arrow). (d) Axial maximum intensity projection of left breast subtraction of precontrast images from three-dimensional spoiled gradient-echo T1-weighted MR images obtained 90 seconds after intravenous injection of 0.1 mmol of gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo) per kilogram of body weight also shows rim enhancement in known malignancy (curved arrows) and intense enhancement in second, 1-cm mass (long straight arrow) in lower inner region of breast. Metastatic axillary nodes were suspected at MR imaging. The patient opted for direct mastectomy; histopathologic analysis confirmed multicentric grade III IDC, the largest of which was 3.0 cm. The second mass was confirmed to be 1.2-cm grade III IDC with a less than 5% DCIS component. A third 0.7-cm grade III IDC (straight short arrow) noted in the immediate retroareolar region was difficult to distinguish from normal nipple enhancement but visible on both PEM and MR images. Two of five sentinel nodes showed metastatic disease. Both PEM and MR imaging correctly depicted multicentric disease that was not seen at mammography.
Figure d:
Images obtained in 68-year-old woman who previously underwent right mastectomy for cancer and was noted to have lump in left breast. (a) Lump was shown to correspond to 2.8-cm indistinctly marginated mass (triangular marker) on craniocaudal mammogram. US-guided core-needle biopsy revealed grade III IDC. (b) Craniocaudal and (c) mediolateral oblique PEM images (5.6-mm section thickness) obtained beginning 70 minutes after intravenous injection of 10.4 mCi (384.8 MBq) of FDG show intense rim uptake in known cancer (curved arrows). Approximately 5 cm medial and inferior to this region, intense FDG uptake was noted in second, 1-cm mass (straight arrow). (d) Axial maximum intensity projection of left breast subtraction of precontrast images from three-dimensional spoiled gradient-echo T1-weighted MR images obtained 90 seconds after intravenous injection of 0.1 mmol of gadoversetamide (OptiMARK; Mallinckrodt, St Louis, Mo) per kilogram of body weight also shows rim enhancement in known malignancy (curved arrows) and intense enhancement in second, 1-cm mass (long straight arrow) in lower inner region of breast. Metastatic axillary nodes were suspected at MR imaging. The patient opted for direct mastectomy; histopathologic analysis confirmed multicentric grade III IDC, the largest of which was 3.0 cm. The second mass was confirmed to be 1.2-cm grade III IDC with a less than 5% DCIS component. A third 0.7-cm grade III IDC (straight short arrow) noted in the immediate retroareolar region was difficult to distinguish from normal nipple enhancement but visible on both PEM and MR images. Two of five sentinel nodes showed metastatic disease. Both PEM and MR imaging correctly depicted multicentric disease that was not seen at mammography.
For 54 (14%) of the 388 women, the local excision was wider than that planned at conventional imaging, and the wider excision was deemed to be appropriate in 33 of these cases (Table 6). Another 20 cases were considered to have had unnecessarily wider local excision prompted by imaging: Nine were prompted by MR imaging only; six, by PEM only; and five, by both. One high-risk lesion (lobular carcinoma in situ and atypical ductal hyperplasia) was suspicious on both PEM and MR imaging, prompting wider local excision.
MR imaging was more accurate than PEM in surgical planning. MR imaging was accurate for 292 (75%) of the 388 ipsilateral breasts, led to an underestimation of disease in 47 (12%) breasts, and led to an overestimation of disease in 49 (13%). The disease extent determined by using PEM was accurate for 262 (67%) ipsilateral breasts, underestimated in 85 (22%) breasts, and overestimated in 41 (11%) (P < .001 that MR imaging was more accurate). In 28 (7.2%) breasts, PEM was more accurate in depicting the extent of disease than was MR imaging, and in 39 (10%) breasts, MR imaging was more accurate than was PEM (P = .22, not significant). The two examinations were equally likely to lead to overestimation of disease, but MR imaging was less likely to lead to underestimation of disease than was PEM.
