Abstract
Glucagon-like peptide-1 (GLP-1) binds its Class II G protein-coupled receptor to stimulate cyclic adenosine monophosphate (cAMP) production and to potentiate the glucose metabolism-dependent secretion of insulin from pancreatic β cells located within the islets of Langerhans. Prior clinical studies demonstrate that this cAMP-mediated action of GLP-1 to potentiate glucose-stimulated insulin secretion (GSIS) is of major therapeutic importance when evaluating the abilities of GLP-1 receptor (GLP-1R) agonists to lower levels of blood glucose in type 2 diabetic subjects. Surprisingly, recent in vitro studies of human or rodent islets of Langerhans provide evidence for the existence of a noncanonical mechanism of β cell cAMP signal transduction, one that may explain how GLP-1R agonists potentiate GSIS. What these studies demonstrate is that a cAMP-regulated guanine nucleotide exchange factor designated as Epac2 couples β cell cAMP production to the protein kinase A-independent stimulation of insulin exocytosis. Provided here is an overview of the Epac2 signal transduction system in β cells, with special emphasis on Rap1, a Ras-related GTPase that is an established target of Epac2.
I. Introduction
Glucagon-like peptide-1 (GLP-1) is an intestinally derived incretin hormone that potentiates the glucose metabolism-dependent secretion of insulin from β cells located within the islets of Langerhans. This action of GLP-1 to potentiate glucose-stimulated insulin secretion (GSIS) is achieved by the binding of GLP-1 to the β cell GLP-1 receptor (GLP-1R), a Class II GTP-binding protein-coupled receptor (GPCR) that is positive coupled to 3′-5′-cyclic adenosine monophosphate (cAMP) production (Thorens, 1992). Since GLP-1R agonists (e.g., exenatide, liraglutide) stimulate pancreatic insulin secretion and lower levels of blood glucose in patients diagnosed with type 2 diabetes mellitus (Campbell and Miller, 2009; Israili, 2009), there is considerable interest in identifying the molecular mechanisms of β cell stimulus–secretion coupling that are regulated by GLP-1 in a cAMP-dependent manner. Summarized here are recent findings that provide evidence for a functional coupling of the GLP-1R to a noncanonical mechanism of cAMP signal transduction, one that is mediated by the cAMP-regulated guanine nucleotide exchange factor designated as Epac2. In this regard, we focus on the likely involvement of Rap1, a Ras-related GTPase that is reported to couple Epac2 activation to the potentiation of GSIS (Shibasaki et al., 2007). For additional information concerning Epac2 and Rap1, the reader is referred to prior reviews of this subject matter (Holz et al., 2006; Seino and Shibasaki, 2005).
II. PKA and Epac2 Regulate Insulin Secretion from β Cells
Although it is widely accepted that cAMP mediates the insulin secretagogue action of GLP-1 in β cells, there is debate concerning the identities of the cAMP signaling pathways that mediate this action of the hormone. The principal issue concerns whether the secretagogue action of GLP-1 is explained by its ability to activate protein kinase A (PKA) or whether GLP-1 also signals through Epac2. A major, if not exclusive, role for PKA as the dominant cAMP-binding protein controlling glucose-dependent insulin secretion has been championed by Hatakeyama et al. (2006) in live-cell imaging studies of mouse β cell exocytosis. These investigators reported that PKA mediated the cAMP-dependent potentiation of large dense core secretory vesicle exocytosis, whereas in these same cells Epac2 was implicated in the cAMP-dependent exocytosis of small synaptic vesicle-like structures (Hatakeyama et al., 2007). Since insulin is present within large vesicles, whereas the neurotransmitter GABA is found within small vesicles of β cells, such findings argue for an unexpected segregation of cAMP signaling such that PKA and Epac2 differentially regulate the exocytosis of two distinct subpopulations of secretory vesicles. Importantly, these findings concerning mouse β cells are in general agreement with one earlier study of human β cells in which it was reported that GLP-1 potentiated exocytosis that was both depolarization-induced and Ca2+-dependent, as determined by the measurement of membrane capacitance, and that this effect of GLP-1 was blocked by a cAMP analog (Rp-cAMPS) that is a selective antagonist of PKA activation (Gromada et al., 1998). Thus, for both human and mouse β cells, it seems clear that PKA plays an important role in the stimulation of exocytosis. Yet, since none of the above-mentioned studies examined insulin secretion per se, and instead relied on biophysical methods to detect exocytosis, no firm conclusions can be reached concerning whether or not PKA is the primary cAMP-binding protein by which GLP-1 potentiates GSIS.
Given that the collective wisdom supports a role for PKA in the regulation of islet insulin secretion, it is remarkable that in studies of mouse islets performed by Kashima and coworkers, a dramatically different story emerged concerning the existence of a noncanonical mechanism of cAMP signaling in β cells. It was reported that an inhibitor of PKA activity (H-89) reduced but did not fully abrogate the action of GLP-1 to potentiate GSIS (Kashima et al., 2001). Moreover, in these same islets, the action of GLP-1 to potentiate GSIS was reduced but not abrogated following treatment of islets with Epac2 antisense (AS) deoxyoligonucleotides. Since combined treatment of islets with H-89 and Epac2 AS suppressed the secretagogue action of GLP-1 in an additive manner, it was concluded that in addition to PKA, it is the cAMP-binding protein Epac2 that couples cAMP production to the potentiation of GSIS (Kashima et al., 2001). This conclusion was reinforced by a parallel study using PKA inhibitors in which a PKA-independent action of GLP-1 to stimulate mouse islet insulin secretion was confirmed (Nakazaki et al., 2002).
One important caveat to the interpretation of findings obtained using PKA inhibitors is that this approach can only establish PKA-independence and cannot definitively establish a role for Epac2 in the potentiation of GSIS by cAMP-elevating agents. A more convincing strategy would be to demonstrate antagonism of GLP-1-stimulated insulin secretion through the use of cAMP analogs that competitively inhibit cAMP-dependent activation of Epac2. Unfortunately, this approach is not feasible at the present time due to the lack of suitable Epac2 antagonists (Holz et al., 2008). There are, however, selective activators of Epac2, and these compounds are known to stimulate insulin secretion (Kelley et al., 2009). Chepurny and coworkers reported that human islet GSIS was potentiated by an acetoxymethyl ester (AM-ester) of a cAMP analog (8-pCPT-2′-O-Me-cAMP-AM) that is a selective activator of Epac (Chepurny et al., 2009, 2010). Importantly, this action of 8-pCPT-2′-O-Me-cAMP-AM was not secondary to PKA activation (Chepurny et al., 2010). Studies of this sort are significant because until recently, the assessment of Epac2 signal transduction was hampered by the poor membrane permeability of first-generation Epac-selective cAMP analogs (ESCAs) that lack the AM-ester moiety (Chepurny et al., 2009). Expanding on this analysis, it has now been demonstrated that 8-pCPT-2′-O-Me-cAMP-AM also exerts profound stimulatory effects on human β cell Ca2+ signaling (Chepurny et al., 2010). As discussed below, this action of the ESCA to raise levels of cytosolic Ca2+ involves not only the mobilization of an intracellular source of Ca2+, but it also results from Ca2+ influx that is secondary to β cell depolarization (Chepurny et al., 2010).
Surprisingly, it has not yet been reported what effect GLP-1 exerts on insulin secretion from the islets of Epac2 knockout (KO) mice. However, Shibasaki et al. (2007) did report that 8-Br-cAMP, a cAMP analog that activates both PKA and Epac2, had a greatly reduced ability to potentiate first-phase GSIS from the β cells of Epac2 KO mice. Assuming 8-Br-cAMP activates PKA in these Epac2 KO mice, it would seem that first-phase GSIS is under the control of Epac2, and that the activation of PKA by 8-Br-cAMP does not allow for the normal cAMP-dependent potentiation of first-phase GSIS in the Epac2 KO mice. This is a remarkable finding, since it is dramatically at odds with the prior study of Hatakeyama and coworkers that was performed using wild-type mouse β cells. In that study, no evidence for Epac2-dependent regulation of GSIS was measurable, and instead it was found that all stimulatory effects of cAMP on GSIS were mediated by PKA (Hatakeyama et al., 2007; Kasai et al., 2010). Although it is not clear why such divergent findings have been reported by different teams of investigators, it seems likely that the source of confusion arises as a consequence of the use of different live-cell imaging techniques when evaluating exocytosis at the single cell level. Clearly, such findings need to be validated using whole islets and conventional immunoassays of secreted insulin. In summary, findings obtained using PKA inhibitors, Epac activators, and Epac2 KO mice provide evidence for an Epac2-mediated action of cAMP to potentiate GSIS from both human and mouse islets. However, it has yet to be definitively established that such a mechanism of cAMP signal transduction mediates the insulin secretagogue action of GLP-1.
