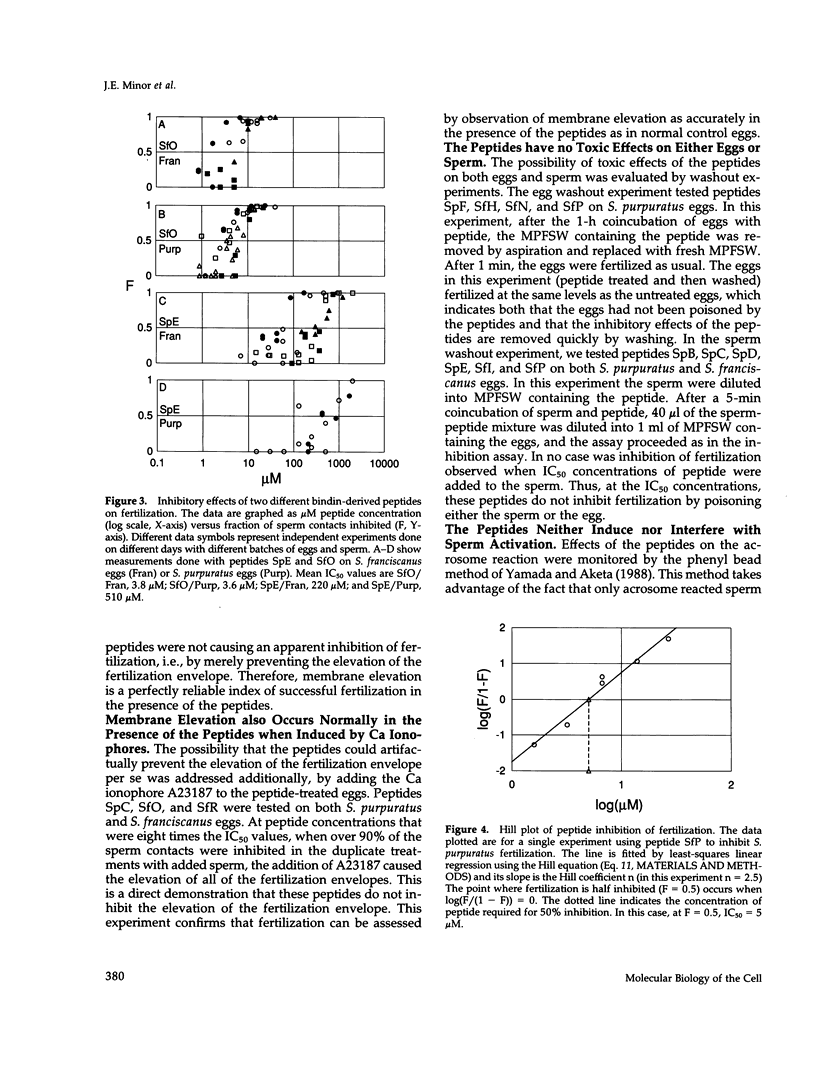
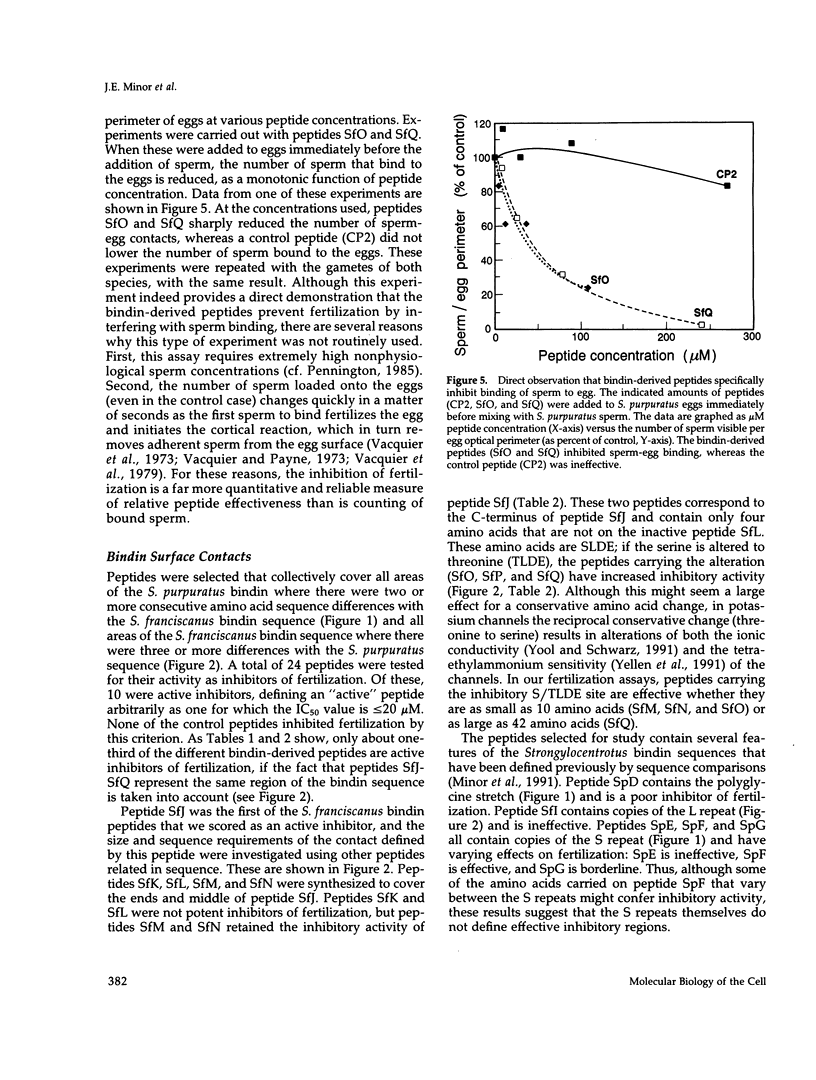
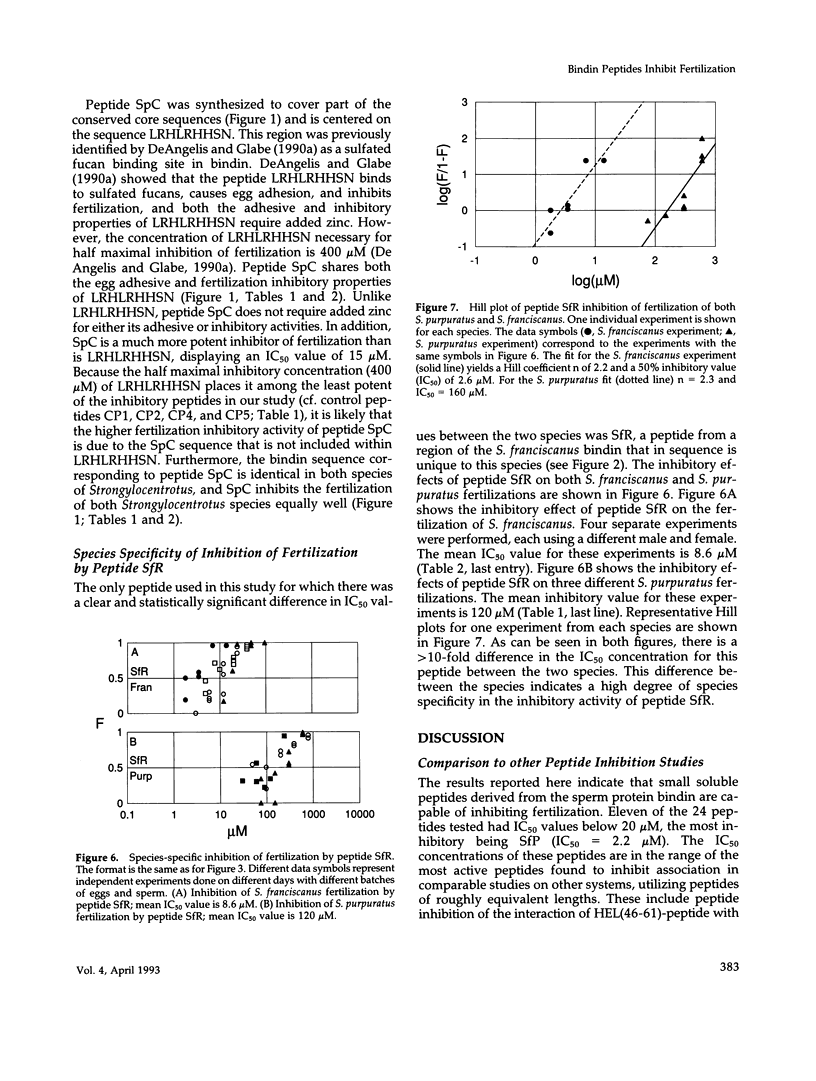
Abstract
The sperm protein bindin is responsible for the species-specific adhesion of the sperm to the egg. The regions of the bindin molecule responsible for forming the contact between the sperm and the egg were investigated by measuring the ability of peptides representing various regions of the bindin sequence to inhibit fertilization. Twenty-four peptides were studied: 7 based on the Strongylocentrotus purpuratus bindin sequence, 11 based on the S. franciscanus bindin sequence, and 6 control peptides. Values for the concentration of peptide required to inhibit 50% of the productive sperm contacts (IC50) were extracted from experimental measurements of the extent of fertilization in the presence of various concentrations. of these peptides. The IC50 value averaged 220 microM for the control peptides. Active peptides representing certain specific subregions of the bindin sequence displayed IC50 values < 10% of the average value for control peptides, and the IC50 for the most potent of the peptides tested was only approximately 1% of the control peptide value (IC50 = 2.2 microM). Furthermore, we found that a peptide representing a particular region of the S. franciscanus bindin sequence that differs from the S. purpuratus bindin sequence inhibits fertilization species specifically. For the reaction of S. purpuratus sperm and eggs, the IC50 of this peptide was approximately 120 microM, whereas for the reaction of S. franciscanus sperm and eggs it was only 8.6 microM. These results demonstrate that a few specific regions of the bindin molecule are involved in the sperm-egg contact and that certain of these regions mediate the species specificity of the interaction in a sequence-specific manner.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buus S., Sette A., Colon S. M., Miles C., Grey H. M. The relation between major histocompatibility complex (MHC) restriction and the capacity of Ia to bind immunogenic peptides. Science. 1987 Mar 13;235(4794):1353–1358. doi: 10.1126/science.2435001. [DOI] [PubMed] [Google Scholar]
- Cameron R. A., Minor J. E., Nishioka D., Britten R. J., Davidson E. H. Locale and level of bindin mRNA in maturing testis of the sea urchin, Strongylocentrotus purpuratus. Dev Biol. 1990 Nov;142(1):44–49. doi: 10.1016/0012-1606(90)90149-d. [DOI] [PubMed] [Google Scholar]