Several factors increased the need for mastectomy. When an extensive intraductal component was present, mastectomy was required for 16 (36%) of 45 breasts, compared with 40 (12%) of 343 breasts requiring mastectomy when an extensive intraductal component was not present (P < .001) (Table 7). Both PEM and MR imaging were more likely to lead to an underestimation of disease extent when an extensive intraductal component was present than when it was not (for comparison of accuracy between breasts with and those without extensive intraductal component: P = .004 for PEM accuracy, P = .012 for MR accuracy), although MR imaging performed slightly better than PEM in such cases (Table 8). Cancer-positive margins were much more common in breasts with an extensive intraductal component: 13 (37%) of 35 women with an extensive intraductal component in whom BCS was attempted had positive margins at initial surgery compared with 30 (11%) of 278 women who had positive margins without an extensive intraductal component (P < .001).
Table 7.
Comparison of 56 Breasts Appropriately Treated with Ipsilateral Mastectomy on the Basis of Disease Extent with 332 Ipsilateral Breasts Needing Only Lumpectomy

Note.—Data are summarized for 56 breasts appropriately treated with ipsilateral mastectomy on the basis of disease extent compared with 332 breasts that did not require mastectomy, among 388 study participants. Unless otherwise noted, data are numbers of breasts, with percentages in parentheses.
ILC = invasive lobular carcinoma.
Numbers in parentheses are age ranges.
Forty-seven invasive tumors with or without DCIS required mastectomy, as did nine participants with pure DCIS.
P = .076 for rate of mastectomy for ILC versus rate of mastectomy for other tumor types.
Other includes one case of invasive tubulobular carcinoma and one case of sarcoma arising in phyllodes tumor.
Median tumor size, in centimeters, with range in parentheses.
Includes multiple tumors that could not be further classified.
Three hundred sixteen participants underwent a single surgery; 59, two surgeries; 10, three surgeries; two, four surgeries; and one, more than four surgeries for treatment of ipsilateral breast cancer.
Table 8.
Accuracy of Surgical Planning Based on PEM and MR Findings, and Initial Margin Status, for 388 Breasts as Functions of Tumor Characteristics and BCS Attempt
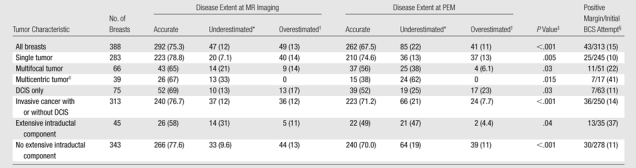
Note.—All data except P values are numbers of breasts, with percentages in parentheses.
Disease extent underestimated by 2 cm or more or by separate tumor foci.
Disease extent overestimated by 2 cm or more or by separate tumor foci.
P values for comparison of MR and PEM data.
Numbers of lesions with cancer-positive margins/numbers of lesions for which BCS was initially attempted.
Includes multicentric tumors, multicentric and multifocal tumors, multiple tumors that could not be further classified, and diffuse tumor.
Other factors that increased the likelihood of mastectomy were positive margins at initial excision or recommendation for mastectomy based on PEM or MR findings (Table 7). Among the 388 participants, mastectomy was also more likely to be needed in 66 (17%) cases with multifocal disease (P = .004 for comparison with unifocal disease) and in 39 (10%) cases with multicentric disease than in the cases of solitary tumor (P < .001) (Table 7). With MR imaging, the disease extent of 13 (33%) of 39 multicentric tumors was less likely underestimated compared with 24 (62%) multicentric tumors whose disease extent was underestimated with PEM (P = .015) (Table 8).
In 283 breasts with a solitary tumor, the tumor size was more often accurately determined with MR imaging (223 [78.8%] breasts) than with PEM (210 [74.6%] breasts) (P = .005), being underestimated by 2 cm or more in 20 (7.1%) breasts with MR imaging and in 36 (13%) breasts with PEM. Initial margins were positive for cancer in 25 (10%) of 245 breasts with solitary tumors for which BCS was attempted initially versus in 11 (22%) of 51 breasts with multifocal tumor (P = .042) and seven (41%) of 17 breasts with multicentric tumor (P < .001 for comparison with solitary tumor) (Table 8) for which BCS was attempted initially. Of the subset of 176 solitary invasive cancers with a lower than 10% in situ component, 166 were seen on PEM images; 163, on MR images; and 153, on both. Pearson correlation coefficients between tumor size at imaging and at histopathologic analysis for the 153 tumors seen on MR and PEM images were 0.55 for PEM and 0.81 for MR imaging (P < .001 for greater accuracy of MR imaging).