III. Epac2 Activates Rap1 GTPase
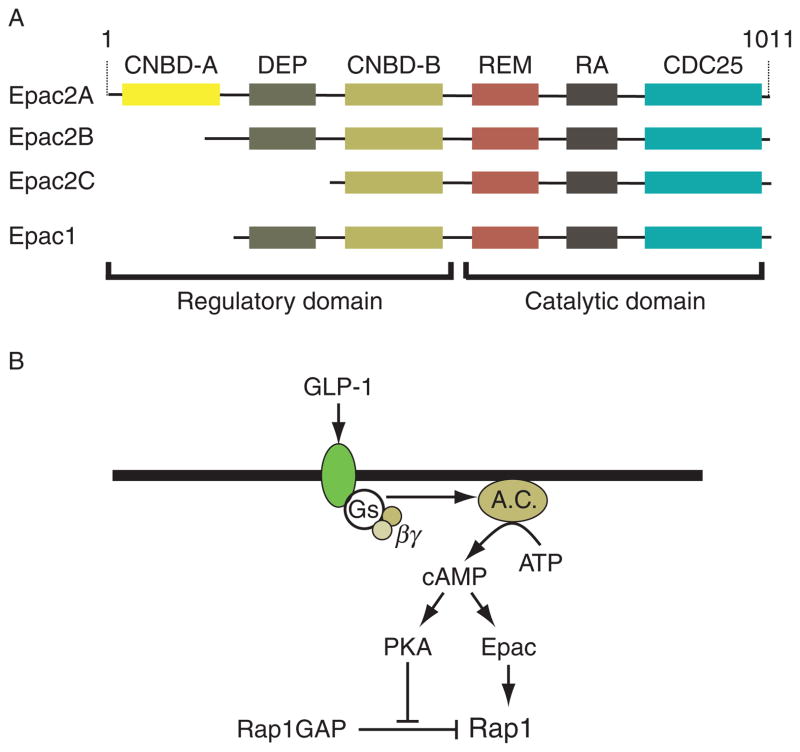
If Epac2 does in fact mediate cAMP-dependent potentiation of GSIS by GLP-1, how is this effect achieved? The most likely explanation is that Epac2 couples cAMP production to the activation of Rap1, a Ras-related GTPase that plays an important role in the control of Ca2+-dependent exocytosis, not only in β cells but also in other cell types (Bos, 2006). In fact, the Epac family of cAMP-regulated guanyl nucleotide exchange factors (GEFs) are cAMP-binding proteins first identified on the basis of their ability to stimulate the exchange of GDP for GTP at the guanine nucleotide-binding site of Rap1 (de Rooij et al., 1998; Kawasaki et al., 1998). Structure–function studies demonstrated that the CDC25 homology domain (CDC25-HD) of Epac2 catalyzes guanine nucleotide exchange on Rap1, thereby activating it (Gloerich and Bos, 2010). This is a regulated process that requires the binding of cAMP to a high-affinity cAMP-binding domain (CNBD-B) located within the regulatory region of Epac2 (Fig. 10.1A).
Figure 10.1.
(A) Domain structure of Epac proteins. The two isoforms of Epac are Epac1 and Epac2, each of which is encoded by its own gene. Epac2 is the major isoform of Epac expressed in islets, and it is encoded by the RAPGEF4 gene located at chromosome 2q31–q32. There are three splice variants of Epac2, with Epac2A being the variant expressed in islets. Epac2A has two cAMP-binding domains, a low-affinity site (CNBD-A), important for cellular localization, and a high-affinity site (CNBD-B), important for cAMP-dependent activation of GEF activity. A disheveled, Egl-10, pleckstrin (DEP) domain is responsible for association of Epac2 with intracellular membranes, a Ras exchange motif (REM) domain stabilizes the tertiary structure of the catalytic region, and a Ras association (RA) domain allows the interaction of Epac2 with activated Ras. The CDC25 homology domain (CDC25) catalyzes guanine nucleotide exchange on Rap1, thereby activating it. Epac2B is specifically expressed in the adrenal cortex and lacks the low-affinity cAMP-binding site (CNBD-A). Epac2C is found in the liver and lacks both CNBD-A and DEP domains. All three isoforms have GEF activity to activate Rap1. (B) Role of cAMP in Rap1 activation. Activation of the GLP-1 receptor stimulates Gs, adenylyl cyclase (AC), and cAMP production. The activation of Epac2 is likely to be the major pathway for Rap1 activation in β cells, although PKA can phosphorylate and inactivate Rap1GAP to prolong the activated state of Rap1.
In the absence of cAMP, the regulatory region of Epac2 is responsible for autoinhibition of the CDC25-HD catalytic function, and this autoinhibition is relieved as a consequence of the binding of cAMP to CNBD-B. Importantly, the lower-affinity cAMP-binding domain (CNBD-A) that is also located in the regulatory region of Epac2 (Fig. 10.1A) does not play a role in the cAMP-dependent disinhibition of Epac2 GEF activity. It is instead reported to play a role in plasma membrane targeting of Epac2 within β cells (Niimura et al., 2009). Just as interesting, within Epac2 there may exist binding sites for the sulfonylurea class of blood glucose-lowering agents, such that the binding of sulfonylureas to Epac2 may disinhibit this exchange factor’s GEF activity. In fact, sulfonylureas such as tolbutamide are reported to activate Rap1, and in Epac2 KO mice there is a diminished ability of sulfonylureas to stimulate pancreatic insulin secretion (Zhang et al., 2009). Although the ability of sulfonylureas to activate Rap1 might result from their direct binding to Epac2, it is also likely that indirect activation of Epac2 occurs as a consequence of the established ability of sulfonylureas to inhibit cyclic nucleotide phosphodiesterases (PDEs) and to elevate levels of cAMP in islets (Goldfine et al., 1971). Thus, it will be of particular interest to validate that sulfonylureas do in fact directly activate Epac2, since this mechanism of Epac2 activation has recently been drawn into question (Leech et al., 2010).
Although largely unexplored, Epac2-independent mechanisms controlling Rap1 activity may also exist in β cells since islets express Rap1 GTPase-activating protein (Rap1GAP), a protein that inactivates Rap1 by stimulating its GTPase activity. Rap1GAP is phosphorylated and inactivated by PKA (McAvoy et al., 2009), and thus elevated PKA activity could prolong the active GTP-bound state of Rap1 (Fig. 10.1B). It remains to be determined whether such an effect of PKA to promote Rap1 activation explains the synergistic interaction of PKA- and Epac-selective cAMP analogs to stimulate insulin secretion, as reported in studies of rat INS-1 insulin-secreting cells (Chepurny et al., 2009).
Epac2-independent activation of Rap1 may also be mediated by the Ras guanyl-releasing proteins (RasGRPs; also known as CalDAG-GEFs) expressed in islets (Ozaki et al., 2005). RasGRPs link intracellular Ca2+ and diacylglycerol (DAG) signaling to Rap1 activation, and in this manner, it is predicted that Rap1-dependent insulin secretion should be synergistically stimulated by agents that activate both RasGRPs and Epac2. However, what role, if any, RasGRPs play in GLP-1R signal transduction remains to be determined. Finally, posttranslational modifications of Rap1 may also contribute to the overall process by which Rap1 activity regulates insulin secretion (Kowluru, 2008).
IV. Rap1 Effectors and Their Potential Roles in the Control of GSIS
A. Activation of phospholipase C-epsilon
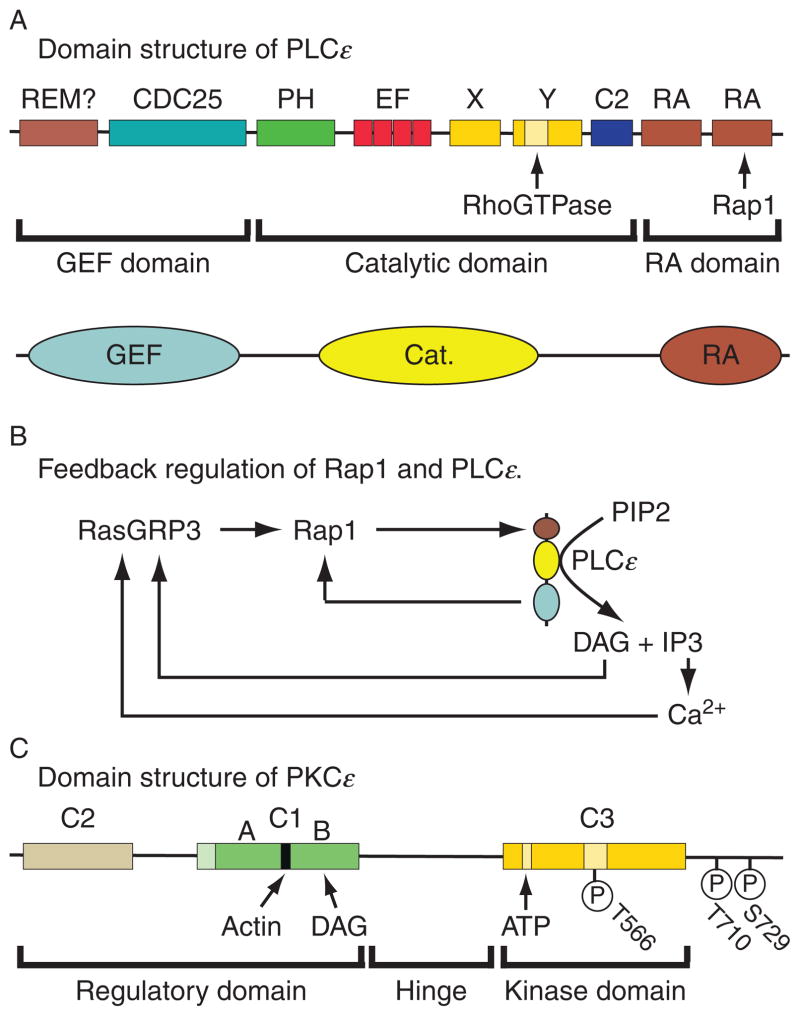
One of the best characterized downstream effectors of Rap GTPase is phospholipase C-epsilon (PLCε). PLCε was first identified as a fourth class of PLC that is directly regulated by Ras (Kelley et al., 2001). Subsequent studies showed that PLCε is also regulated by Rap (Schmidt et al., 2001; Song et al., 2002). Two isoforms of PLCε are generated from the PLCE1 gene as a consequence of the alternative splicing of N-terminal exons resulting in the expression of long (2302 a.a.) and short (1994 a.a.) forms of the enzyme, each with similar domain structures (Fig. 10.2A). Both isoforms of PLCε are expressed in the pancreas (Sorli et al., 2005), but their relative levels of expression within islets are not currently known. Although it was originally reported that Rap2B is the specific activator of PLCε (Schmidt et al., 2001), more recent studies demonstrated that Rap1 is also capable of activating this phospholipase (Song et al., 2002). Less well understood are the reported roles of Rho GTPase and heterotrimeric G protein subunits (Gα12/13 and Gβγ) in the regulation of PLCε (Wing et al., 2001, 2003).
Figure 10.2.
