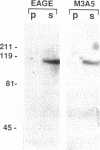
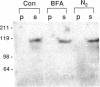
Abstract
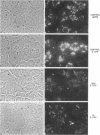
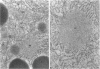
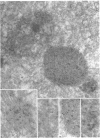
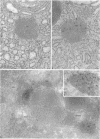
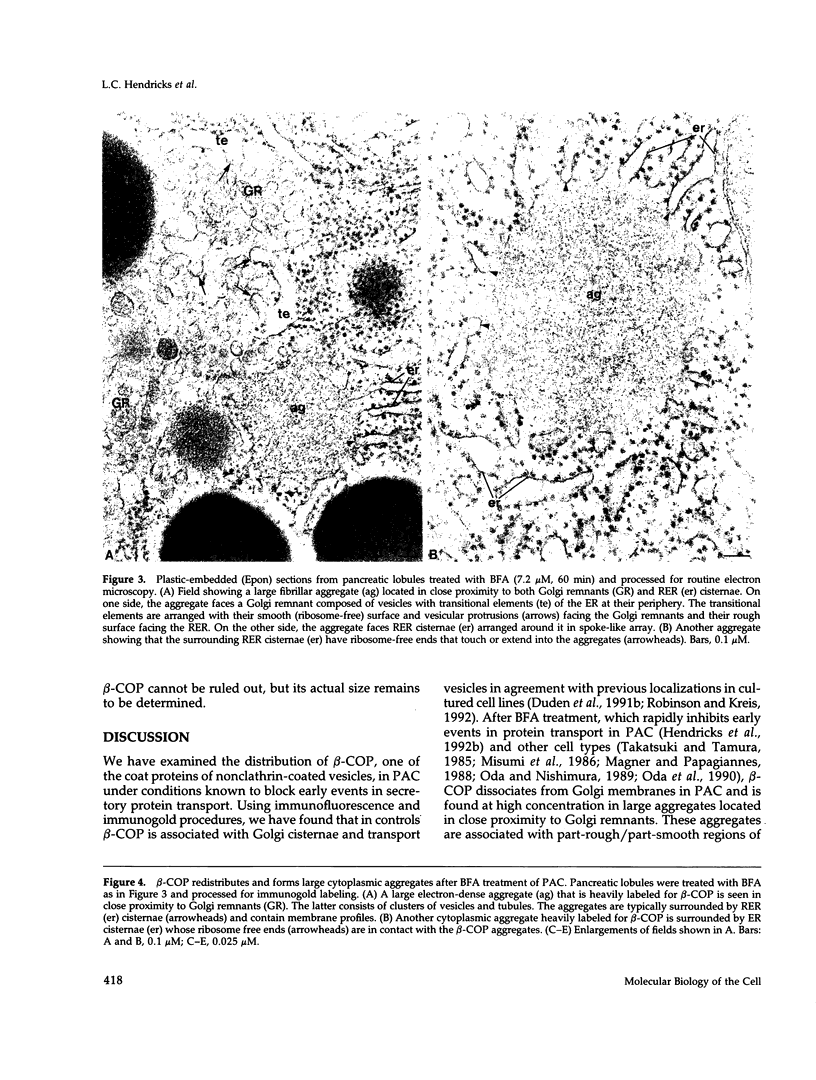
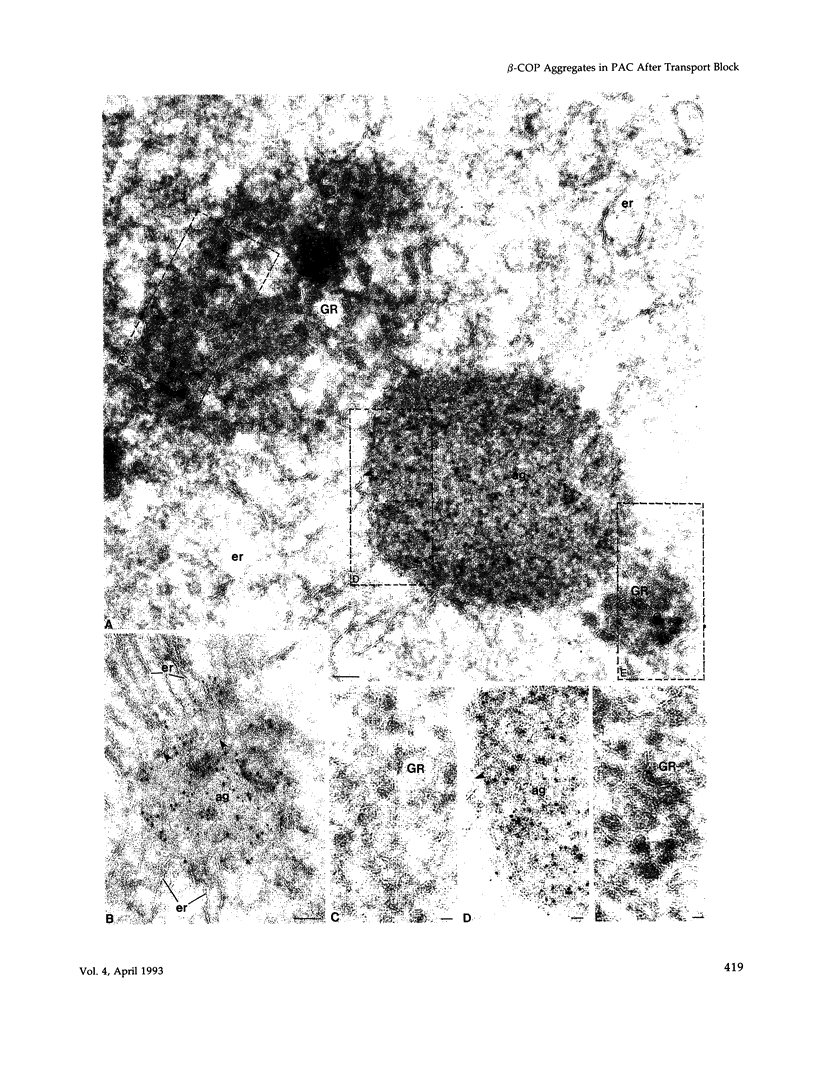
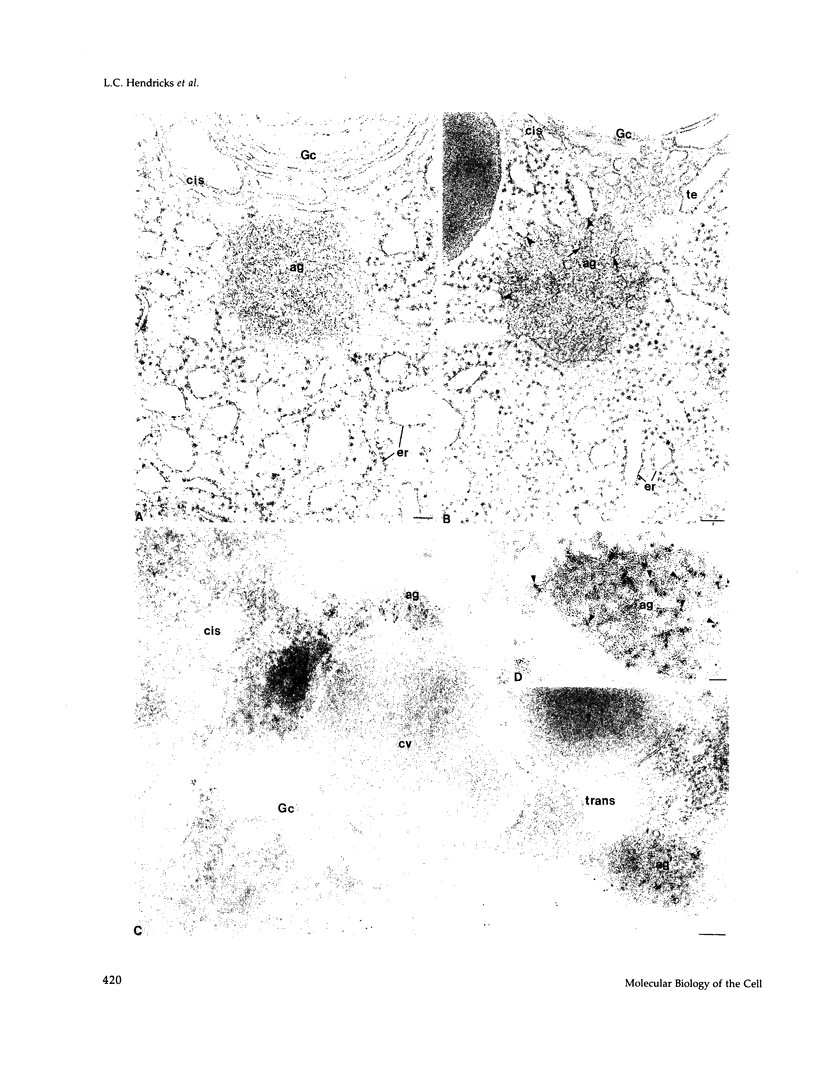
When transport between the rough endoplasmic reticulum (ER) and Golgi complex is blocked by Brefeldin A (BFA) treatment or ATP depletion, the Golgi apparatus and associated transport vesicles undergo a dramatic reorganization. Because recent studies suggest that coat proteins such as beta-COP play an important role in the maintenance of the Golgi complex, we have used immunocytochemistry to determine the distribution of beta-COP in pancreatic acinar cells (PAC) in which ER to Golgi transport was blocked by BFA treatment or ATP depletion. In controls, beta-COP was associated with Golgi cisternae and transport vesicles as expected. Upon BFA treatment, PAC Golgi cisternae are dismantled and replaced by clusters of remnant vesicles surrounded by typical ER transitional elements that are generally assumed to represent the exit