Discussion
In this prospective trial, we found that among 388 women anticipating BCS, additional tumor was depicted by MR imaging in 13% (n = 49) of them, by PEM in 11% (n = 42), and with conventional imaging review in 5.7% (n = 22). Our MR imaging results are similar to the 16% rate of detecting additional foci in a meta-analysis involving 2610 women with newly diagnosed cancer (5). We found that 21% (n = 82) of the 388 women had additional unsuspected ipsilateral tumor foci, and MR imaging missed additional foci in 40% (33 of 82) of these women, or 8.5% of all the participants.
We found that PEM was more specific than MR imaging at the lesion level (151 [79.9%] compared with 124 [65.6%] of 189 benign lesions correctly characterized as imaging negative) and less likely to prompt unnecessary biopsies. At the lesion level, PEM was less sensitive than MR imaging (47 [41%] compared with 61 [53%] of 116 malignant lesions imaging positive), although there was no difference in sensitivity at the participant level. Furthermore, PEM proved to be complementary to MR imaging, increasing the additional ipsilateral cancer detection rate from 49 (60%) to 61 (74%) of 82 participants with additional cancer and depicting additional ipsilateral malignancies that were not seen on MR images in 14 (3.6%) of the 388 participants.
We found that conventional image review significantly improved the detection of additional malignancies, with additional ipsilateral tumor foci (all DCIS) in seven participants (1.8%) seen only at conventional image review. Our results suggest that a coordinated review of all breast imaging studies at the time of PEM or MR image interpretation is important for improved diagnosis. Even after the combination of conventional imaging review, PEM, and MR imaging, additional ipsilateral foci remained undetected in 12 (15%) of the 82 women with additional tumors (3.1% of all the women examined), suggesting that there is room for further improvement. This emphasizes the need for caution when making decisions about adjuvant radiation therapy, especially partial breast irradiation, in women with negative imaging results.
The use of MR imaging for preoperative evaluation is under close scrutiny owing to resulting increased rates of mastectomy (10,12–14) and treatment delays, which averaged 22 days in one series (10), particularly in the absence of decreased rates of repeat surgery (10,15), decreased recurrences (16,17), or improved survival (16,17). False-positive results of biopsies prompted by MR findings result in extra testing and stress for the patient, add to costs, and delay treatment. Our reported PPVs of 53% for biopsies prompted by MR imaging and 69% for conventional imaging were within expected ranges for breasts ipsilateral to malignancy (5,18). The PPV of 66% for biopsies prompted by PEM was slightly lower than previously reported values (11) but significantly higher than the PPV of MR imaging. Nevertheless, if additional suspicious findings are identified, presurgical biopsy should be performed to confirm the need for wider excision or mastectomy. An important result was the significantly improved specificity of PEM (91.2%) compared with the specificity of MR imaging (86.3%) (P = .032), with six unnecessary mastectomies prompted by PEM and 10 prompted by MR imaging (P = .45, not significantly different). In our series, we observed a median 7-day delay in the time to surgery for participants with suspicious findings at imaging compared with those with no suspicious findings.
Several factors may have contributed to the reduced sensitivity of PEM in our series. In our study, if the index malignancies had been included, the overall sensitivity of PEM would have been 80.5% (404 of all 502 malignant lesions vs the observed 41% [47 of 116 lesions] we report herein for the detection of unknown malignancies), which is nearly identical to the 80.7% (405 of 502 lesions) sensitivity for the detection of combined index and unknown malignancies with MR imaging (P = .92). Avril et al (19) found that the sensitivity of whole-body FDG PET was highly dependent on the size of the breast cancer, with only three (25%) of 12 cancers 1 cm or smaller identified compared with greater than 90% sensitivity for the detection of tumors 2 cm or larger. We found PEM sensitivity increased with increasing size of malignancy in this series. The section thickness of PEM images increases with increasing breast thickness, as 12 sections are always generated with PEM: Section thickness varied from 3 to 8 mm in our current series. As such, the sensitivity of PEM for the detection of smaller lesions may be reduced in larger breasts owing to volume averaging. PEM-guided percutaneous biopsy (20) became available late in this protocol and at only two sites. This may have discouraged investigators from calling small or vague malignant foci positive at PEM. While posterior lesions can be seen, there is some loss of linearity up to 1.3 cm from the chest wall (21) due to scatter and required coincidence detection. The positioning with earlier versions of the PEM device (used at all but one site) required three hands: This may have reduced the inclusion of very posterior tissues. Indeed, six (1.5%) of 388 index lesions were believed to be outside the field of view on both the craniocaudal and the mediolateral oblique projections. With a field of view of 23 × 17 mm for PEM (11), very large breasts require tiling for complete imaging at PEM (noted for 11 [2.8%] of the 388 participants in the current study), and this may hamper interpretation. Other positioning issues were the most common limitation of PEM, seen in 36 (9.3%) of 388 participants.