Domain structures of PLCε and PKCε. (A) The N-terminal region of PLCε has GEF activity and contains a CDC25 homology domain and possibly a REM domain. The catalytic region contains a pleckstrin homology (PH) domain, EF hand domains, the X and Y boxes, and a core C2 catalytic domain. The C-terminal Ras association (RA) domain contains two Ras association motifs that interact with Ras and Rap. Figure adapted from Bunney and Katan (2006). (B) Illustrated are feedback loops that might be important for sustained activation of Rap1 and PLCε. Rap1 activates PLCε and the GEF domain of PLCε activates Rap1. Note that PLCε-catalyzed hydrolysis of PIP2 generates DAG and Ca2+, both of which activate RasGRP3, thereby catalyzing additional activation of Rap1. (C) Located within the regulatory domain of PKCε is a C1 domain that contains an actin-binding motif and a DAG-binding site. The regulatory domain also contains a C2 domain that binds phospholipids. The kinase domain of PKCε contains a C3 domain, and within it there is an ATP-binding site. Note that phosphorylation of T566 in the activation loop of the C3 domain is essential for PKCε activity. A pseudosubstrate motif at which autophosphorylation occurs is located at the C-terminus of PKCε. Figure adapted from Akita (2002).
As indicated in the domain structure of PLCε (Fig. 10.2A), activated Rap binds to the phospholipase’s RA2 domain to activate the enzyme. Intrinsic GEF activity at the N-terminal CDC25-HD of PLCε is then responsible for additional Rap activation (Fig. 10.2B). This constitutes a mechanism of positive feedback that is important for sustained Rap activation (Jin et al., 2001). Once activated, PLCε hydrolyzes phosphatidylinositol 4,5-bisphosphate (PIP2) to generate the Ca2+-mobilizing second messenger inositol 1,4,5-trisphosphate (IP3) and the lipid metabolite DAG (Fig. 10.2B). Ca2+ and DAG then act via RasGRP3 to promote additional Rap activation with concomitant stimulation of PLCε (Fig. 10.2B). Ca2+ and DAG also participate in the activation of protein kinase C (PKC), and for cell types expressing the PLCε isozyme, it appears that DAG may be a particularly effective stimulator of PKC-epsilon (PKCε) activity (Akita, 2002) (Fig. 10.2C).
Given that both Ca2+ and PKC are key stimulators of islet insulin secretion, it is clear that the activation of PLCε by Rap1 could explain, at least in part, the action of cAMP-elevating agents to potentiate GSIS. However, it should be pointed out that earlier studies of islet phosphoinositide metabolism failed to demonstrate a stimulatory effect of GLP-1 on 3H-inositol phosphate production (Fridolf and Ahren, 1991), as would be expected if GLP-1 activates any of the multiple PLC isozymes expressed in islets. This negative finding might be explained by the low sensitivity of biochemical assays of inositol phosphate production since PLCε is expressed in relatively low abundance in islets. In fact, in cardiac myocytes that express PLCε at low abundance, the activation of Epac1 by an ESCA results in the PLCε-mediated stimulation of sarcoplasmic reticulum Ca2+ release (Oestreich et al., 2009), and this effect is not accompanied by significant inositol phosphate production. However, using more sensitive methods of live-cell imaging in combination with a fluorescent phosphoinositide biosensor (PHD-PLCδ-EGFP) expressed in rat INS-1 insulin-secreting cells, it was recently demonstrated that phosphoinositide hydrolysis at the plasma membrane can be stimulated by an ESCA (Leech et al., 2010). Such findings are reminiscent of prior studies in which glucose metabolism-stimulated cAMP production in β cells was found to be measurable using imaging techniques (Dyachok et al., 2008; Landa et al., 2005), whereas conventional biochemical assays of bulk cAMP content failed to support a major role for glucose metabolism in the stimulation of β cell cAMP production (Schuit and Pipeleers, 1985).
B. Stimulation of PIP2 hydrolysis
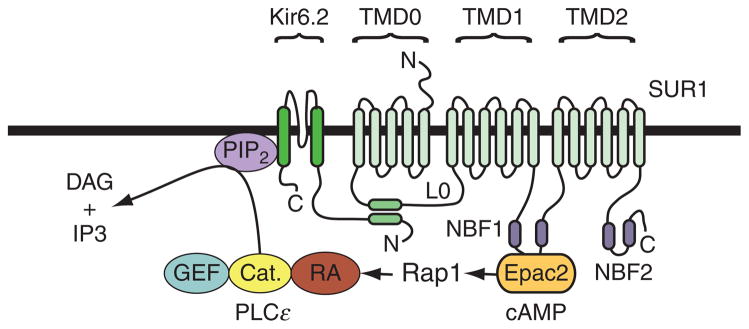
Since activation of PLCε by Rap1 results in PIP2 hydrolysis, the resultant depletion of PIP2 in the β cell plasma membrane might have important functional consequences, even without further downstream signaling. We have proposed a model (Fig. 10.3) in which the hydrolysis of PIP2 by PLCε favors the ATP-dependent closure of ATP-sensitive potassium (K-ATP) channels in β cells (Kang et al., 2006). This model is based on the established fact that the activity of this channel type, as well as its ATP sensitivity, is under the control of PIP2 (Baukrowitz et al., 1998; Shyng and Nichols, 1998). The model also takes into account the demonstration that Epac2 binds to nucleotide-binding fold-1 (NBF-1) of the sulfonylurea receptor-1 (SUR1) subunit of K-ATP channels (Kang et al., 2006). Thus, the model predicts that there can exist a macromolecular signaling complex comprising K-ATP channels, Epac2, Rap1, and PLCε. The existence of this signaling complex is of primary significance because the model predicts that cAMP-dependent activation of Eapc2 associated with SUR1 will selectively deplete PIP2 in the immediate vicinity of K-ATP channels, without exerting a global effect on PIP2 content throughout the β cell plasma membrane (Fig. 10.3).
Figure 10.3.
A model for Epac/Rap1 regulation of K-ATP channels. The K-ATP channel in β cells is a hetero-octamer formed by four SUR1 subunits and four Kir6.2 subunits. SUR1 has 17 transmembrane domains that are grouped into three units (TMD0, TMD1, and TMD2). The intracellular L0 loop between TMD0 and TMD1 interacts with the N-terminal of Kir6.2 (Bryan et al., 2007). Note that Epac2 binds to nucleotide-binding fold-1 (NBF1) of SUR1 and that this interaction may allow cAMP to activate Rap1 and PLCε located within the immediate vicinity of K-ATP channels. Therefore, cAMP is predicted to regulate K-ATP channel activity by stimulating hydrolysis of PIP2 associated with Kir6.2. Although not shown, it is also possible that the binding of Epac2 to NBF-1 of SUR1 allows Epac2 to allosterically regulate K-ATP channel activity.
One additional prediction of this model is that activation of PLCε by GLP-1 will enhance glucose-induced closure of K-ATP channels, and that this action of GLP-1 will explain how the hormone potentiates GSIS. To understand this model, it is must be realized that K-ATP channels close in response to a glucose metabolism-dependent increase of cytosolic [ATP]/[ADP] ratio. Furthermore, the association of PIP2 with K-ATP channels increases the likelihood that these channels will open, while it also decreases the channel’s apparent affinity for ATP (Baukrowitz et al., 1998; Shyng and Nichols, 1998). Thus, the model predicts that under conditions in which PIP2 hydrolysis is stimulated by GLP-1, a majority of K-ATP channels will be sensitized to the inhibitory action of ATP, and for this reason more K-ATP channels will close in response to β cell glucose metabolism (Kang et al., 2008). In view of the fact that K-ATP channel closure promotes β cell depolarization and Ca2+ influx that initiates exocytosis, this model provides one simple explanation for how glucose metabolism and GLP-1 signal transduction interact to stimulate islet insulin secretion. In fact, the model also provides an explanation for how GLP-1 acts as a β cell glucose sensitizer (Holz et al., 1993).
Interestingly, the above-summarized model may also provide an explanation for how GLP-1R agonists functionally interact with sulfonylureas to stimulate insulin secretion. This concept derives from the established observation that combined administration of a sulfonylurea with the GLP-1R agonist exenatide can induce excessive pancreatic insulin secretion and hypoglycemia in patients diagnosed with type 2 diabetes mellitus (Kendall et al., 2005). We proposed that such clinical findings are explained, at least in part, by the synergistic interaction of exenatide and sulfonylureas to inhibit K-ATP channels (Leech et al., 2010). This concept is advanced because PIP2 hydrolysis is expected to enhance the inhibitory action of sulfonylureas at K-ATP channels (Koster et al., 1999), whereas exenatide is expected to activate PLCε in β cells. Thus, there may exist a mechanism of GLP-1R signal transduction that promotes PIP2 hydrolysis, and that modulates K-ATP channel responsiveness to sulfonylureas while also controlling the channel’s sensitivity to ATP derived from glucose metabolism.
C. Activation of protein kinase C-epsilon
PKCε is a member of the “novel” family of PKC isoforms, and it serves as an immediate effector of PLCε in various cell types, although this has yet to be demonstrated for β cells. A role for PKCε in the control of insulin biosynthesis is reported (Warwar et al., 2008), but there are conflicting data concerning what role PKCε plays in regulated insulin secretion. Published findings demonstrate that PKCε translocates to insulin-containing secretory granules in response to elevated levels of glucose, whereas over expression of a dominant-negative PKCε abolishes GSIS (Mendez et al., 2003; Zaitsev et al., 1995). Interestingly, PKCε is also reported to mediate the inositol hexakisphosphate-induced exocytosis of insulin in β cells (Hoy et al., 2003). What is surprising is that the findings summarized above are contradicted by reports that genetic deletion or inhibition of PKCε fails to influence GSIS (Cantley et al., 2009; Schmitz-Peiffer et al., 2007). For example, in PKCε KO mice, or when PKCε activity was inhibited, insulin secretion in response to glucose alone was not altered (Cantley et al., 2009; Schmitz-Peiffer et al., 2007).