- DeAngelis P. L., Glabe C. G. Role of basic amino acids in the interaction of bindin with sulfated fucans. Biochemistry. 1988 Oct 18;27(21):8189–8194. doi: 10.1021/bi00421a030. [DOI] [PubMed] [Google Scholar]
- DeAngelis P. L., Glabe C. G. Specific recognition of sulfate esters by bindin, a sperm adhesion protein from sea urchins. Biochim Biophys Acta. 1990 Jan 19;1037(1):100–105. doi: 10.1016/0167-4838(90)90107-q. [DOI] [PubMed] [Google Scholar]
- DeAngelis P. L., Glabe C. G. Zinc specifically stimulates the selective binding of a peptide analog of bindin to sulfated fucans. Pept Res. 1990 Mar-Apr;3(2):62–68. [PubMed] [Google Scholar]
- Foltz K. R., Lennarz W. J. Purification and characterization of an extracellular fragment of the sea urchin egg receptor for sperm. J Cell Biol. 1990 Dec;111(6 Pt 2):2951–2959. doi: 10.1083/jcb.111.6.2951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao B., Klein L. E., Britten R. J., Davidson E. H. Sequence of mRNA coding for bindin, a species-specific sea urchin sperm protein required for fertilization. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8634–8638. doi: 10.1073/pnas.83.22.8634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. G. A supramolecular theory for specificity in intracellular adhesion. J Theor Biol. 1979 Jun 7;78(3):417–423. doi: 10.1016/0022-5193(79)90340-0. [DOI] [PubMed] [Google Scholar]
- Glabe C. G., Clark D. The sequence of the Arbacia punctulata bindin cDNA and implications for the structural basis of species-specific sperm adhesion and fertilization. Dev Biol. 1991 Feb;143(2):282–288. doi: 10.1016/0012-1606(91)90078-h. [DOI] [PubMed] [Google Scholar]
- Glabe C. G. Interaction of the sperm adhesive protein, bindin, with phospholipid vesicles. I. Specific association of bindin with gel-phase phospholipid vesicles. J Cell Biol. 1985 Mar;100(3):794–799. doi: 10.1083/jcb.100.3.794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. G. Interaction of the sperm adhesive protein, bindin, with phospholipid vesicles. II. Bindin induces the fusion of mixed-phase vesicles that contain phosphatidylcholine and phosphatidylserine in vitro. J Cell Biol. 1985 Mar;100(3):800–806. doi: 10.1083/jcb.100.3.800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glabe C. G., Vacquier V. D. Egg surface glycoprotein receptor for sea urchin sperm bindin. Proc Natl Acad Sci U S A. 1978 Feb;75(2):881–885. doi: 10.1073/pnas.75.2.