The National Lung Screening Trial is a multicenter, randomized controlled trial comparing low-dose helical CT with chest radiography in the screening of current and former heavy smokers for lung cancer.
Abstract
The National Lung Screening Trial (NLST) is a randomized multicenter study comparing low-dose helical computed tomography (CT) with chest radiography in the screening of older current and former heavy smokers for early detection of lung cancer, which is the leading cause of cancer-related death in the United States. Five-year survival rates approach 70% with surgical resection of stage IA disease; however, more than 75% of individuals have incurable locally advanced or metastatic disease, the latter having a 5-year survival of less than 5%. It is plausible that treatment should be more effective and the likelihood of death decreased if asymptomatic lung cancer is detected through screening early enough in its preclinical phase. For these reasons, there is intense interest and intuitive appeal in lung cancer screening with low-dose CT. The use of survival as the determinant of screening effectiveness is, however, confounded by the well-described biases of lead time, length, and overdiagnosis. Despite previous attempts, no test has been shown to reduce lung cancer mortality, an endpoint that circumvents screening biases and provides a definitive measure of benefit when assessed in a randomized controlled trial that enables comparison of mortality rates between screened individuals and a control group that does not undergo the screening intervention of interest. The NLST is such a trial. The rationale for and design of the NLST are presented.
© RSNA, 2010
Clinical trial registration no. NCT 00047385
Supplemental material: http://radiology.rsna.org/lookup/suppl/doi:10.1148/radiol.10091808/-/DC1
Introduction
The National Lung Screening Trial (NLST) is a multicenter, randomized controlled trial (RCT) comparing low-dose helical computed tomography (CT) with chest radiography in the screening of current and former heavy smokers for lung cancer. This is the largest randomized study of lung cancer screening in a high-risk population to date. The trial was launched in September 2002 and had enrolled 53 456 participants when recruitment goals were reached in April 2004. Each NLST participant was randomized to undergo a baseline and two annual screenings by using either low-dose CT or chest radiography. Lung cancer mortality is the primary endpoint of the study.
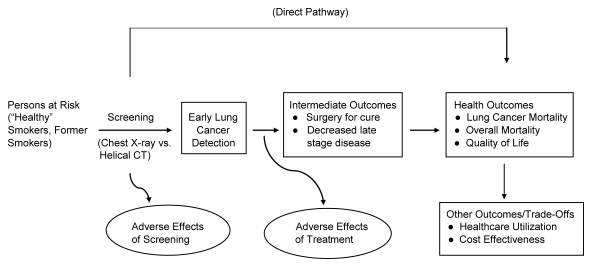
The purpose of this article was to provide a concise but comprehensive description of the NLST. The design, conduct, and analysis of the NLST follow the framework depicted in the Figure.
Process and outcomes in the NLST. (Adapted and reprinted, with permission, from reference (1).
The Magnitude of the Lung Cancer Problem
Lung cancer is the leading cause of cancer-related death in the United States. In 2002, it was estimated that 169 400 new lung cancer cases and 154 900 lung cancer deaths would occur and that lung cancer deaths would represent about 25% of all cancer deaths (2). Despite decreasing trends in smoking and resulting decrease in lung cancer mortality, the population at risk for lung cancer continues to be very large. In 2007, roughly 90 million individuals in the United States had a history of cigarette smoking, with about half reporting to be current smokers (3–6). While smoking cessation reduces the elevated risk of lung cancer, former smokers remain at elevated risk relative to never smokers (7). These data portend the continued dominance of lung cancer as a major public health problem for the next several decades and underscore the need for strategies beyond smoking cessation to reduce the toll (8).
Methodologic Issues in Lung Cancer Screening
Clinical stage at diagnosis is a major determinant of survival after therapy (9). Accordingly, there is intense interest and intuitive appeal in lung cancer screening with low-dose CT, a highly sensitive imaging modality. The use of survival as the determinant of screening effectiveness is, however, confounded by the well-known screening biases of lead time, length, and overdiagnosis (10). No screening modality has been shown to reduce lung cancer mortality, an endpoint that circumvents these screening biases. A prospective RCT enables the comparison of mortality rates between screened individuals and a control group that does not undergo the screening intervention of interest and therefore provides a definitive measure of benefit (10).
Previous Research
Experience from Early Chest Radiographic Screening RCTs
Early RCTs in lung cancer screening evaluated chest radiography with or without sputum cytology. In the 1970s, the U.S. National Cancer Institute sponsored three RCTs of lung cancer screening in male smokers (Table 1). Two trials, the Memorial Sloan-Kettering Study (11,12) and the Johns Hopkins Study (13,14), evaluated the incremental benefit of adding frequent sputum cytology to annual chest radiography. Neither study showed a decrease in lung cancer mortality in the experimental arm. The third trial, the Mayo Lung Project, examined the effects of intense chest radiography and sputum cytology screening (15–17). All participants underwent screening chest radiography and sputum cytology at the prevalence screening, and those with lung cancer were excluded from further follow-up and analyses. The remaining 9211 participants were randomized to undergo both chest radiography and sputum cytology examination at 4-month intervals (experimental arm) or were advised at trial entry only to undergo annual chest radiography and sputum cytology examination, although these examinations were not provided as part of the trial. A fourth trial, conducted in Czechoslovakia around the same time (18,19), compared chest radiography and sputum cytology every 6 months for 3 years and annually thereafter versus the same examinations annually and also observed no reduction in lung cancer mortality. None of the trials tested the efficacy of chest radiography alone. These four trials were conducted in an era in which squamous cell histology, generally centrally located and more difficult to detect with chest radiography, was the most prevalent lung cancer histologic finding (20). However, adenocarcinoma of the lung, which tends to be peripherally located (21) and thus more amenable to detection with chest radiography, has now become the most prevalent histologic type (22). Therefore, the direct applicability of these four studies to early detection of the current distribution of lung cancers may be questioned.
Table 1.