It remains to be determined whether stimulatory effects of GLP-1 on insulin secretion involve its putative Epac2-mediated ability to activate PKCε. This might be the case in view of the fact that PKCε is involved in the regulation of Cdc42 (Akita, 2008), a Rho-family GTPase that stimulates cytoskeletal reorganization during exocytosis. Cdc42 is activated in β cells in response to glucose metabolism, and it activates a Rac1 GTPase that promotes actin remodeling (Wang et al., 2007). Remodeling under the control of Cdc42 and Rac1 might favor exocytosis because the actin-containing cytoskeleton is a barrier that restricts access of secretory granules to the plasma membrane, whereas disruption of this barrier enhances insulin secretion (Orci et al., 1972). Importantly, it is now known that Rac1 GEFs can be activated by Rap1 (Arthur et al., 2004), and that Rap1 regulates insulin secretory granule dynamics (Shibasaki et al., 2007). Thus, Rap1 acting through Rac1–GEFs and Rac1 itself might stimulate cytoskeletal reorganization and the trafficking of secretory granules to the plasma membrane. Furthermore, such a signaling mechanism might be under the control of GLP-1 acting through Epac2. This hypothetical Rap1 and Rac1–GEF-mediated effect of GLP-1 would complement its putative ability to activate PKCε and to directly regulate Cdc42 function. Finally, it is interesting to note that in neurons, the activation of PKCε enables this kinase to bind to actin and to regulate synaptic vesicle exocytosis (Prekeris et al., 1996). This finding leads to the prediction that in β cells, the GLP-1R-mediated activation of PKCε should facilitate Cdc42 dependent trafficking of insulin granules to their docking sites at the plasma membrane where insulin exocytosis occurs.
D. Elevation of cytosolic [Ca2+]
Considerable evidence exists that stimulatory effects of GLP-1 on islet insulin secretion are attributable, at least in part, to its ability to increase levels of cytosolic [Ca2+] in β cells. This action of GLP-1 to increase [Ca2+]i requires exposure of β cells to elevated concentrations of glucose. One mechanism that underlies this action of GLP-1 involves its ability to enhance the action of glucose metabolism to trigger Ca2+ influx through voltage-dependent Ca2+ channels (VDCCs). Such an ability of GLP-1 to sensitize β cells to the stimulatory action of glucose is consistent with its established action to promote glucose metabolism-dependent closure of K-ATP channels, and to also promote β cell depolarization (Holz et al., 1993). These effects of GLP-1 to inhibit K-ATP channel activity, induce β depolarization, and promote Ca2+ influx through VDCCs are likely to be Epac2-mediated, since all such actions of GLP-1 are mimicked by ESCAs (Chepurny et al., 2010; Kang et al., 2006, 2008).
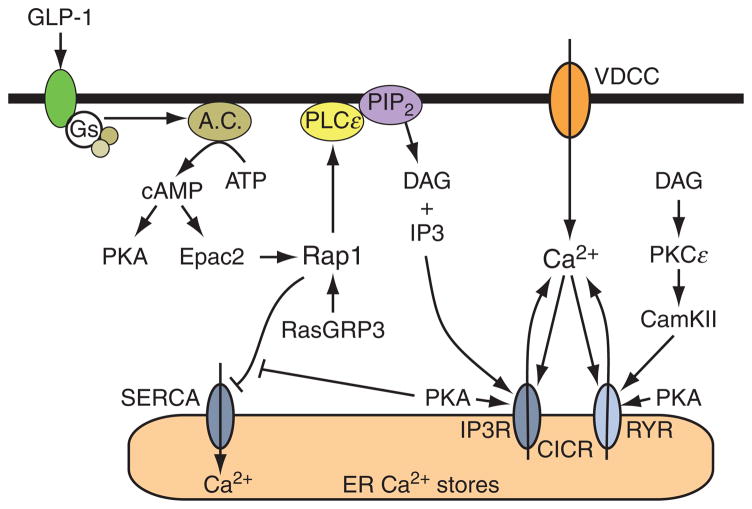
There is also good evidence that GLP-1 acts through cAMP to mobilize an intracellular source of Ca2+ in β cells (Fig. 10.4). This intracellularly released Ca2+ is likely to be an effective stimulus for insulin secretion, as is already established to be the case for Ca2+ that enters by way of VDCCs (Fig. 10.5). Although there was some earlier debate concerning whether or not the Ca2+ mobilizing action of GLP-1 is mediated by PKA or Epac2, it is now established that both cAMP-binding proteins participate in the regulation of [Ca2+]i by the hormone (Kang et al., 2005). Thus, prior to the discovery of Epac2, a role for PKA as the target of GLP-1 was demonstrated in studies of rat and human β cells (Holz et al., 1999), and this finding was subsequently validated in studies of Ca2+ oscillations and intracellular Ca2+-induced Ca2+ release (CICR) in mouse β cells (Dyachok and Gylfe, 2004; Kang et al., 2005). The consensus that developed was that PKA promotes the release of Ca2+ from intracellular Ca2+ stores by sensitizing intracellular Ca2+ release channels to the stimulatory effects of Ca2+-mobilizing second messengers (e.g., IP3) or even Ca2+ itself (Fig. 10.4).
Figure 10.4.
Regulation of CICR by cAMP. Ca2+ influx through VDCCs generates an increase of [Ca2+]i, and this acts as a stimulus for CICR from the ER. The Ca2+ release channel that mediates CICR is the ryanodine receptor (RYR). However, Ca2+ released from the ER acts as a coagonist with IP3 to stimulate additional Ca2+ release from IP3 receptor-regulated Ca2+ stores (IP3R). Ca2+ release via the RYR and IP3R is facilitated by PKA, possibly as a consequence of the phosphorylation of RYR and IP3R. It is also likely that PKA increases the ER Ca2+ load by promoting ER Ca2+ uptake. Epac2 acts through a pathway involving Rap1, PLCε, PKCε, and CamKII to exert a stimulatory effect at RYR. This action of Epac2 favors additional CICR. Note that Rap1 activation may be stimulated by RasGRP3 since the GEF activity of RasGRP3 is itself stimulated by Ca2+ and DAG derived from PLCε-catalyzed hydrolysis of PIP2. Activated Rap1 may inhibit SERCA, so that leakage of Ca2+ from the ER may raise levels of [Ca2+]i and favor CICR. The association of Rap1 with SERCA is inhibited by PKA.
Figure 10.5.
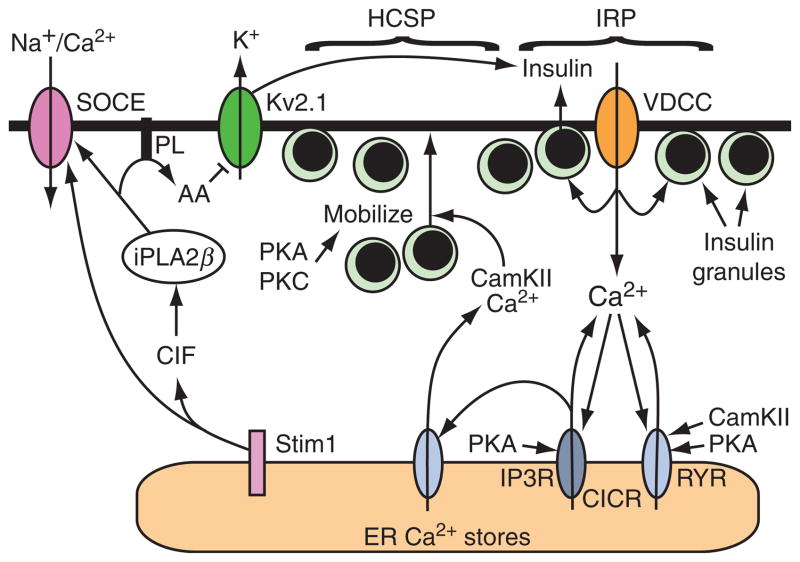
CICR regulates insulin secretion. Ca2+ influx through VDCCs is the main stimulus for insulin secretion under conditions in which β cells are exposed to elevated levels of glucose. Secretory granules located in an immediate releasable pool (IRP) adjacent to these VDCCs undergo exocytosis in response to the high [Ca2+] that exists at the inner mouth of each VDCC. When β cells are exposed to both glucose and GLP-1, exocytosis is amplified due to the fact that CICR is initiated. Amplification of exocytosis occurs because PKA and PKC increase the number of secretory granules within a highly Ca2+-sensitive pool (HCSP). These granules are then able to undergo exocytosis in response to CICR that is facilitated by both PKA and Epac2. Ca2+ released from the ER also plays a major role in stimulating the mobilization of insulin granules from the reserve pool, an effect mediated by CamKII. The role of Stim1 in β cells is not fully understood but upon store depletion it translocates to the plasma membrane where it may activate store-operated Ca2+ entry (SOCE) and also Na+ entry through nonselective cation channels. An alternative model suggests that Stim1 activation triggers the formation of a Ca2+ influx factor (CIF) that activates iPLA2β, and that iPLA2β regulates SOCE activity. iPLA2β hydrolyzes phospholipids (PL) in the plasma membrane to liberate arachidonic acid (AA) and this lipid metabolite can inhibit Kv2.1 delayed rectifier channels to potentiate membrane depolarization, Ca2+ influx, and insulin secretion.
Following the discovery of Epac2 in 1998, it was reported that the action of GLP-1 to raise [Ca2+]i in mouse islets was not entirely explained by its ability to activate PKA (Bode et al., 1999). Soon after, it was demonstrated that the action of cAMP-elevating agent forskolin to raise [Ca2+]i was inhibited by transfection of INS-1 insulin-secreting cells with a dominant-negative Epac2 (DN-Epac2) that fails to bind cAMP (Kang et al., 2001). More extensive studies revealed that the action of a GLP-1R agonist to raise [Ca2+]i was similarly blocked by DN-Epac2 (Kang et al., 2005). Final confirmation that Epac2 does in fact participate in the regulation of [Ca2+]i was provided by studies in which it was demonstrated that ESCAs reproduced the Ca2+-elevating action of GLP-1 in mouse and human β cells (Chepurny et al., 2010; Kang et al., 2003; Leech et al., 2010).