Noncalcified DCIS remains problematic. While MR imaging was reported to depict 92% of DCIS lesions in the work of Kuhl et al (22), it remains the source of nearly half of all false-negative MR imaging results (23). In our series, sensitivity for the detection of additional DCIS foci was relatively low for both MR imaging and PEM, at 39% and 41%, respectively. The addition of PEM to MR imaging significantly improved DCIS detection (to 57%, P = .001 for comparison with MR imaging alone). Another seven DCIS foci were seen only at conventional imaging review. (Across all imaging, sensitivity was 70%.)
There were several limitations to this study, which are discussed more fully in Appendix E1 (online). Because PEM is a recently introduced technology, surgeons may have been hesitant to directly excise findings that were seen only on PEM images, particularly without initial core-needle biopsy, and PEM-guided core biopsy was not available until late in the protocol. While we used explicit criteria to determine eligibility for BCS, the amount of tissue removed, including full mastectomy, is a complex decision based on a combination of medical, cosmetic, surgical, and participant concerns. The strict attribution of surgical management to any particular imaging modality, combination of modalities, or patient wishes was, at times, challenging. Finally, not evaluating the influence of findings from the contralateral breast was a limitation that we plan to address in the future.
In summary, PEM proved to be complementary to MR imaging for defining preoperative disease extent in the ipsilateral breast of women with newly diagnosed breast cancer. PEM was more specific than MR imaging and less likely to prompt unnecessary biopsies, but MR imaging was more sensitive to additional malignant lesions and more accurate for assessing disease extent and the need for mastectomy than was PEM. An important finding, however, was that even the combination of PEM and MR imaging did not fully depict the disease extent, particularly in cases with an extensive intraductal component, multifocal disease, or multicentric disease—that is, the very patient populations anticipated to benefit most from accurate preoperative assessment of disease extent. Overall, 61 (16%) of the 388 participants had an appropriate change in surgical management based on MR findings; this was more than the 41 (11%) women with an appropriate change based on PEM findings (P = .003) and fewer than the 71 (18%) women with an appropriate change based on combined PEM and MR findings (P = .004 for comparison with MR imaging alone). Twenty-five (6.4%) participants had excessive excisions based on MR findings, as did 19 (4.9%) on the basis of PEM findings (P = .26) and 32 (8.2%) after combined PEM and MR imaging (P = .023 for comparison with MR imaging alone).
Advances in Knowledge.
Positron emission mammography (PEM) proved to be complementary to MR imaging in defining the preoperative disease extent in the ipsilateral breasts of women with newly diagnosed breast cancer.
Of 388 participants anticipating breast-conserving surgery, 82 (21%) were ultimately found to have additional tumor foci after all imaging examinations and surgery: 49 (13%) participants had additional disease depicted at MR imaging, 42 (11%) had additional disease depicted at PEM, and 70 (18%) were identified after review of combined conventional (mammographic and ultrasonographic), PEM, and MR images.
Combined conventional imaging and PEM depicted additional disease in 53 (14%) participants—not significantly different from the detection achieved with review of combined conventional and MR images (in 59 [15%] participants) (P = .26).
Implications for Patient Care.
PEM is an alternative for women who cannot tolerate MR imaging.
PEM has improved specificity compared with MR imaging and is therefore less likely to prompt unnecessary biopsies.
Review of mammograms together with MR or PEM images, or both, improves the detection of additional disease.