RCTs of Lung Cancer Screening with Chest Radiography with or without Sputum Cytology
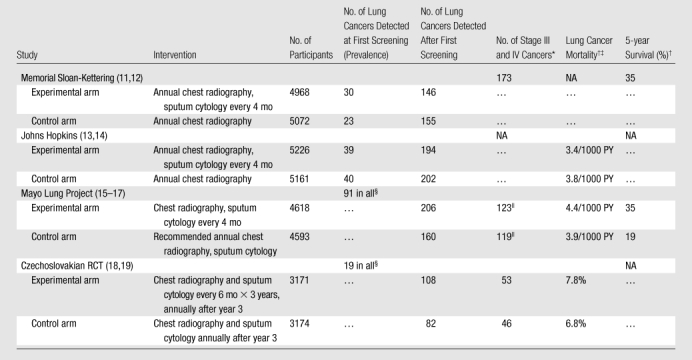
Note.—NA = not available.
Numbers of stage III and IV lung cancers include all cancers (prevalence and later) for the Memorial Sloan-Kettering RCT. Numbers of stage III and IV lung cancers do not include prevalence cancers for the Mayo Lung Project and Czechoslovakian RCT.
Lung cancer mortality and 5-year survival was at 5–8 years of follow-up for the Memorial Sloan-Kettering RCT, at 8 years of follow-up for the Johns Hopkins Study, at 20 years of follow-up for the Mayo Lung Project, and at 15 years of follow-up for the Czechoslovakian RCT.
Mortality data for the Johns Hopkins RCT include prevalence cases. Mortality data for the Mayo Lung Project and the Czechoslovakian RCT do not include prevalence cases. PY = person-years.
Not included in remainder of Table.
Includes all unresected cancers plus resected cancers that were international stage III.
The Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
In 1992, the U.S. National Cancer Institute initiated the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial (PLCO), a large RCT designed to assess the ability of multiple screening modalities to reduce disease-specific cancer mortality (23). The PLCO enrolled nearly 155 000 participants (both men and women), aged 55–74 years. Participants randomized to the intervention arm were offered either three (never smokers) or four (ever smokers) annual posteroanterior chest radiographic screenings; participants randomized to the control arm were told to receive their usual medical care (24). Lung cancer detection results from the chest radiographic baseline screening round were published in 2005 (25). Follow-up of the trial is ongoing for the primary endpoint of lung cancer mortality.
Single-Arm Studies Evaluating Low-Dose CT Screening
Multidetector helical CT represents a major advance in cross-sectional imaging due to increased scan speed, improved spatial resolution, and capacity to reconstruct multiple series from a single data acquisition. CT affords several advantages over chest radiography as a potential screening examination, primarily because of its cross-sectional data acquisition and display, which reduces the problem of overlying structures obscuring the detection of lung nodules. Moreover, the inherent contrast in the lung parenchyma is substantially greater with CT, thus enabling the visualization of more subtle abnormalities. As discussed below, the radiation exposure with low-dose CT is approximately 20%–25% that of diagnostic CT.
Six prospective single-arm studies of low-dose CT screening for lung cancer were published by 2002 (Table 2). Widespread interest in low-dose CT as a lung cancer screening modality began in Japan (26–28). In the United States, interest in low-dose CT increased in 1999 with the publication of findings from the Early Lung Cancer Action Project (29,30). Two additional single-arm studies were conducted in the United States (31) and in Germany (32). These six studies contributed valuable preliminary information on the performance of low-dose CT screening. The fraction of participants with positive results from low-dose CT screenings at baseline ranged from 5.1% (26) to 51.4% (31). This wide range was probably in part because of differences in study populations, criteria for a positive result, and the manner in which data were reported. However, the variability also may point toward meaningful differences in diagnostic performance of low-dose CT and radiologists’ interpretation practices. In addition, false-positive rates were substantial.
Table 2.
Single-Arm Low-Dose CT Studies of Lung Cancer Screening Published by 2002
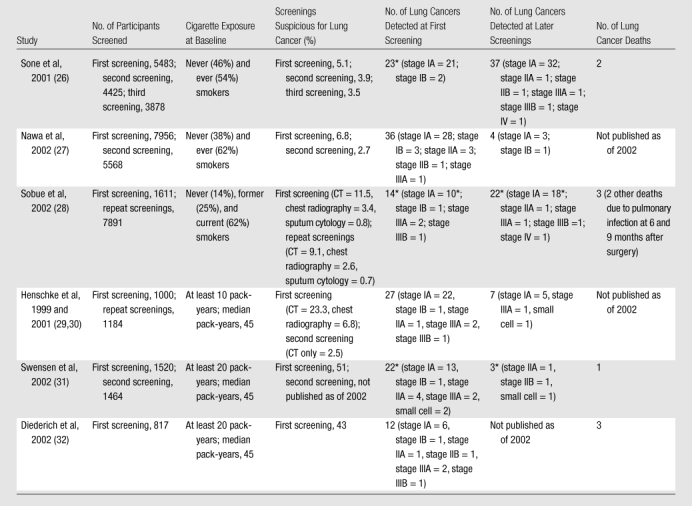
One or more cancers had a positive result only at sputum cytology examination (one negative result at CT in Sone et al, one negative result at CT and chest radiography at first screening in Sobue et al, three negative results at CT and chest radiography at repeated screenings in Sobue et al, one negative result at CT in Swensen et al at first screening, one negative result in Swensen et al at second screening).
Two of the studies in Table 2 screened participants with both low-dose CT and chest radiography (28,29). The rates of positive screening results from low-dose CT at baseline were roughly three times higher than those for chest radiography. Limited information on survival of persons with a diagnosis of lung cancer associated with low-dose CT screening had been reported for three single-arm studies as of 2002 (26,28,32), and while those data were promising, none of the study designs permitted a reliable assessment of the effect of screening on lung cancer mortality.