The potential role of PLCε in the regulation of β cell Ca2+ handling by GLP-1 is an area of ongoing investigation, and preliminary studies favor such a mechanism. Thus, the ability of Epac activators to promote PIP2 hydrolysis and to enhance K-ATP channel closure is fully consistent with the established action of GLP-1 to depolarize β cells and to stimulate Ca2+ influx. In view of the fact that PIP2 hydrolysis produces IP3, it may be speculated that PLCε plays a significant role as a determinant of Ca2+ release from an intracellular compartment where IP3 receptors are located (Fig. 10.4). This concept is consistent with the demonstrated ability of GLP-1R agonists and ESCAs to mobilize Ca2+ in β cells (Chepurny et al., 2010; Kang et al., 2001, 2003).
The source of Ca2+ mobilized by Epac activators is not exclusively IP3 receptor regulated, but also includes those Ca2+ stores that under the control of ryanodine receptors (RYRs). This is particularly true for human and rat β cells that express the type-2 isoform of RYR (Dror et al., 2008), whereas it may be less significant for mouse β cells, since the expression of RYR in this cell type is low (Beauvois et al., 2004). Exactly how Epac2 activation leads to RYR-dependent release of intracellular Ca2+ remains to be elucidated, but studies of cardiac myocytes seem to indicate a role for PLCε, PKCε, and calcium–calmodulin kinase II (CamKII) (Oestreich et al., 2009). Available evidence indicates that Ca2+ is mobilized from RYR-regulated Ca2+ stores via a process of CICR, and that this Ca2+ acts as a direct stimulus for insulin secretion from β cells (Kang et al., 2003).
Since PKA and Epac2 activators mobilize Ca2+ from endoplasmic reticulum (ER) Ca2+ stores, it is significant that ER Ca2+ release plays a major role in regulating insulin granule trafficking (Hao et al., 2005). This effect is achieved through the activation of CamKII (Gromada et al., 1999). Thus, we propose a unifying model to explain how cAMP regulates insulin secretion (Fig. 10.5). In this model, cAMP acting through PKA and Epac2 enhances glucose-dependent influx of Ca2+ through VDCCs while also promoting CICR. Simultaneously, CamKII promotes insulin granule translocation to the plasma membrane. In the absence of cAMP, exocytosis is limited to the small numbers of docked secretory granules that are located in close to proximity to VDCCs. However, in the combined presence of cAMP and glucose, exocytosis can occur at a distance from VDCCs, and for this reason a larger number of secretory granules are released. This model is based on studies that demonstrated an ability of CICR to amplify exocytosis in an insulin-secreting β cell line (Kang et al., 2003). Although the model emphasizes the role of CICR originating from the ER, the real situation is likely to be considerably more complicated because the ER is not the only Ca2+ store in β cells (Duman et al., 2006), and the release of Ca2+ from these stores may be governed by additional Ca2+ mobilizing second messengers such as cyclic-ADP-ribose and NAADP (Kim et al., 2008).
Finally, it is interesting to note that depletion of ER Ca2+ stores induces the reversible translocation of the ER Ca2+ sensor Stim1 with concomitant activation of store-operated Ca2+ entry (SOCE) in β cells (Tamarina et al., 2008). Activation of Stim1 by store depletion is proposed to activate SOCE either directly through a conformational coupling model, or indirectly by inducing the generation of a soluble Ca2+ influx factor (CIF) that activates Ca2+-independent phospholipase A2β (iPLA2β) (Bolotina, 2008). This raises the possibility that iPLA2β mediates an indirect effect of ER Ca2+ store depletion on insulin secretion (Fig. 10.5). It is known that β cells express iPLA2β and that it plays a role in GSIS (Turk and Ramanadham, 2004). It is also known that iPLA2β plays a role in generating arachidonic acid in β cells and that this lipid metabolite regulates Kv2.1 delayed rectifier potassium channels to facilitate Ca2+ entry and to stimulate insulin secretion (Jacobson et al., 2007).
E. Activation of protein kinases
The ability of cAMP-elevating agents such as GLP-1 to enhance islet insulin secretion is not simply a consequence of their acute stimulatory influences on β cell GSIS. Instead, in vivo studies of rodents demonstrate that chronic administration of a GLP-1R agonist (exendin-4) stimulates the proliferation of β cells while also protecting against β cell death (apoptosis) (Drucker, 2003). This remarkable growth factor-like effect of exendin-4 leads to an increase of “β cell mass” with associated islet hypertrophy, and these effects are measurable as a long-term increase of pancreatic insulin secretory capacity (Holz and Chepurny, 2005). Although it has yet to be established that such growth factor-like effects of exendin-4 occur in the human pancreas, evidence exists that this may in fact be the case (Farilla et al., 2003; Lupi et al., 2008). Mechanistically, a role for Rap in this process of islet hypertrophy is indicated on the basis of new findings demonstrating that in rodent islets and INS-1 insulin-secreting cells, Rap1A promotes the activation of a serine/threonine protein kinase designated as ribosomal protein S6 kinase (S6K1) (Kelly et al., 2010). This kinase is a substrate of the mitochondrial target of rapamycin (mTOR), and it stimulates both protein synthesis and cellular proliferation in numerous cell types. Intriguingly, Rap1 may also play a significant role in the stimulation of the mitogen-activated protein kinases (MAPKs) that are known to control β cell growth. For example, in human islets, GLP-1 is reported to act through Rap to activate the serine/threonine protein kinase B-Raf that couples cAMP production to the activation of MAPKs of the extracellular signal-regulated kinase (ERK) family (Trümper et al., 2005). Finally, it is important to note that one recent study provides evidence for Epac-mediated “noncanonical” activation of PKB in β cells (Widenmaier et al., 2009). PKB is a serine/threonine protein kinase that has an established role as a stimulator of β cell growth (Assmann et al., 2009), so it could be that cAMP-elevating agents such as GLP-1 act through Epac2 and Rap1 to promote PKB activation with attendant expansion of β cell mass.
V. Interactions of Epac2 with Secretory Granule-Associated Proteins
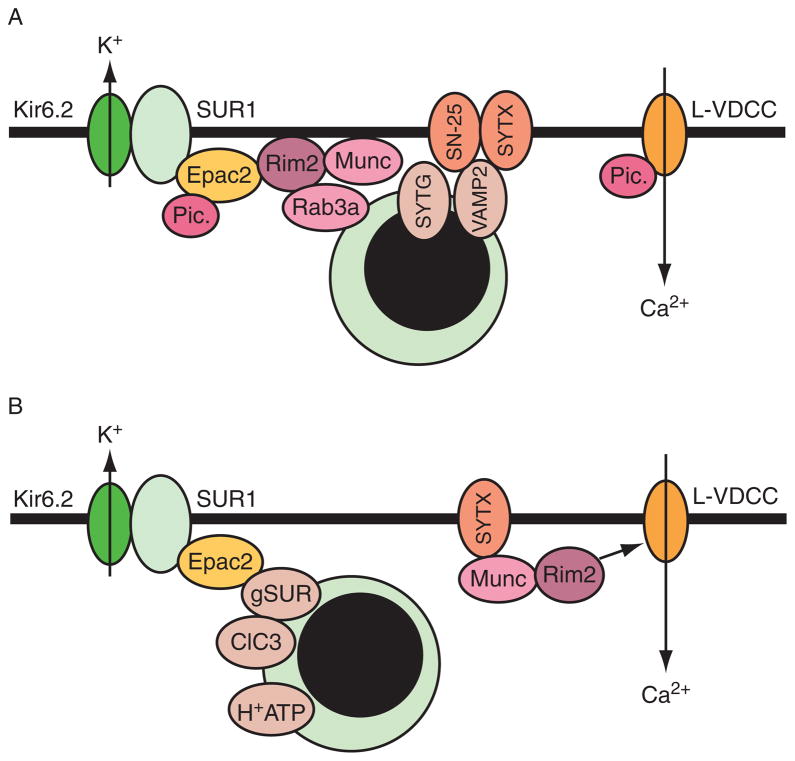
Epac2 binds directly to secretory granule-associated proteins and also to SNARE apparatus proteins that are involved in the regulation of exocytosis. These direct protein–protein interactions may explain, at least in part, how Epac2 regulates insulin secretion (Fig. 10.6). The proteins that Epac2 interacts with include Rim2 (Ozaki et al., 2000), Piccolo (Fujimoto et al., 2002), Rap1 (Shibasaki et al., 2007), and SNAP-25 (Vikman et al., 2009). Interestingly, the binding of Epac2 to the SUR1 subunit of K-ATP channels may influence exocytosis not simply by altering K-ATP channel activity, but by promoting the cAMP-dependent acidification of secretory granules, a priming step that renders the granules competent to undergo exocytosis (Eliasson et al., 2003). A role for Epac2 in the priming of secretory granules is also suggested based on the observation that Epac2 exists within a macro-molecular complex comprising Rim2, Munc13-1, and the SNARE protein syntaxin. Available evidence indicates that this complex is under the dual regulation of PKA and Epac2, and that priming of secretory granules results from the action of Munc13-1 to unfold syntaxin (Leung et al., 2007). Although electrophysiological findings first indicated that Epac2 increases the number of primed granules available for exocytosis (Eliasson et al., 2003; Renstrom et al., 1997), it should be noted that in studies of neurons, it was reported that Epac2 acts presynaptically to increase the probability that individual synaptic vesicles will undergo exocytosis (Gekel and Neher, 2008). Intriguingly, this action of Epac2 might reflect its ability to increase the number of vesicles located in close proximity to VDCCs. Thus, the action of Epac2 to influence exocytosis appears to be complex and may even be contingent on simultaneous activation of PKA, as suggested by the finding that Epac agonist-stimulated insulin secretion is abrogated after treatment of human islets with PKA inhibitors (Chepurny et al., 2010).
Figure 10.6.