Supplementary Material
Acknowledgments
We thank Irving Weinberg, MD, PhD, for his contributions to the initial study concepts and design. We also thank the following individuals for their invaluable assistance: Lorraine Tafra, MD, and Daina Pack, MD, Anne Arundel Medical Center, Annapolis, Md; Paul Mirabella, Linda Ebling, Edward Anashkin, Rochelle Keen, Victor Nusic, Steve Payne, and Larry Lugo, Naviscan, San Diego, Calif; Lisa Hall, Denise Lawson, Kathy Turner, Roy Sorbet, Nancy Fish, Dwight DiMartino, Lisa Marshall, Fred Quimpo, and Edward Aten, MD, Certus International, St Louis, Mo, and Bedford, NH; Peggy Livingston, RT(NM), Chris Cullings, RT(M), Barbara Levit, RT(M), and Sandra Griffith, RT(NM), American Radiology Services, Baltimore, Md; Kathy Wetzel, Cecilia Brennecke, MD, Susan Harvey, MD, Bruce Copeland, MD, Lisa Mullen, MD, Fariba Asrari, MD, Theodore Tsangaris, MD, and Lisa Jacobs, MD, Johns Hopkins Medical Institutions at Green Spring, Lutherville, Md; Michael Schultz, MD, and Jim Eagan, MD, St Joseph Hospital, Towson, Md; Kathy Almquist, RT(R), (T), (CT), Simone Kahn Griff, MD, Maria Velasquez, MD, Matthew Saady, MD, Juliette The, MD, and Ravinder Mahal, MD, Boca Raton Community Hospital, Boca Raton, Fla; Susan Heath, Laurie Sampson, Amy Nance-Thompson, RN, MSN, OCN, Richard Abello, MD, Ann Nguyen, MD, Symphorosa Williams, MD, Ugne Skripkus, MD, and Amy Wu, MD, Scripps Clinic Medical Group, La Jolla, Calif; Pamela Kurtzhals, MD, Joan Kroener, MD, and Ray Lin, MD, Scripps Green Hospital, La Jolla, Calif; Laura Tuttle, MA, Doreen Steed, RT(M), Cherie Kuzmiak, MD, Dag Pavic, MD, Marcia Koomen, MD, and Marija Ivanovic, MD, University of North Carolina, Chapel Hill, NC; and Christina Kiss, CCRP, Pulin Sheth, MD, and Mary Yamashita, MD, Keck School of Medicine, University of Southern California, Los Angeles, Calif. We thank Nehmat Houssami, PhD, University of Sydney, Sydney, Australia, and Christopher Comstock, MD, Memorial Sloan-Kettering Cancer Center, New York, NY, for helpful comments.
Received February 27, 2010; revision requested April 5; revision received May 20; accepted June 9; final version accepted July 7.
Supported by Naviscan and the National Institutes of Health.
Current addresses: American College of Radiology Imaging Network, Lutherville, Md.
Department of Radiology, Medical University of South Carolina, Charleston, SC.
National Cancer Institute, Bethesda, Md.
Clinical Research Program, Children’s Hospital, Boston, Mass.
Funding: This research was supported by National Institutes of Health (grant 5 R44 CA103102-05).
See Materials and Methods for pertinent disclosures.
Abbreviations:
- BCS
- breast-conserving surgery
- BI-RADS
- Breast Imaging Reporting and Data System
- CI
- confidence interval
- DCIS
- ductal carcinoma in situ
- FDG
- fluorine 18 fluorodeoxyglucose
- IDC
- invasive ductal carcinoma
- PEM
- positron emission mammography
- PPV
- positive predictive value
References
- 1.Clarke M, Collins R, Darby S, et al. Effects of radiotherapy and of differences in the extent of surgery for early breast cancer on local recurrence and 15-year survival: an overview of the randomised trials. Lancet 2005;366(9503):2087–2106 [DOI] [PubMed] [Google Scholar]
- 2.Berg WA, Gutierrez L, NessAiver MS, et al. Diagnostic accuracy of mammography, clinical examination, US, and MR imaging in preoperative assessment of breast cancer. Radiology 2004;233(3):830–849 [DOI] [PubMed] [Google Scholar]
- 3.Fischer U, Kopka L, Grabbe E. Breast carcinoma: effect of preoperative contrast-enhanced MR imaging on the therapeutic approach. Radiology 1999;213(3):881–888 [DOI] [PubMed] [Google Scholar]
- 4.Holland R, Veling SH, Mravunac M, Hendriks JH. Histologic multifocality of Tis, T1-2 breast carcinomas: implications for clinical trials of breast-conserving surgery. Cancer 1985;56(5):979–990 [DOI] [PubMed] [Google Scholar]