site of vesicular carriers for ER to Golgi transport. In BFA-treated PAC, beta-COP was concentrated in large (0.5-1.0 micron) aggregates closely associated with remnant Golgi membranes. In addition to typical ER transitional elements, we detected a new type of transitional element that consists of specialized regions of rough ER (RER) with ribosome-free ends that touched or extended into the beta-COP containing aggregates. In ATP-depleted PAC, beta-COP was not detected on Golgi membranes but was concentrated in similar large aggregates found on the cis side of the Golgi stacks. The data indicate that upon arrest of ER to Golgi transport by either BFA treatment or energy depletion, beta-COP dissociates from PAC Golgi membranes and accumulates as large aggregates closely associated with specialized ER elements. The latter may correspond to either the site of entry or exit for vesicles recycling between the Golgi and the RER.
Full text
PDF


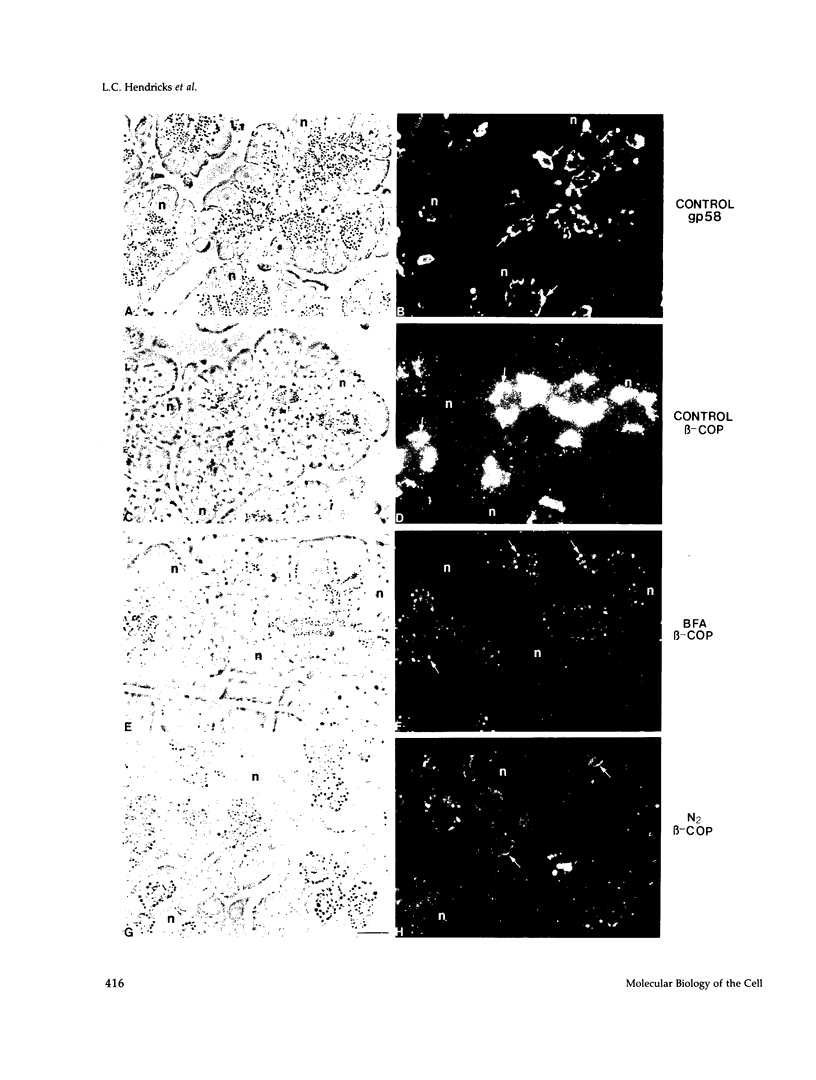
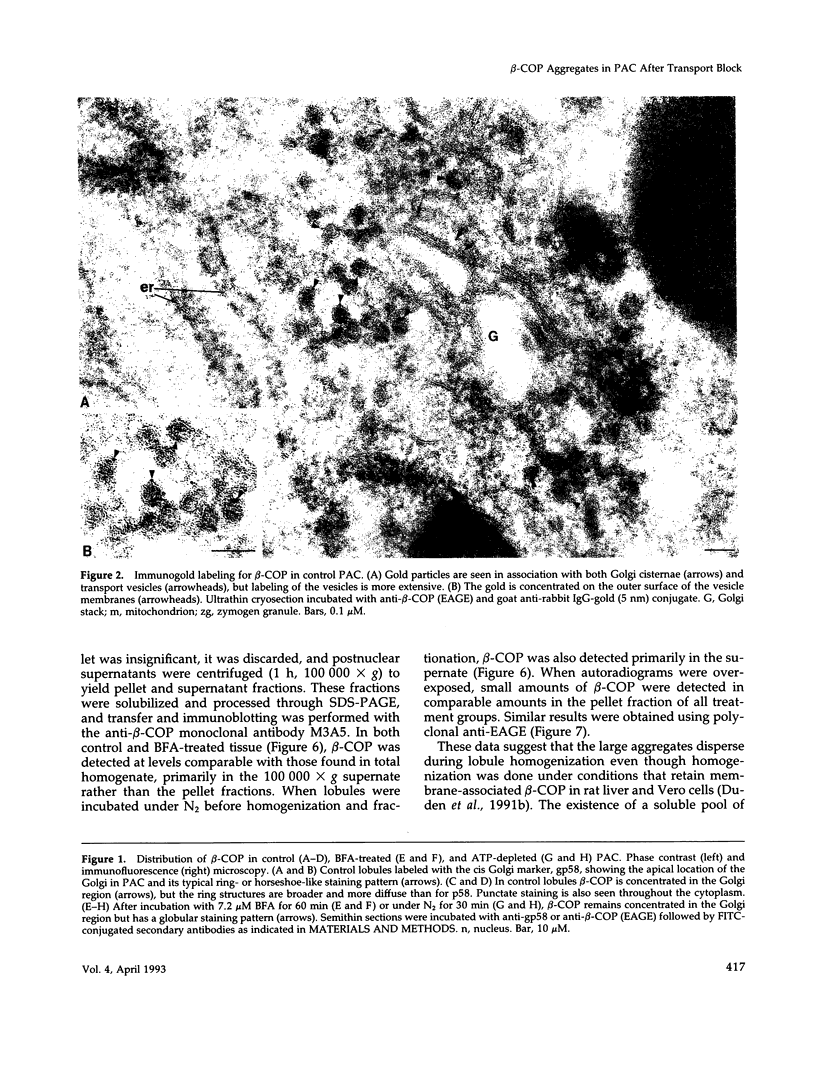