(A) Protein interactions that regulate exocytosis. Epac2 stimulates exocytosis by directly interacting with the Ca2+ sensor Piccolo (Pic.), Rim2 (Rab GTPase-interacting molecule), and SNAP-25 (SN-25; interaction not shown). Epac2 also indirectly interacts with secretory granule-associated proteins and SNARE apparatus proteins that control exocytosis. These include Rab3A, synaptotagmin (SYTG), VAMP2, and syntaxin (SYTX). Piccolo also interacts with L-type voltage-dependent Ca2+ channels (L-VDCC), but the significance of this interaction is not known. (B) Priming of secretory granules by Epac2. In addition to binding plasma membrane SUR1, Epac2 is proposed to bind granular SUR (gSUR) and regulate ClC3 chloride channels to promote granular acidification through the v-type H+-ATPase (H+ATP). Also shown is the interaction of Munc13-1 with SYTX and Rim2. The C-terminal tail of Rim2 binds weakly to L-VDCC but the significance of this interaction is not known.
The above-summarized protein interactions are also likely to explain the ability of Epac2 to influence insulin secretory granule dynamics. Using methods of live-cell imaging, Shibasaki et al. (2007) provided evidence for an Epac2- and Rap1-mediated action of cAMP to potentiate first-phase GSIS. Such studies revealed that cAMP potentiated GSIS by increasing the number of secretory granules newly recruited to the plasma membrane in response to glucose metabolism, and that these granules underwent exocytosis without pausing in a docked mode. Such granules were designated as “restless newcomers” since they not only appeared at the plasma membrane suddenly, but also underwent exocytosis without a pause. This cAMP-dependent stimulation of restless newcomer exocytosis was Epac2-mediated, since the action of cAMP was dramatically reduced in β cells of Epac2 KO mice. Moreover, the potentiation of GSIS by cAMP was abrogated following treatment of β cells with siRNA for Rap1, or after overexpression of Rap1GAP (Shibasaki et al., 2007). Importantly, the existence of Epac2-regulated restless newcomer exocytosis is unanticipated in view of prior electrophysiological studies in which the action of Epac2 to enhance insulin secretion was demonstrated to result from its ability to facilitate the exocytosis of granules that were already docked and primed at the plasma membrane (Eliasson et al., 2003; Renstrom et al., 1997). Thus, considerable debate exists as to the nature of the secretory granule pool that undergoes exocytosis in an Epac2-regulated manner.
VI. Conclusions
From the data reviewed above, it is apparent that both Epac2 and Rap1 play a highly significant role in the cAMP-dependent stimulation of insulin secretion from pancreatic β cells. Complex feedback loops, possibly involving Ca2+, DAG, and RasGRPs, may dictate the spatiotemporal activation of Rap1 that is required for an appropriate insulin-secretory response. Furthermore, the possibility that Rap1 regulates the activity of PLCε provides an unexpected explanation for how cAMP influences K-ATP channel activity, cytosolic Ca2+ signaling, and PKC activation in β cells. It may be anticipated that a molecular genetics approach involving the use of Epac2 and PLCε KO mice will more fully reveal what roles these signaling proteins play as determinants of incretin hormone action.
Acknowledgments
Funding was provided by the National Institutes of Health (DK045817 and DK069575 to G. G. H.).
References
- Akita Y. Protein kinase C-epsilon (PKC-epsilon): Its unique structure and function. J Biochem. 2002;132:847–852. doi: 10.1093/oxfordjournals.jbchem.a003296. [DOI] [PubMed] [Google Scholar]
- Akita Y. Protein kinase C-epsilon: Multiple roles in the function of, and signaling mediated by, the cytoskeleton. FEBS J. 2008;275:3995–4004. doi: 10.1111/j.1742-4658.2008.06557.x. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Quilliam LA, Cooper JA. Rap1 promotes cell spreading by localizing Rac guanine nucleotide exchange factors. J Cell Biol. 2004;167:111–122. doi: 10.1083/jcb.200404068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann A, Hinault C, Kulkarni RN. Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes. 2009;10:14–32. doi: 10.1111/j.1399-5448.2008.00468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T, Schulte U, Oliver D, Herlitze S, Krauter T, Tucker SJ, Ruppersberg JP, Fakler B. PIP2 and PIP as determinants for ATP inhibition of K-ATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Beauvois MC, Arredouani A, Jonas JC, Rolland JF, Schuit F, Henquin JC, Gilon P. Atypical Ca2+-induced Ca2+ release from a sarco-endoplasmic reticulum Ca2+-ATPase 3-dependent Ca2+ pool in mouse pancreatic beta-cells. J Physiol. 2004;559:141–156. doi: 10.1113/jphysiol.2004.067454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bode HP, Moormann B, Dabew R, Goke B. Glucagon-like peptide-1 elevates cytosolic calcium in pancreatic beta-cells independently of protein kinase A. Endocrinology. 1999;140:3919–3927. doi: 10.1210/endo.140.9.6947. [DOI] [PubMed] [Google Scholar]
- Bolotina VM. Orai, STIM1 and iPLA2beta: A view from a different perspective. J Physiol. 2008;586:3035–3042. doi: 10.1113/jphysiol.2008.154997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bos JL. Epac proteins: Multi-purpose cAMP targets. Trends Biochem Sci. 2006;31:680–686. doi: 10.1016/j.tibs.2006.10.002. [DOI] [PubMed] [Google Scholar]
- Bryan J, Munoz A, Zhang X, Dufer M, Drews G, Krippeit-Drews P, Aguilar-Bryan L. ABCC8 and ABCC9: ABC transporters that regulate K+ channels. Pflugers Arch. 2007;453:703–718. doi: 10.1007/s00424-006-0116-z. [DOI] [PubMed] [Google Scholar]
- Bunney TD, Katan M. Phospholipase C epsilon: Linking second messengers and small GTPases. Trends Cell Biol. 2006;16:640–648. doi: 10.1016/j.tcb.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Campbell RK, Miller S. New therapeutic horizons: Mapping the future of glycemic control with incretin-based therapy. Diabetes Educ. 2009;35:731–734. 738–740, 742–744. doi: 10.1177/0145721709342900. passim. [DOI] [PubMed] [Google Scholar]
- Cantley J, Burchfield JG, Pearson GL, Schmitz-Peiffer C, Leitges M, Biden TJ. Deletion of PKC-epsilon selectively enhances the amplifying pathways of glucose-stimulated insulin secretion via increased lipolysis in mouse beta-cells. Diabetes. 2009;58:1826–1834. doi: 10.2337/db09-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Leech CA, Kelley GG, Dzhura I, Dzhura E, Li X, Rindler MJ, Schwede F, Genieser HG, Holz GG. Enhanced Rap1 activation and insulin secretagogue properties of an acetoxymethyl ester of an Epac-selective cyclic AMP analog in rat INS-1 cells: Studies with 8-pCPT-2′-O-Me-cAMP-AM. J Biol Chem. 2009;284:10728–10736. doi: 10.1074/jbc.M900166200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chepurny OG, Kelley GG, Dzhura I, Leech CA, Roe MW, Dzhura E, Li X, Schwede F, Genieser HG, Holz GG. PKA-dependent potentiation of glucose-stimulated insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM in human islets of Langerhans. Am J Physiol Endocrinol Metab. 2010;298:E622–E633. doi: 10.1152/ajpendo.00630.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Rooij J, Zwartkruis FJ, Verheijen MH, Cool RH, Nijman SM, Wittinghofer A, Bos JL. Epac is a Rap1 guanine-nucleotide-exchange factor directly activated by cyclic AMP. Nature. 1998;396:474–477. doi: 10.1038/24884. [DOI] [PubMed] [Google Scholar]
- Dror V, Kalynyak TB, Bychkivska Y, Frey MH, Tee M, Jeffrey KD, Nguyen V, Luciani DS, Johnson JD. Glucose and endoplasmic reticulum calcium channels regulate HIF-1beta via presenilin in pancreatic beta-cells. J Biol Chem. 2008;283:9909–9916. doi: 10.1074/jbc.M710601200. [DOI] [PubMed] [Google Scholar]
- Drucker DJ. Glucagon-like peptides: Regulators of cell proliferation, differentiation, and apoptosis. Mol Endocrinol. 2003;17:161–171. doi: 10.1210/me.2002-0306. [DOI] [PubMed] [Google Scholar]
- Duman JG, Chen L, Palmer AE, Hille B. Contributions of intracellular compartments to calcium dynamics: Implicating an acidic store. Traffic. 2006;7:859–872. doi: 10.1111/j.1600-0854.2006.00432.x. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Gylfe E. Ca2+-induced Ca2+ release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J Biol Chem. 2004;279:45455–45461. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Idevall-Hagren O, Sagetorp J, Tian G, Wuttke A, Arrieumerlou C, Akusjarvi G, Gylfe E, Tengholm A. Glucose-induced cyclic AMP oscillations regulate pulsatile insulin secretion. Cell Metab. 2008;8:26–37. doi: 10.1016/j.cmet.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Eliasson L, Ma X, Renstrom E, Barg S, Berggren PO, Galvanovskis J, Gromada J, Jing X, Lundquist I, Salehi A, Sewing S, Rorsman P. SUR1 regulates PKA-independent cAMP-induced granule priming in mouse pancreatic b-cells. J Gen Physiol. 2003;121:181–197. doi: 10.1085/jgp.20028707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farilla L, Bulotta A, Hirshberg B, Li Calzi S, Khoury N, Noushmehr H, Bertolotto C, Di Mario U, Harlan DM, Perfetti R. Glucagon-like peptide-1 inhibits cell apoptosis and improves glucose responsiveness of freshly isolated human islets. Endocrinology. 2003;144:5149–5158. doi: 10.1210/en.2003-0323. [DOI] [PubMed] [Google Scholar]