The Lung Screening Study Feasibility Phase
In 2000, the U.S. National Cancer Institute conducted a feasibility study, in which a relatively small number of individuals were recruited, randomized to either low-dose CT or chest radiography, and screened at baseline and a year later (33,34). This study, now referred to as the Lung Screening Study (LSS) Feasibility Phase, enrolled 3318 participants within a 2½-month period at six PLCO screening centers. Trial accrual targets were met ahead of schedule, and the percentage of participants randomized to chest radiography who underwent low-dose CT outside the study was low, thus establishing feasibility. The LSS Feasibility Phase confirmed that low-dose CT could help detect more lung cancer than chest radiography (Table 3). Forty cancers were diagnosed in the low-dose CT arm and 20 in the chest radiographic arm (34). Stage I cancers accounted for 48% of cancers in the low-dose CT arm and 40% in the chest radiographic arm. Roughly equal percentages of advanced (stage III or IV) cancers were observed in the two study arms: 40% in the low-dose CT arm, compared with 45% in the chest radiographic arm.
Table 3.
Data from the LSS Feasibility Phase RCT
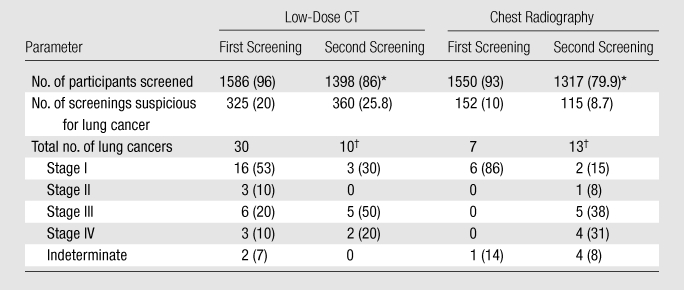
Source.—References 33,34.
Note.—Data in parentheses are percentages. Number of participants randomized was 1660 for low-dose CT and 1658 for chest radiography.
Among those eligible to be screened.
Includes one interval cancer in the low-dose CT arm (stage IV) and four interval cancers in the chest radiographic arm (one stage III and three stage IV cancers).
The design of the LSS Feasibility Phase, as well as a formal protocol written by American College of Radiology Imaging Network (ACRIN) investigators, served as the basis for the NLST.
NLST Description
Design Overview
The NLST participants were randomized to either low-dose CT or chest radiography in equal proportions. Screening was offered three times to participants in each of the NLST arms (at baseline and two annual follow-up examinations). The primary endpoint of the NLST is lung cancer mortality. The study arms also will be compared with regard to the following: overall mortality, lung cancer incidence, and screening and treatment-related morbidity. Health care utilization, quality of life, and cost-effectiveness will be assessed in substudies (Figure).
The rationale for the choice of a two-arm study design, with chest radiography as the comparator, has been described in detail previously (35). In brief, the comparison to usual care is currently addressed by PLCO. In addition, chest radiographic screening is currently often recommended to persons at elevated risk for lung cancer, even though available evidence does not support the practice and no U.S. medical organization currently recommends it.
Study Organization
The NLST is a collaborative effort of the National Cancer Institute’s Division of Cancer Prevention, which funds and administers the NLST-LSS, and the ACRIN, which administers the NLST-ACRIN (Figure E1 [online]). ACRIN is funded by National Cancer Institute’s Cancer Imaging Program, Division of Cancer Treatment and Diagnosis. The day-to-day management of the study is the responsibility of the NLST-ACRIN Executive Committee and the NLST-LSS Steering Committee. A trial-wide Executive Committee coordinates the entire process. Study progress is monitored by an independent Data and Safety Monitoring Board, which meets every 6 months. Reports to the Data and Safety Monitoring Board are prepared jointly from a combined database by the statistical teams of NLST-ACRIN and NLST-LSS.
Participant Cohort
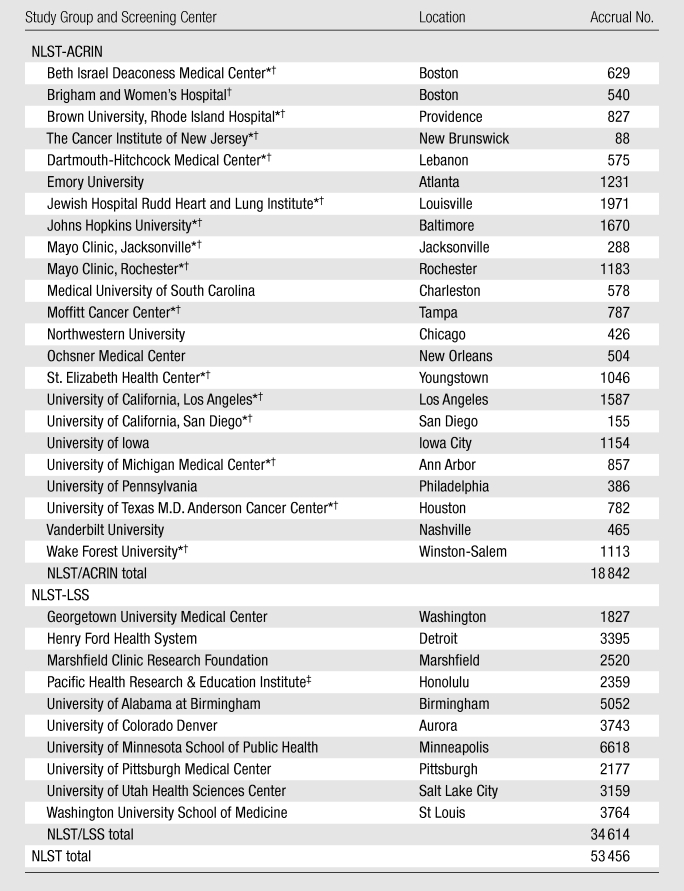
Participants were recruited by and enrolled at 33 screening centers across the country (Table 4). Each screening center received institutional review board approval before the onset of recruitment. The target accrual was 50 000 participants, although rapid and highly successful recruitment resulted in enrollment of 3456 additional participants. The NLST was officially launched on September 18, 2002, although some participants were enrolled as early as August 2002. At each screening center, potential participants were made aware of the trial through numerous methods, including direct mailings and use of mass media (Appendix E1 [online]).
Table 4.
NLST Accrual according to Study Group and Screening Center
NLST-ACRIN site participating in the biospecimen collection.
NLST-ACRIN site participating in the quality of life data collection.
Formerly known as Pacific Health Research Institute.