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamm H. E., Deretic D., Arendt A., Hargrave P. A., Koenig B., Hofmann K. P. Site of G protein binding to rhodopsin mapped with synthetic peptides from the alpha subunit. Science. 1988 Aug 12;241(4867):832–835. doi: 10.1126/science.3136547. [DOI] [PubMed] [Google Scholar]
- Kennedy L., DeAngelis P. L., Glabe C. G. Analysis of the membrane-interacting domain of the sea urchin sperm adhesive protein bindin. Biochemistry. 1989 Nov 14;28(23):9153–9158. doi: 10.1021/bi00449a029. [DOI] [PubMed] [Google Scholar]
- Kinsey W. H., Rubin J. A., Lennarz W. J. Studies on the specificity of sperm binding in echinoderm fertilization. Dev Biol. 1980 Jan;74(1):245–250. doi: 10.1016/0012-1606(80)90067-6. [DOI] [PubMed] [Google Scholar]
- König B., Arendt A., McDowell J. H., Kahlert M., Hargrave P. A., Hofmann K. P. Three cytoplasmic loops of rhodopsin interact with transducin. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6878–6882. doi: 10.1073/pnas.86.18.6878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lentz T. L., Hawrot E., Donnelly-Roberts D., Wilson P. T. Synthetic peptides in the study of the interaction of rabies virus and the acetylcholine receptor. Adv Biochem Psychopharmacol. 1988;44:57–71. [PubMed] [Google Scholar]
- Matsumura K., Aketa K. Proteasome (multicatalytic proteinase) of sea urchin sperm and its possible participation in the acrosome reaction. Mol Reprod Dev. 1991 Jun;29(2):189–199. doi: 10.1002/mrd.1080290215. [DOI] [PubMed] [Google Scholar]
- Minor J. E., Fromson D. R., Britten R. J., Davidson E. H. Comparison of the bindin proteins of Strongylocentrotus franciscanus, S. purpuratus, and Lytechinus variegatus: sequences involved in the species specificity of fertilization. Mol Biol Evol. 1991 Nov;8(6):781–795. doi: 10.1093/oxfordjournals.molbev.a040690. [DOI] [PubMed] [Google Scholar]
- Moy G. W., Vacquier V. D. Immunoperoxidase localization of bindin during the adhesion of sperm to sea urchin eggs. Curr Top Dev Biol. 1979;13(Pt 1):31–44. doi: 10.1016/s0070-2153(08)60688-2. [DOI] [PubMed] [Google Scholar]
- Nishioka D., Ward R. D., Poccia D., Kostacos C., Minor J. E. Localization of bindin expression during sea urchin spermatogenesis. Mol Reprod Dev. 1990 Nov;27(3):181–190. doi: 10.1002/mrd.1080270302. [DOI] [PubMed] [Google Scholar]