- Fridolf T, Ahren B. GLP-1(7–36) amide stimulates insulin secretion in rat islets: Studies on the mode of action. Diabetes Res. 1991;16:185–191. [PubMed] [Google Scholar]
- Fujimoto K, Shibasaki T, Yokoi N, Kashima Y, Matsumoto M, Sasaki T, Tajima N, Iwanaga T, Seino S. Piccolo, a Ca2+ sensor in pancreatic beta-cells. Involvement of cAMP-GEFII.Rim2.Piccolo complex in cAMP-dependent exocytosis. J Biol Chem. 2002;277:50497–50502. doi: 10.1074/jbc.M210146200. [DOI] [PubMed] [Google Scholar]
- Gekel I, Neher E. Application of an Epac activator enhances neurotransmitter release at excitatory central synapses. J Neurosci. 2008;28:7991–8002. doi: 10.1523/JNEUROSCI.0268-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloerich M, Bos JL. Epac: Defining a new mechanism for cAMP action. Annu Rev Pharmacol Toxicol. 2010;50:355–375. doi: 10.1146/annurev.pharmtox.010909.105714. [DOI] [PubMed] [Google Scholar]
- Goldfine ID, Perlman R, Roth J. Inhibition of cyclic 3′,5′-AMP phosphodiesterase in islet cells and other tissues by tolbutamide. Nature. 1971;234:295–297. doi: 10.1038/234295a0. [DOI] [PubMed] [Google Scholar]
- Gromada J, Bokvist K, Ding WG, Holst JJ, Nielsen JH, Rorsman P. Glucagon-like peptide-1(7–36)amide stimulates exocytosis in human pancreatic β-cells by both proximal and distal regulatory steps in stimulus–secretion coupling. Diabetes. 1998;47:57–65. doi: 10.2337/diab.47.1.57. [DOI] [PubMed] [Google Scholar]
- Gromada J, Hoy M, Renstrom E, Bokvist K, Eliasson L, Gopel S, Rorsman P. CaM kinase II-dependent mobilization of secretory granules underlies acetylcholine-induced stimulation of exocytosis in mouse pancreatic B-cells. J Physiol. 1999;518:745–759. doi: 10.1111/j.1469-7793.1999.0745p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao M, Li X, Rizzo MA, Rocheleau JV, Dawant BM, Piston DW. Regulation of two insulin granule populations within the reserve pool by distinct calcium sources. J Cell Sci. 2005;118:5873–5884. doi: 10.1242/jcs.02684. [DOI] [PubMed] [Google Scholar]
- Hatakeyama H, Kishimoto T, Nemoto T, Kasai H, Takahashi N. Rapid glucose sensing by protein kinase A for insulin exocytosis in mouse pancreatic islets. J Physiol. 2006;570:271–282. doi: 10.1113/jphysiol.2005.096560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatakeyama H, Takahashi N, Kishimoto T, Nemoto T, Kasai H. Two cAMP-dependent pathways differentially regulate exocytosis of large dense-core and small vesicles in mouse β-cells. J Physiol. 2007;582:1087–1098. doi: 10.1113/jphysiol.2007.135228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG. Diabetes outfoxed by GLP-1? Sci STKE. 2005;268:pe2. doi: 10.1126/stke.2682005pe2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kuhtreiber WM, Habener JF. Pancreatic beta cells are rendered glucose-competent by the insulinotropic hormone glucagon-like peptide-1 (7–37) Nature. 1993;361:362–365. doi: 10.1038/361362a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Leech CA, Heller RS, Castonguay M, Habener JF. cAMP-dependent mobilization of intracellular Ca2+ stores by activation of ryanodine receptors in pancreatic beta-cells. A Ca2+ signaling system stimulated by the insulinotropic hormone glucagon-like peptide-1-(7–37) J Biol Chem. 1999;274:14147–14156. doi: 10.1074/jbc.274.20.14147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Kang G, Harbeck M, Roe MW, Chepurny OG. Cell physiology of cAMP sensor Epac. J Physiol. 2006;577:5–15. doi: 10.1113/jphysiol.2006.119644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz GG, Chepurny OG, Schwede F. Epac-selective cAMP analogs: New tools with which to evaluate the signal transduction properties of cAMP-regulated guanine nucleotide exchange factors. Cell Signal. 2008;20:10–20. doi: 10.1016/j.cellsig.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoy M, Berggren PO, Gromada J. Involvement of protein kinase C-epsilon in inositol hexakisphosphate-induced exocytosis in mouse pancreatic beta-cells. J Biol Chem. 2003;278:35168–35171. doi: 10.1074/jbc.M303927200. [DOI] [PubMed] [Google Scholar]
- Israili ZH. Advances in the treatment of type 2 diabetes mellitus. Am J Ther. 2009 doi: 10.1097/MJT.0b013e3181afbf51. (in press) [DOI] [PubMed] [Google Scholar]
- Jacobson DA, Weber CR, Bao S, Turk J, Philipson LH. Modulation of the pancreatic islet beta-cell-delayed rectifier potassium channel Kv2.1 by the polyunsaturated fatty acid arachidonate. J Biol Chem. 2007;282:7442–7449. doi: 10.1074/jbc.M607858200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin TG, Satoh T, Liao Y, Song C, Gao X, Kariya K, Hu CD, Kataoka T. Role of the CDC25 homology domain of phospholipase C-epsilon in amplification of Rap1-dependent signaling. J Biol Chem. 2001;276:30301–30307. doi: 10.1074/jbc.M103530200. [DOI] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Holz GG. cAMP-regulated guanine nucleotide exchange factor II (Epac2) mediates Ca2+-induced Ca2+ release in INS-1 pancreatic β cells. J Physiol. 2001;536:375–385. doi: 10.1111/j.1469-7793.2001.0375c.xd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Joseph JW, Chepurny OG, Monaco M, Wheeler MB, Bos JL, Schwede F, Genieser HG, Holz GG. Epac-selective cAMP analog 8-pCPT-2′-O-Me-cAMP as a stimulus for Ca2+-induced Ca2+ release and exocytosis in pancreatic beta cells. J Biol Chem. 2003;278:8279–8285. doi: 10.1074/jbc.M211682200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Rindler MJ, Collis L, Chepurny Z, Li WH, Harbeck M, Roe MW, Holz GG. A cAMP and Ca2+ coincidence detector in support of Ca2+-induced Ca2+ release in mouse pancreatic β cells. J Physiol. 2005;566:173–188. doi: 10.1113/jphysiol.2005.087510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Chepurny OG, Malester B, Rindler MJ, Rehmann H, Bos JL, Schwede F, Coetzee WA, Holz GG. cAMP sensor Epac as a determinant of ATP-sensitive potassium channel activity in human pancreatic β cells and rat INS-1 cells. J Physiol. 2006;573:595–609. doi: 10.1113/jphysiol.2006.107391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang G, Leech CA, Chepurny OG, Coetzee WA, Holz GG. Role of the cAMP sensor Epac as a determinant of K-ATP channel ATP sensitivity in human pancreatic beta cells and rat INS-1 cells. J Physiol. 2008;586:1307–1319. doi: 10.1113/jphysiol.2007.143818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasai H, Hatakeyama H, Ohno M, Takahashi N. Exocytosis in islet beta-cells. Adv Exp Med Biol. 2010;654:305–338. doi: 10.1007/978-90-481-3271-3_14. [DOI] [PubMed] [Google Scholar]
- Kashima Y, Miki T, Shibasaki T, Ozaki N, Miyazaki M, Yano H, Seino S. Critical role of cAMP–GEFII–Rim2 complex in incretin-potentiated insulin secretion. J Biol Chem. 2001;276:46046–46053. doi: 10.1074/jbc.M108378200. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Springett GM, Mochizuki N, Toki S, Nakaya M, Matsuda M, Housman DE, Graybiel AM. A family of cAMP-binding proteins that directly activate Rap1. Science. 1998;282:2275–2279. doi: 10.1126/science.282.5397.2275. [DOI] [PubMed] [Google Scholar]
- Kelley GG, Reks SE, Ondrako JM, Smrcka AV. Phospholipase C-epsilon: A novel Ras effector. EMBO J. 2001;20:743–754. doi: 10.1093/emboj/20.4.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley GG, Chepurny OG, Leech CA, Roe MW, Li X, Dzhura I, Dzhura E, Afshari P, Holz GG. Glucose-dependent potentiation of mouse islet insulin secretion by Epac activator 8-pCPT-2′-O-Me-cAMP-AM. Islets. 2009;1:260–265. doi: 10.4161/isl.1.3.9645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P, Bailey CL, Fueger PT, Newgard CB, Casey PJ, Kimple ME. Rap1 promotes multiple pancreatic islet cell functions and signals through mTOR complex 1 to enhance proliferation. J Biol Chem. 2010 Mar 25; doi: 10.1074/jbc.M109.069112. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendall DM, Riddle MC, Rosenstock J, Zhuang D, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in patients with type 2 diabetes treated with metformin and a sulfonylurea. Diabetes Care. 2005;28:1083–1091. doi: 10.2337/diacare.28.5.1083. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Park KH, Yim CY, Takasawa S, Okamoto H, Im MJ, Kim UH. Generation of nicotinic acid adenine dinucleotide phosphate and cyclic ADP-ribose by glucagon-like peptide-1 evokes Ca2+ signal that is essential for insulin secretion in mouse pancreatic islets. Diabetes. 2008;57:868–878. doi: 10.2337/db07-0443. [DOI] [PubMed] [Google Scholar]