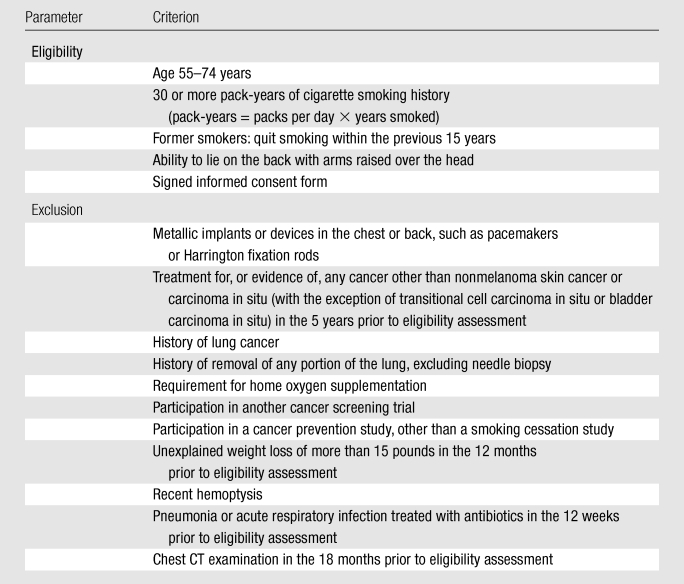
Interested participants contacted screening centers and were assessed for eligibility. Participants who were eligible and signed an informed consent form were randomized into one of the two trial arms. Blocked randomization was used, with stratification according to age, sex, and screening center. Blocks were randomly assigned to be of length six or eight; the order of assignments within each block was random as well. Study inclusion and exclusion criteria are listed in Table 5.
Table 5.
Entry Criteria for the NLST
Low-Dose CT Screening
Low-Dose CT Techniques and Procedures
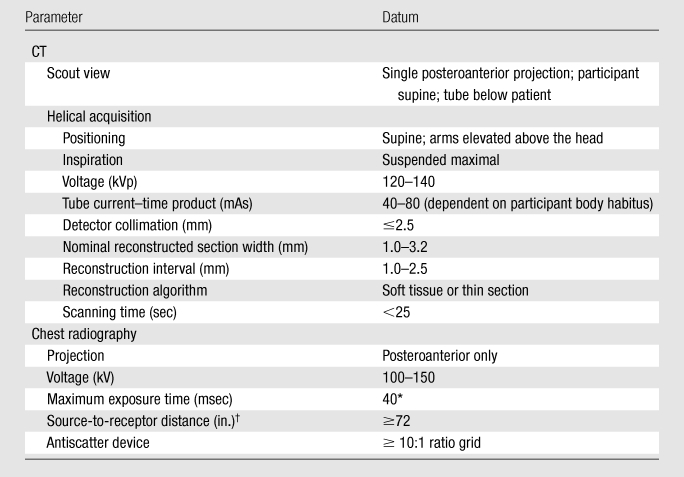
All low-dose CT scanners were certified for use in the NLST, meeting NLST protocol requirements (Table 6) and American College of Radiology guidelines (36). Multidetector (ie, at least four detectors) scanners were used to ensure that the whole chest could be scanned in a single maximal breath hold and to achieve good spatial resolution. All NLST acquisitions utilized a low radiation exposure protocol consistent with lung cancer screening protocols in use at the time the study began (29) and defined as a protocol to minimize patient radiation exposure while maintaining the performance of CT for the detection of lung nodules (37). Although the acquisition parameters for low-dose CT are not explicitly defined in the imaging community, each of the scanners used in NLST was individually tested by using a CT dose index phantom and patient data to achieve comparable subjective image quality by using tube current–time products of 40 mAs or lower for the average-sized patient. Depending on the scanner, the effective tube current–time product (tube current–time products/scan pitch) range varied from 20 to 30 mAs for an average-sized patient (38,39). It is estimated that each NLST low-dose CT resulted in an average effective dose of 1.5 mSv, whereas the effective dose from conventional chest CT varies considerably in clinical practice but is on the order of 8 mSv (40). Low-dose CT acquisitions were electronically transmitted to ACRIN- or LSS-maintained central repositories.
Table 6.
CT and Chest Radiographic Acquisition Parameters
LSS-specified tube current–time product, 0.1 to 20.0 mAs; ACRIN-specified maximum skin entrance dose, 0.4 mGy.
≥182.9 cm
Low-Dose CT Interpretations and Recommendations
All low-dose CT acquisitions were interpreted at the screening center by radiologists approved to read for the NLST (Appendix E2 [online]). Interpretations were made by using soft-copy display at lung and soft-tissue windows, without computer-assisted diagnosis. Measurements were obtained at a minimum of full view, one-on-one image display. Images were first interpreted independently and then “in context,” meaning that any available historical images from the NLST or other sources were utilized in a comparative manner. Most baseline images were interpreted without historical images because none were available. The protocol mandated that second and third screening images initially be read in isolation but then read in conjunction with prior NLST images.
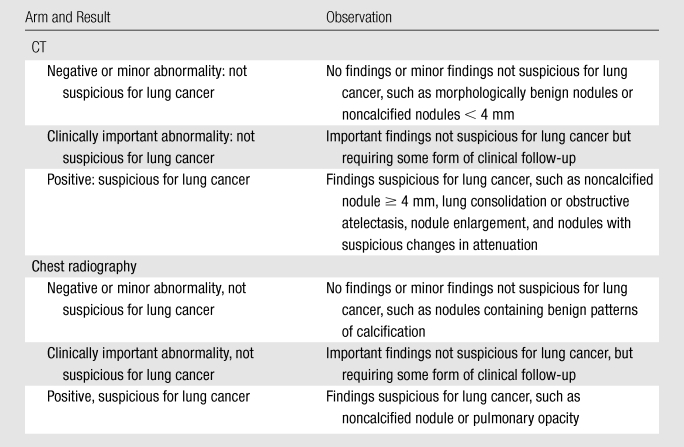
Images were reviewed for the presence of lung nodules, masses, or other abnormalities or constellations of abnormalities suspicious for lung cancer (positive screening results), as well as other findings of potential clinical importance (Table 7). For all noncalcified nodules and masses with a maximum diameter of 4 mm or greater, the following features were recorded: anatomic location, longest axial perpendicular diameter, margin characteristics (spiculated, smooth, poorly defined, indeterminant), and attenuation (soft tissue, ground glass, mixed, fluid and/or water, fat, other indeterminant).