- Roe J. L., Farach H. A., Jr, Strittmatter W. J., Lennarz W. J. Evidence for involvement of metalloendoproteases in a step in sea urchin gamete fusion. J Cell Biol. 1988 Aug;107(2):539–544. doi: 10.1083/jcb.107.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol D. P., Earles B. J., Decker G. L., Lennarz W. J. Characterization of the sperm receptor on the surface of eggs of Strongylocentrotus purpuratus. Dev Biol. 1984 Aug;104(2):308–321. doi: 10.1016/0012-1606(84)90086-1. [DOI] [PubMed] [Google Scholar]
- SeGall G. K., Lennarz W. J. Chemical characterization of the component of the jelly coat from sea urchin eggs responsible for induction of the acrosome reaction. Dev Biol. 1979 Jul;71(1):33–48. doi: 10.1016/0012-1606(79)90080-0. [DOI] [PubMed] [Google Scholar]
- Summers R. G., Hylander B. L. Species-specificity of acrosome reaction and primary gamete binding in echinoids. Exp Cell Res. 1975 Nov;96(1):63–68. doi: 10.1016/s0014-4827(75)80037-1. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D. Handling, labeling, and fractionating sea urchin spermatozoa. Methods Cell Biol. 1986;27:15–40. doi: 10.1016/s0091-679x(08)60340-4. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Moy G. W. Isolation of bindin: the protein responsible for adhesion of sperm to sea urchin eggs. Proc Natl Acad Sci U S A. 1977 Jun;74(6):2456–2460. doi: 10.1073/pnas.74.6.2456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacquier V. D., Payne J. E. Methods for quantitating sea urchin sperm-egg binding. Exp Cell Res. 1973 Nov;82(1):227–235. doi: 10.1016/0014-4827(73)90265-6. [DOI] [PubMed] [Google Scholar]
- Vacquier V. D., Tegner M. J., Epel D. Protease released from sea urchin eggs at fertilization alters the vitelline layer and aids in preventing polyspermy. Exp Cell Res. 1973 Jul;80(1):111–119. doi: 10.1016/0014-4827(73)90281-4. [DOI] [PubMed] [Google Scholar]
- Ward G. E., Brokaw C. J., Garbers D. L., Vacquier V. D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985 Dec;101(6):2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G., Jurman M. E., Abramson T., MacKinnon R. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 1991 Feb 22;251(4996):939–942. doi: 10.1126/science.2000494. [DOI] [PubMed] [Google Scholar]
- Yool A. J., Schwarz T. L. Alteration of ionic selectivity of a K+ channel by mutation of the H5 region. Nature. 1991 Feb 21;349(6311):700–704. doi: 10.1038/349700a0. [DOI] [PubMed] [Google Scholar]