- Koster JC, Sha Q, Nichols CG. Sulfonylurea and K+-channel opener sensitivity of K-ATP channels. Functional coupling of Kir6.2 and SUR1 subunits. J Gen Physiol. 1999;114:203–213. doi: 10.1085/jgp.114.2.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowluru A. Protein prenylation in glucose-induced insulin secretion from the pancreatic islet beta cell: A perspective. J Cell Mol Med. 2008;12:164–173. doi: 10.1111/j.1582-4934.2007.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landa LR, Jr, Harbeck M, Kaihara K, Chepurny O, Kitiphongspattana K, Graf O, Nikolaev VO, Lohse MJ, Holz GG, Roe MW. Interplay of Ca2+ and cAMP signaling in the insulin-secreting MIN6 beta cell line. J Biol Chem. 2005;280:31294–31302. doi: 10.1074/jbc.M505657200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leech CA, Dzhura I, Chepurny OG, Schwede F, Genieser HG, Holz GG. Facilitation of β-cell KATP channel sulfonylurea sensitivity by a cAMP analog selective for the cAMP-regulated guanine nucleotide exchange factor Epac. Islets. 2010;2:72–81. doi: 10.4161/isl.2.2.10582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung YM, Kwan EP, Ng B, Kang Y, Gaisano HY. SNAREing voltage-gated K+ and ATP-sensitive K+ channels: Tuning beta cell excitability with syntaxin-1A and other exocytotic proteins. Endocr Rev. 2007;28:653–663. doi: 10.1210/er.2007-0010. [DOI] [PubMed] [Google Scholar]
- Lupi R, Mancarella R, Del Guerra S, Bugliani M, Del Prato S, Boggi U, Mosca F, Filipponi F, Marchetti P. Effects of exendin-4 on islets from type 2 diabetes patients. Diabetes Obes Metab. 2008;10:515–519. doi: 10.1111/j.1463-1326.2007.00838.x. [DOI] [PubMed] [Google Scholar]
- McAvoy T, Zhou MM, Greengard P, Nairn AC. Phosphorylation of Rap1GAP, a striatally enriched protein, by protein kinase A controls Rap1 activity and dendritic spine morphology. Proc Natl Acad Sci USA. 2009;106:3531–3536. doi: 10.1073/pnas.0813263106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendez CF, Leibiger IB, Leibiger B, Hoy M, Gromada J, Berggren PO, Bertorello AM. Rapid association of protein kinase C-epsilon with insulin granules is essential for insulin exocytosis. J Biol Chem. 2003;278:44753–44757. doi: 10.1074/jbc.M308664200. [DOI] [PubMed] [Google Scholar]
- Nakazaki M, Crane A, Hu M, Seghers V, Ullrich S, Aguilar-Bryan L, Bryan J. cAMP-activated protein kinase-independent potentiation of insulin secretion by cAMP is impaired in SUR1 null islets. Diabetes. 2002;51:3440–3449. doi: 10.2337/diabetes.51.12.3440. [DOI] [PubMed] [Google Scholar]
- Niimura M, Miki T, Shibasaki T, Fujimoto W, Iwanaga T, Seino S. Critical role of the N-terminal cyclic AMP-binding domain of Epac2 in its subcellular localization and function. J Cell Physiol. 2009;219:652–658. doi: 10.1002/jcp.21709. [DOI] [PubMed] [Google Scholar]
- Oestreich EA, Malik S, Goonasekera SA, Blaxall BC, Kelley GG, Dirksen RT, Smrcka AV. Epac and phospholipase C-epsilon regulate Ca2+ release in the heart by activation of protein kinase C-epsilon and calcium–calmodulin kinase II. J Biol Chem. 2009;284:1514–1522. doi: 10.1074/jbc.M806994200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orci L, Gabbay KH, Malaisse WJ. Pancreatic beta-cell web: Its possible role in insulin secretion. Science. 1972;175:1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Shibasaki T, Kashima Y, Miki T, Takahashi K, Ueno H, Sunaga Y, Yano H, Matsuura Y, Iwanaga T, Takai Y, Seino S. cAMP–GEFII is a direct target of cAMP in regulated exocytosis. Nat Cell Biol. 2000;2:805–811. doi: 10.1038/35041046. [DOI] [PubMed] [Google Scholar]
- Ozaki N, Miura Y, Yamada T, Kato Y, Oiso Y. RasGRP3 mediates phorbol ester-induced, protein kinase C-independent exocytosis. Biochem Biophys Res Commun. 2005;329:765–771. doi: 10.1016/j.bbrc.2005.02.031. [DOI] [PubMed] [Google Scholar]
- Prekeris R, Mayhew MW, Cooper JB, Terrian DM. Identification and localization of an actin-binding motif that is unique to the epsilon isoform of protein kinase C and participates in the regulation of synaptic function. J Cell Biol. 1996;132:77–90. doi: 10.1083/jcb.132.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic β cells. J Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M, Evellin S, Weernink PA, von Dorp F, Rehmann H, Lomasney JW, Jakobs KH. A new phospholipase-C-calcium signalling pathway mediated by cyclic AMP and a Rap GTPase. Nat Cell Biol. 2001;3:1020–1024. doi: 10.1038/ncb1101-1020. [DOI] [PubMed] [Google Scholar]
- Schmitz-Peiffer C, Laybutt DR, Burchfield JG, Gurisik E, Narasimhan S, Mitchell CJ, Pedersen DJ, Braun U, Cooney GJ, Leitges M, Biden TJ. Inhibition of PKC-epsilon improves glucose-stimulated insulin secretion and reduces insulin clearance. Cell Metab. 2007;6:320–328. doi: 10.1016/j.cmet.2007.08.012. [DOI] [PubMed] [Google Scholar]
- Schuit FC, Pipeleers DG. Regulation of adenosine 3′,5′-monophosphate levels in the pancreatic B cell. Endocrinology. 1985;117:834–840. doi: 10.1210/endo-117-3-834. [DOI] [PubMed] [Google Scholar]
- Seino S, Shibasaki T. PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis. Physiol Rev. 2005;85:1303–1342. doi: 10.1152/physrev.00001.2005. [DOI] [PubMed] [Google Scholar]
- Shibasaki T, Takahashi H, Miki T, Sunaga Y, Matsumura K, Yamanaka M, Zhang C, Tamamoto A, Satoh T, Miyazaki J, Seino S. Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc Natl Acad Sci USA. 2007;104:19333–19338. doi: 10.1073/pnas.0707054104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyng SL, Nichols CG. Membrane phospholipid control of nucleotide sensitivity of K-ATP channels. Science. 1998;282:1138–1141. doi: 10.1126/science.282.5391.1138. [DOI] [PubMed] [Google Scholar]
- Song C, Satoh T, Edamatsu H, Wu D, Tadano M, Gao X, Kataoka T. Differential roles of Ras and Rap1 in growth factor-dependent activation of phospholipase C-epsilon. Oncogene. 2002;21:8105–8113. doi: 10.1038/sj.onc.1206003. [DOI] [PubMed] [Google Scholar]
- Sorli SC, Bunney TD, Sugden PH, Paterson HF, Katan M. Signaling properties and expression in normal and tumor tissues of two phospholipase C-epsilon splice variants. Oncogene. 2005;24:90–100. doi: 10.1038/sj.onc.1208168. [DOI] [PubMed] [Google Scholar]
- Tamarina NA, Kuznetsov A, Philipson LH. Reversible translocation of EYFP-tagged STIM1 is coupled to calcium influx in insulin secreting beta-cells. Cell Calcium. 2008;44:533–544. doi: 10.1016/j.ceca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Thorens B. Expression cloning of the pancreatic beta cell receptor for the gluco-incretin hormone glucagon-like peptide 1. Proc Natl Acad Sci USA. 1992;89:8641–8645. doi: 10.1073/pnas.89.18.8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trümper J, Ross D, Jahr H, Brendel MD, Göke R, Hörsch D. The Rap-B-Raf signalling pathway is activated by glucose and glucagon-like peptide-1 in human islet cells. Diabetologia. 2005;48:1534–1540. doi: 10.1007/s00125-005-1820-5. [DOI] [PubMed] [Google Scholar]
- Turk J, Ramanadham S. The expression and function of a group VIA calcium-independent phospholipase A2 (iPLA2beta) in beta-cells. Can J Physiol Pharmacol. 2004;82:824–832. doi: 10.1139/y04-064. [DOI] [PubMed] [Google Scholar]
- Vikman J, Svensson H, Huang YC, Kang Y, Andersson SA, Gaisano HY, Eliasson L. Truncation of SNAP-25 reduces the stimulatory action of cAMP on rapid exocytosis in insulin-secreting cells. Am J Physiol Endocrinol Metab. 2009;297:E452–E461. doi: 10.1152/ajpendo.90585.2008. [DOI] [PubMed] [Google Scholar]
- Wang Z, Oh E, Thurmond DC. Glucose-stimulated Cdc42 signaling is essential for the second phase of insulin secretion. J Biol Chem. 2007;282:9536–9546. doi: 10.1074/jbc.M610553200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warwar N, Dov A, Abramovitch E, Wu R, Jmoudiak M, Haber E, Cerasi E, Nesher R. PKC-epsilon mediates glucose-regulated insulin production in pancreatic beta-cells. Biochim Biophys Acta. 2008;1783:1929–1934. doi: 10.1016/j.bbamcr.2008.04.007. [DOI] [PubMed] [Google Scholar]
- Widenmaier SB, Sampaio AV, Underhill TM, McIntosh CH. Noncanonical activation of Akt/protein kinase B in {beta}-cells by the incretin hormone glucose-dependent insulinotropic polypeptide. J Biol Chem. 2009;284:10764–10773. doi: 10.1074/jbc.M809116200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing MR, Houston D, Kelley GG, Der CJ, Siderovski DP, Harden TK. Activation of phospholipase C-epsilon by heterotrimeric G protein beta-gamma-subunits. J Biol Chem. 2001;276:48257–48261. doi: 10.1074/jbc.C100574200. [DOI] [PubMed] [Google Scholar]
- Wing MR, Snyder JT, Sondek J, Harden TK. Direct activation of phospholipase C-epsilon by Rho. J Biol Chem. 2003;278:41253–41258. doi: 10.1074/jbc.M306904200. [DOI] [PubMed] [Google Scholar]
- Zaitsev SV, Efendic S, Arkhammar P, Bertorello AM, Berggren PO. Dissociation between changes in cytoplasmic free Ca2+ concentration and insulin secretion as evidenced from measurements in mouse single pancreatic islets. Proc Natl Acad Sci USA. 1995;92:9712–9716. doi: 10.1073/pnas.92.21.9712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang CL, Katoh M, Shibasaki T, Minami K, Sunaga Y, Takahashi H, Yokoi N, Iwasaki M, Miki T, Seino S. The cAMP sensor Epac2 is a direct target of antidiabetic sulfonylurea drugs. Science. 2009;325:607–610. doi: 10.1126/science.1172256. [DOI] [PubMed] [Google Scholar]