- 5.Houssami N, Ciatto S, Macaskill P, et al. Accuracy and surgical impact of magnetic resonance imaging in breast cancer staging: systematic review and meta-analysis in detection of multifocal and multicentric cancer. J Clin Oncol 2008;26(19):3248–3258 [DOI] [PubMed] [Google Scholar]
- 6.Liberman L, Morris EA, Dershaw DD, Abramson AF, Tan LK. MR imaging of the ipsilateral breast in women with percutaneously proven breast cancer. AJR Am J Roentgenol 2003;180(4):901–910 [DOI] [PubMed] [Google Scholar]
- 7.Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM. Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 2002;179(5):1193–1199 [DOI] [PubMed] [Google Scholar]
- 8.Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM. MRI vs. histologic measurement of breast cancer following chemotherapy: comparison with x-ray mammography and palpation. J Magn Reson Imaging 2001;13(6):868–875 [DOI] [PubMed] [Google Scholar]
- 9.Davis PL, Staiger MJ, Harris KB, et al. Breast cancer measurements with magnetic resonance imaging, ultrasonography, and mammography. Breast Cancer Res Treat 1996;37(1):1–9 [DOI] [PubMed] [Google Scholar]
- 10.Bleicher RJ, Ciocca RM, Egleston BL, et al. Association of routine pretreatment magnetic resonance imaging with time to surgery, mastectomy rate, and margin status. J Am Coll Surg 2009;209(2):180–187; quiz 294–295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Berg WA, Weinberg IN, Narayanan D, et al. High-resolution fluorodeoxyglucose positron emission tomography with compression (“positron emission mammography”) is highly accurate in depicting primary breast cancer. Breast J 2006;12(4):309–323 [DOI] [PubMed] [Google Scholar]
- 12.Katipamula R, Degnim AC, Hoskin T, et al. Trends in mastectomy rates at the Mayo Clinic Rochester: effect of surgical year and preoperative magnetic resonance imaging. J Clin Oncol 2009;27(25):4082–4088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mameri CS, Kemp C, Goldman SM, Sobral LA, Ajzen S. Impact of breast MRI on surgical treatment, axillary approach, and systemic therapy for breast cancer. Breast J 2008;14(3):236–244 [DOI] [PubMed] [Google Scholar]
- 14.Pettit K, Swatske ME, Gao F, et al. The impact of breast MRI on surgical decision-making: are patients at risk for mastectomy? J Surg Oncol 2009;100(7):553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet 2010;375(9714):563–571 [DOI] [PubMed] [Google Scholar]
- 16.Houssami N, Hayes DF. Review of preoperative magnetic resonance imaging (MRI) in breast cancer: should MRI be performed on all women with newly diagnosed, early stage breast cancer? CA Cancer J Clin 2009;59(5):290–302 [DOI] [PubMed] [Google Scholar]
- 17.Solin LJ, Orel SG, Hwang WT, Harris EE, Schnall MD. Relationship of breast magnetic resonance imaging to outcome after breast-conservation treatment with radiation for women with early-stage invasive breast carcinoma or ductal carcinoma in situ. J Clin Oncol 2008;26(3):386–391 [DOI] [PubMed] [Google Scholar]
- 18.Schnall MD, Blume J, Bluemke DA, et al. MRI detection of distinct incidental cancer in women with primary breast cancer studied in IBMC 6883. J Surg Oncol 2005;92(1):32–38 [DOI] [PubMed] [Google Scholar]
- 19.Avril N, Rosé CA, Schelling M, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol 2000;18(20):3495–3502 [DOI] [PubMed] [Google Scholar]
- 20.Kalinyak JE, Schilling K, Berg WA, et al. PET-guided breast biopsy. Breast J (in press) [DOI] [PubMed] [Google Scholar]
- 21.MacDonald L, Edwards J, Lewellen T, Haseley D, Rogers J, Kinahan P. Clinical imaging characteristics of the positron emission mammography camera: PEM Flex Solo II. J Nucl Med 2009;50(10):1666–1675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuhl CK, Schrading S, Bieling HB, et al. MRI for diagnosis of pure ductal carcinoma in situ: a prospective observational study. Lancet 2007;370(9586):485–492 [DOI] [PubMed] [Google Scholar]
- 23.Schnall MD, Blume J, Bluemke DA, et al. Diagnostic architectural and dynamic features at breast MR imaging: multicenter study. Radiology 2006;238(1):42–53 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