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan V. J., Kreis T. E. A microtubule-binding protein associated with membranes of the Golgi apparatus. J Cell Biol. 1986 Dec;103(6 Pt 1):2229–2239. doi: 10.1083/jcb.103.6.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lemos-Chiarandini C., Ivessa N. E., Black V. H., Tsao Y. S., Gumper I., Kreibich G. A Golgi-related structure remains after the brefeldin A-induced formation of an ER-Golgi hybrid compartment. Eur J Cell Biol. 1992 Aug;58(2):187–201. [PubMed] [Google Scholar]
- Donaldson J. G., Kahn R. A., Lippincott-Schwartz J., Klausner R. D. Binding of ARF and beta-COP to Golgi membranes: possible regulation by a trimeric G protein. Science. 1991 Nov 22;254(5035):1197–1199. doi: 10.1126/science.1957170. [DOI] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Bloom G. S., Kreis T. E., Klausner R. D. Dissociation of a 110-kD peripheral membrane protein from the Golgi apparatus is an early event in brefeldin A action. J Cell Biol. 1990 Dec;111(6 Pt 1):2295–2306. doi: 10.1083/jcb.111.6.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donaldson J. G., Lippincott-Schwartz J., Klausner R. D. Guanine nucleotides modulate the effects of brefeldin A in semipermeable cells: regulation of the association of a 110-kD peripheral membrane protein with the Golgi apparatus. J Cell Biol. 1991 Feb;112(4):579–588. doi: 10.1083/jcb.112.4.