Table 7.
Interpretation of Findings at CT or Chest Radiographic Screening
Participants with positive screening results always received follow-up recommendations from NLST radiologists. Some NLST screening centers developed practice guidelines for subsequent evaluation of abnormalities suspicious for lung cancer on the basis of current best practices. However, no NLST-wide diagnostic algorithms were established, and any diagnostic evaluation recommendations that were available served only as guidelines for participants’ health care providers. Diagnostic evaluation was performed outside the context of the NLST, although information on diagnostic evaluation performed in response to a positive screening result was collected.
Chest Radiographic Screening
Chest Radiographic Techniques and Acquisition Parameters
All chest radiographic machines were certified for use and met the NLST protocol requirements (Table 6) and American College of Radiology guidelines (41). Chest radiographic devices varied across sites and included screen-film, computed radiographic, and digital radiographic systems. Images were obtained with deep inspiration with a posteroanterior projection. It is estimated that each NLST chest radiographic examination resulted in an average effective dose of 0.02 mSv. NLST-ACRIN chest radiographic images were either transmitted electronically or mailed (in the case of screen-film images, which were utilized at several screening centers early in the trial) to ACRIN headquarters for permanent archiving. NLST-LSS chest radiographs are stored at the screening centers, although, in the future, a central repository for these images may be established.
Chest Radiograph Interpretations and Recommendations
Protocol requirements for interpretation of chest radiographs were identical to those for low-dose CT interpretation: Radiologists had to be approved to read trial acquisitions (Appendix E2 [online]), historical images were used after an isolation read, no trial-wide diagnostic algorithms were established, diagnostic evaluation was performed outside the context of the NLST, and information on diagnostic evaluation in response to a positive screening result was collected. Chest radiographic interpretations were made by using film- or soft-copy versions, depending on institutional practice. Abnormalities were classified as indicated in Table 7.
Follow-up of Abnormalities
Participants and their health care providers were promptly informed of trial examination results by mail. Participants with abnormalities suspicious for lung cancer received further contact, the format of which varied across study sites. Diagnostic work-up was determined by the participant’s personal health care provider. All screening centers developed mechanisms to assist participants who were under- or uninsured with medical follow-up. The NLST screening centers maintained close contact with participants and collected medical records to abstract information on diagnostic evaluation, its adverse effects, and initial treatment (Appendixes E3, E4, E5 [online]).
Biospecimen Collection
Serial specimens of blood, urine, and sputum were collected at 15 NLST-ACRIN screening centers from a total of 10 208 participants. In addition, paraffin blocks from resected lung tumors are collected across NLST (Appendix E6 [online]).
Quality Control: Data Collection, Screening Equipment and Imaging, and Personnel
Extensive quality control processes were implemented in the study (Appendix E7 [online]). Standardized data collection procedures and data elements were used. Data accuracy and completeness checks were implemented in the data collection. Regular site audits were conducted. Image and screening equipment quality assurance programs were implemented by study physicists. Radiologists and technical personnel met criteria specified in the protocol.
Primary Endpoint
The primary endpoint of the NLST is lung cancer mortality. Secondary endpoints include all-cause mortality, incidence of lung cancer, lung cancer case survival (as measured from date of diagnosis), and lung cancer stage distribution. An algorithm-driven endpoint verification process was established to ascertain deaths due to lung cancer as well as deaths from adverse outcomes of the screening process. The review is performed by a panel of independent experts, blinded to the randomization arm.
Sample Size Considerations
Preliminary computations of the required sample size for the NLST were made by using the approach of Taylor and Fontana (42), which is based on several simplifying assumptions and does not account for the number of screenings. The final computations were based on an elaboration of the approach of Hu and Zelen (43), modified to allow for staggered entry of participants and analyses based on calendar time instead of time on study.
Parameters for the Hu-Zelen model are listed in Appendix E8 (online) and were estimated by using data from the Mayo Lung Project (44). With 25 000 participants enrolled in each of years 1 and 2 of the trial, statistical power of 90% for detecting a 21% reduction in lung cancer mortality in the low-dose CT arm relative to the chest radiographic arm may be achieved in an analysis conducted on events occurring through August 2008. Because of lags in data availability and entry, such an analysis would not occur until 2010. Therefore, we continued to collect information on lung cancer cases and deaths occurring through December 2009 so that information would not have to be obtained retroactively if needed.
Study Monitoring
In consultation with the Data and Safety Monitoring Board, a strategy to monitor the primary endpoint of the study for efficacy and futility was put into effect for the NLST. The approach uses a weighted log-rank statistic and constructs stopping boundaries by using the Lan and DeMets approach with an O’Brien-Fleming spending function (45). Formal interim analyses for efficacy and futility are presented to the Data and Safety Monitoring Board on an annual basis.
Secondary Endpoints
Incidence and Screening Findings
The incidence of lung cancer and the lung cancer stage distribution will be compared across study arms. Variation in screening results, cancer incidence, and stage distributions across screening centers also will be examined.
Diagnostic Performance
The sensitivity, specificity, positive predictive value, and negative predictive value of the two screening modalities will be estimated and compared overall, as well as for subsets defined by participant characteristics and screening center.
NLST-ACRIN Substudies
NLST-ACRIN screening centers collect data on health care utilization and outcomes for a broad group of participants to permit cost-effectiveness analyses. Health-related quality-of-life data are collected from participants at 16 of the NLST-ACRIN screening centers to compare the effect of low-dose CT versus chest radiography on quality of life and to inform the cost-effectiveness analysis by using a societal perspective (46). The effectiveness of each arm will be measured in terms of quality-adjusted life-years.
Detailed questionnaires on smoking behavior and beliefs are administered on a routine basis to all NLST-ACRIN participants. NLST-ACRIN will assess the effect of being screened for lung cancer on smoking behavior, as well as the effect of screening result (positive or negative) on current smokers’ readiness to quit, efforts to quit, and success in quitting.
Study Update
The first participants were randomized in August 2002, and randomization of all 53 456 participants was completed in April 2004. The NLST-LSS group randomized 34 614 participants. The NLST-ACRIN group randomized 18 842 participants, 10 208 of whom were part of the biospecimen substudy. The number of randomized participants varied widely by screening center, from a low of 88 to a high of 6618 (Table 4).
The last screening round was completed in the summer of 2007. A substantial portion of the collection of data on medical outcomes, health care utilization, and deaths has been completed, and endpoint verification process review of deaths occurring by the end of 2009 continues.
Discussion
The NLST is a multicenter RCT designed and conducted by a multidisciplinary team of investigators. Trial participants represent cohorts who likely will be targeted for population-based screening should it be shown to reduce lung cancer mortality. The radiologic techniques utilized in the trial were developed with careful consideration for precision, standardization, and quality control and were updated on an ongoing basis to match the current state of the science.
The NLST is the only RCT of lung cancer screening with low-dose CT currently underway in the United States that has adequate statistical power to detect a modest reduction in lung cancer mortality should one exist. Other trials are underway in Europe. The largest of these, the NELSON trial, is being conducted in the Netherlands and Belgium; a companion trial is underway in Denmark as well (47,48). Enrollment of the NELSON trial is 15 428 participants, and the enrollment for the companion trial is 4104 participants (Jesper Pedersen, personal communication, March 1, 2009). Other RCTs, including ItaLung (planned enrollment of roughly 3000 participants) (49) and DANTE (enrollment of roughly 2472 participants) (50) in Italy, are underway. These trials differ from the NLST, in that control-arm participants are not undergoing annual chest radiography. For example, participants in the NELSON trial are randomized to low-dose CT or community care. This diversity of approaches makes the NLST, in conjunction with the other trials, well-poised to provide a definitive assessment of harms and benefits associated with low-dose CT screening.
The primary endpoint of the NLST is lung cancer mortality. Other outcomes, including all-cause mortality, lung cancer stage at diagnosis, lung cancer survival, and adverse effects of diagnostic evaluation, are important metrics of a screening program and are being monitored in the NLST. Data on quality of life and cost-effectiveness are also being collected, which will allow the NLST researchers to answer additional important questions concerning screening effects.
In addition, the NLST will provide a unique opportunity to explore and provide early phase validation of emerging molecular technologies in a well-characterized cohort of people at elevated risk of lung cancer, because biospecimens, including urine and blood, are being collected in a subset of participants. Tumor tissue also is being collected on a subset of lung cancers diagnosed during the trial. These specimens will enable numerous comparisons at the molecular level, including comparisons between persons with a diagnosis of lung cancer and those who remain free of the disease, persons with a diagnosis of lung cancer through screening and those with a diagnosis due to symptoms, and those who die of their disease compared with those who survive it.
There is no substitute for the current intense, ongoing efforts to eliminate cigarette smoking, the single most important cause of lung cancer. Forty-five years have passed since the first Surgeon General’s Report regarding smoking was published, and despite endeavors during these years, the smoking problem remains. Many adolescents and young adults are starting to smoke, and a portion will undoubtedly become addicted. Those who quit smoking do experience some reduction in lung cancer risk, but they remain at elevated risk compared with never smokers. In fact, it is likely that the majority of lung cancer cases diagnosed in the United States today are occurring in former smokers (51). Advances in lung cancer chemotherapy have been made for nearly all stages of disease, but most persons with a diagnosis of symptomatic lung cancer ultimately die of their disease. It is clear that we must pursue all possible avenues to reduce the lung cancer burden.
Most lung cancer researchers agree that lung cancer screening is of unproved benefit and associated with inescapable harms. Results of past lung cancer screening trials have been disappointing, but changes in radiologic technology and a shift from centrally located squamous cell lung cancers to peripherally located adenocarcinomas give reason for optimism and provide a strong rationale for readdressing the issue.
The most robust and efficient means to determine the balance of benefit versus harm and to help the public make informed personal decisions about lung cancer screening is through an RCT with a disease-specific mortality endpoint. The NLST is such a trial; it will contribute unique and critical information that will result in major advances in lung cancer knowledge, thus helping to tackle this serious public health problem.
Advances in Knowledge.
Although there is intense interest in lung cancer screening and intuitive appeal for screening with low-dose helical CT, which is a highly sensitive modality, a prospective randomized controlled trial with an endpoint that avoids the common screening biases is necessary to establish the benefit of screening.
The National Lung Screening Trial (NLST) is a randomized multicenter study comparing low-dose helical CT with chest radiography in the screening of older current and former heavy smokers for early detection of lung cancer.
Beginning in 2002, more than 50 000 patients have been randomized to one of the two study arms of NLST and offered screening at three time points—baseline and two annual screenings.
Participants have been followed up to assess the primary endpoint of the study, which is lung cancer mortality.
The study will also assess a comprehensive list of secondary endpoints, including disease incidence and screening findings, diagnostic and predictive accuracy of the imaging modalities, health care utilization and health outcomes, health-related quality of life, cost and cost-effectiveness, and smoking cessation.
Supplementary Material
Acknowledgments
The authors thank the Screening Center investigators and staff of the NLST. Most important, we acknowledge the study participants, whose contributions made this study possible. A list of the writing group for this article and for the entire NLST team of investigators can be found in Appendix E9 (online).
Received November 30, 2009; revision requested December 16; revision received June 21, 2010; accepted June 25; final version accepted July 20.
Funding: This research was supported by the National Institutes of Health (grants U01 CA079778 and U01 CA080098).
A list of the writing group for this article and for the entire NLST team of investigators can be found in Appendix E9 (online).
Supported by the Department of Health and Human Services via contracts from the Division of Cancer Prevention, National Cancer Institute, and grants to the American College of Radiology Imaging Network and Brown University under a cooperative agreement with the Cancer Imaging Program, Division of Cancer Treatment and Diagnosis, National Cancer Institute.
Clinical trial registration no. NCT 0047385
Authors stated no financial relationship to disclose.
Abbreviations:
- ACRIN
- American College of Radiology Imaging Network
- LSS
- Lung Screening Study
- NLST
- National Lung Screening Trial
- PLCO
- Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial
- RCT
- randomized controlled trial
References
- 1.Harris RP, Helfand M, Woolf SH, et al. Current methods of the US Preventive Services Task Force: a review of the process. Am J Prev Med 2001;20(3 suppl):21–35 [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Thomas A, Murray T, Thun M. Cancer statistics, 2002. CA Cancer J Clin 2002;52(1):23–47 [DOI] [PubMed] [Google Scholar]
- 3.National Cancer Institute, US National Institutes of Health, Surveillance, Epidemiology and End Results. (SEER Program) CSR section 15: lung and bronchus cancer pdf. http://seer.cancer.gov/csr/1975_2002. Accessed January 7, 2009.
- 4.Jemal A, Thun MJ, Ries LA, et al. Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 2008;100(23):1672–1694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults: United States, 2002. MMWR Morb Mortal Wkly Rep 2004;53(20):427–431 [PubMed] [Google Scholar]
- 6.Centers for Disease Control and Prevention (CDC) Cigarette smoking among adults: United States, 2007. MMWR Morb Mortal Wkly Rep 2008;57(45):1221–1226 [Published correction appears in MMWR Morb Mortal Wkly Rep 2008;57(47):1281.] [PubMed] [Google Scholar]
- 7.Blot WJ, Fraumeni JF., Jr Cancer of the lung and pleura. In: Schottenfeld D, Fraumeni JF, eds. Cancer epidemiology and prevention. New York, NY: Oxford University Press, 1996;637–665 [Google Scholar]
- 8.Crispo A, Brennan P, Jöckel KH, et al. The cumulative risk of lung cancer among current, ex- and never-smokers in European men. Br J Cancer 2004;91(7):1280–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mountain CF. Revisions in the international system for staging lung cancer. Chest 1997;111(6):1710–1717 [DOI] [PubMed] [Google Scholar]
- 10.Black WC, Welch HG. Screening for disease. AJR Am J Roentgenol 1997;168(1):3–11 [DOI] [PubMed] [Google Scholar]
- 11.Flehinger BJ, Melamed MR, Zaman MB, Heelan RT, Perchick WB, Martini N. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Memorial Sloan-Kettering study. Am Rev Respir Dis 1984;130(4):555–560 [DOI] [PubMed] [Google Scholar]
- 12.Melamed MR. Lung cancer screening results in the National Cancer Institute New York study. Cancer 2000;89(11 suppl):2356–2362 [DOI] [PubMed] [Google Scholar]
- 13.Frost JK, Ball WC, Jr, Levin ML, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Johns Hopkins study. Am Rev Respir Dis 1984;130(4):549–554 [DOI] [PubMed] [Google Scholar]
- 14.Tockman MS. Survival and mortality from lung cancer in a screened population: The Johns Hopkins Study. Chest 1986;89(suppl):324S–325S3956302 [Google Scholar]
- 15.Fontana RS, Sanderson DR, Taylor WF, et al. Early lung cancer detection: results of the initial (prevalence) radiologic and cytologic screening in the Mayo Clinic study. Am Rev Respir Dis 1984;130(4):561–565 [DOI] [PubMed] [Google Scholar]
- 16.Fontana RS, Sanderson DR, Woolner LB, et al. Screening for lung cancer: a critique of the Mayo Lung Project. Cancer 1991;67(4 suppl):1155–1164 [DOI] [PubMed] [Google Scholar]
- 17.Marcus PM, Bergstralh EJ, Fagerstrom RM, et al. Lung cancer mortality in the Mayo Lung Project: impact of extended follow-up. J Natl Cancer Inst 2000;92(16):1308–1316 [DOI] [PubMed] [Google Scholar]
- 18.Kubík A, Polák J. Lung cancer detection: results of a randomized prospective study in Czechoslovakia. Cancer 1986;57(12):2427–2437 [DOI] [PubMed] [Google Scholar]
- 19.Kubík AK, Parkin DM, Zatloukal P. Czech study on lung cancer screening: post-trial follow-up of lung cancer deaths up to year 15 since enrollment. Cancer 2000;89(11 suppl):2363–2368 [DOI] [PubMed] [Google Scholar]
- 20.Wynder EL, Muscat JE. The changing epidemiology of smoking and lung cancer histology. Environ Health Perspect 1995;103(suppl 8):143–148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schrump DS, Giaccone G, Kelsey CR, Marks LB. Non small-cell lung cancer. In: DeVita VT, Lawrence TS, Rosenberg SA, eds. Cancer: principles and practice of oncology. Philadelphia, Pa: Lippincott, Williams & Wilkins, 2008;896–946 [Google Scholar]
- 22.National Cancer Institute, US National Institutes of Health, Surveillance, Epidemiology and End Results. (SEER Program) CSR section 15: lung and bronchus cancer pdf. http://seer.cancer.gov/csr/1975_2005. Accessed January 27, 2009.
- 23.Gohagan JK, Prorok PC, Hayes RB, Kramer BS, Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial Project Team The Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial of the National Cancer Institute: history, organization, and status. Control Clin Trials 2000;21(6 suppl):251S–272S [DOI] [PubMed] [Google Scholar]
- 24.Prorok PC, Andriole GL, Bresalier RS, et al. Design of the Prostate, Lung, Colorectal and Ovarian (PLCO) Cancer Screening Trial. Control Clin Trials 2000;21(6 suppl):273S–309S [DOI] [PubMed] [Google Scholar]
- 25.Oken MM, Marcus PM, Hu P, et al. Baseline chest radiograph for lung cancer detection in the randomized Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. J Natl Cancer Inst 2005;97(24):1832–1839 [DOI] [PubMed] [Google Scholar]
- 26.Sone S, Li F, Yang ZG, et al. Results of three-year mass screening programme for lung cancer using mobile low-dose spiral computed tomography scanner. Br J Cancer 2001;84(1):25–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nawa T, Nakagawa T, Kusano S, Kawasaki Y, Sugawara Y, Nakata H. Lung cancer screening using low-dose spiral CT: results of baseline and 1-year follow-up studies. Chest 2002;122(1):15–20 [DOI] [PubMed] [Google Scholar]
- 28.Sobue T, Moriyama N, Kaneko M, et al. Screening for lung cancer with low-dose helical computed tomography: anti-lung cancer association project. J Clin Oncol 2002;20(4):911–920 [DOI] [PubMed] [Google Scholar]
- 29.Henschke CI, McCauley DI, Yankelevitz DF, et al. Early lung cancer action project: overall design and findings from baseline screening. Lancet 1999;354(9173):99–105 [DOI] [PubMed] [Google Scholar]
- 30.Henschke CI, Naidich DP, Yankelevitz DF, et al. Early lung cancer action project: initial findings on repeat screenings. Cancer 2001;92(1):153–159 [DOI] [PubMed] [Google Scholar]
- 31.Swensen SJ, Jett JR, Sloan JA, et al. Screening for lung cancer with low-dose spiral computed tomography. Am J Respir Crit Care Med 2002;165(4):508–513 [DOI] [PubMed] [Google Scholar]
- 32.Diederich S, Wormanns D, Semik M, et al. Screening for early lung cancer with low-dose spiral CT: prevalence in 817 asymptomatic smokers. Radiology 2002;222(3):773–781 [DOI] [PubMed] [Google Scholar]
- 33.Gohagan J, Marcus P, Fagerstrom R, et al. Baseline findings of a randomized feasibility trial of lung cancer screening with spiral CT scan vs chest radiograph: the Lung Screening Study of the National Cancer Institute. Chest 2004;126(1):114–121 [DOI] [PubMed] [Google Scholar]
- 34.Gohagan JK, Marcus PM, Fagerstrom RM, et al. Final results of the Lung Screening Study, a randomized feasibility study of spiral CT versus chest X-ray screening for lung cancer. Lung Cancer 2005;47(1):9–15 [DOI] [PubMed] [Google Scholar]
- 35.Church TR, National Lung Screening Trial Executive Committee Chest radiography as the comparison for spiral CT in the National Lung Screening Trial. Acad Radiol 2003;10(6):713–715 [DOI] [PubMed] [Google Scholar]
- 36.American College of Radiology ACR standard for performance of pediatric and adult thoracic computed tomography (CT). In: Standards 2003-2004. Reston, Va: American College of Radiology, 2002;115–119 [Google Scholar]
- 37.Zhu X, Yu J, Huang Z. Low-dose chest CT: optimizing radiation protection for patients. AJR Am J Roentgenol 2004;183(3):809–816 [DOI] [PubMed] [Google Scholar]
- 38.ACRIN/NLST CT Technique Comparison Chart American College of Radiology Imaging Network. http://www.acrin.org/Portals/0/Protocols/6654/forms/qcforms/CT_Technique_Chart.pdf. Accessed July 5, 2010.
- 39.Cagnon CH, Cody DD, McNitt-Gray MF, Seibert JA, Judy PF, Aberle DR. Description and implementation of a quality control program in an imaging-based clinical trial. Acad Radiol 2006;13(11):1431–1441 [DOI] [PubMed] [Google Scholar]
- 40.International Commission on Radiological Protection Managing patient dose in computed tomography. Publication 87. Oxford, England: Elsevier, 2001 [Google Scholar]
- 41.American College of Radiology ACR standard for performance of pediatric and adult chest radiography. In: Standards 2002-2003. Reston, Va: American College of Radiology, 2001;105–109 [Google Scholar]
- 42.Taylor WF, Fontana RS. Biometric design of the Mayo Lung Project for early detection and localization of bronchogenic carcinoma. Cancer 1972;30(5):1344–1347 [DOI] [PubMed] [Google Scholar]
- 43.Hu P, Zelen M. Planning clinical trials to evaluate early detection programmes. Biometrika 1997;84(4):817–829 [Google Scholar]
- 44.Fontana R. Early detection of lung cancer: The Mayo Lung Project. In: Prorok PC, Miller AB, eds. Screening for cancer. I. General principles on evaluation of screening for cancer and screening for lung, bladder and oral cancer. Geneva, Switzerland: International Union Against Cancer, 1984;107–122 [Google Scholar]
- 45.Jennison C, Turnbull BW. Group sequential methods with applications to clinical trials. Boca Raton, Fla: Chapman & Hall/CRC, 2000 [Google Scholar]
- 46.Gold MR, Siegel JE, Russell LB, Weinstein MC, eds. Cost-effectiveness in health and medicine. New York, NY: Oxford University Press, 1996 [Google Scholar]
- 47.van Iersel CA, de Koning HJ, Draisma G, et al. Risk-based selection from the general population in a screening trial: selection criteria, recruitment and power for the Dutch-Belgian randomised lung cancer multi-slice CT screening trial (NELSON). Int J Cancer 2007;120(4):868–874 [DOI] [PubMed] [Google Scholar]
- 48.van den Bergh KA, Essink-Bot ML, Bunge EM, et al. Impact of computed tomography screening for lung cancer on participants in a randomized controlled trial (NELSON trial). Cancer 2008;113(2):396–404 [DOI] [PubMed] [Google Scholar]
- 49.Picozzi G, Paci E, Lopez Pegna A, et al. Screening of lung cancer with low dose spiral CT: results of a three year pilot study and design of the randomised controlled trial “Italung-CT”. Radiol Med (Torino) 2005;109(1-2):17–26 [PubMed] [Google Scholar]
- 50.Infante M, Lutman FR, Cavuto S, et al. Lung cancer screening with spiral CT: baseline results of the randomized DANTE trial. Lung Cancer 2008;59(3):355–363 [DOI] [PubMed] [Google Scholar]
- 51.Burns DM. Primary prevention, smoking, and smoking cessation: implications for future trends in lung cancer prevention. Cancer 2000;89(11 suppl):2506–2509 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.