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duden R., Allan V., Kreis T. Involvement of beta-COP in membrane traffic through the Golgi complex. Trends Cell Biol. 1991 Jul;1(1):14–19. doi: 10.1016/0962-8924(91)90064-g. [DOI] [PubMed] [Google Scholar]
- Duden R., Griffiths G., Frank R., Argos P., Kreis T. E. Beta-COP, a 110 kd protein associated with non-clathrin-coated vesicles and the Golgi complex, shows homology to beta-adaptin. Cell. 1991 Feb 8;64(3):649–665. doi: 10.1016/0092-8674(91)90248-w. [DOI] [PubMed] [Google Scholar]
- Farquhar M. G., Palade G. E. The Golgi apparatus (complex)-(1954-1981)-from artifact to center stage. J Cell Biol. 1981 Dec;91(3 Pt 2):77s–103s. doi: 10.1083/jcb.91.3.77s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendricks L. C., Gabel C. A., Suh K., Farquhar M. G. A 58-kDa resident protein of the cis Golgi cisterna is not terminally glycosylated. J Biol Chem. 1991 Sep 15;266(26):17559–17565. [PubMed] [Google Scholar]
- Hendricks L. C., McClanahan S. L., McCaffery M., Palade G. E., Farquhar M. G. Golgi proteins persist in the tubulovesicular remnants found in brefeldin A-treated pancreatic acinar cells. Eur J Cell Biol. 1992 Aug;58(2):202–213. [PubMed] [Google Scholar]
- Hendricks L. C., McClanahan S. L., Palade G. E., Farquhar M. G. Brefeldin A affects early events but does not affect late events along the exocytic pathway in pancreatic acinar cells. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7242–7246. doi: 10.1073/pnas.89.15.7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo J., Garcia-Navarro R., Gracia-Navarro F., Perez-Vilar J., Velasco A. Presence of Golgi remnant membranes in the cytoplasm of brefeldin A-treated cells. Eur J Cell Biol. 1992 Aug;58(2):214–227. [PubMed] [Google Scholar]
- Hobman T. C., Woodward L., Farquhar M. G. The rubella virus E1 glycoprotein is arrested in a novel post-ER, pre-Golgi compartment. J Cell Biol. 1992 Aug;118(4):795–811. doi: 10.1083/jcb.118.4.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. I. Role of the peripheral elements of the Golgi complex. J Cell Biol. 1967 Aug;34(2):577–596. doi: 10.1083/jcb.34.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. II. Transport to condensing vacuoles and zymogen granules. J Cell Biol. 1967 Aug;34(2):597–615. doi: 10.1083/jcb.34.2.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson J. D., Palade G. E. Intracellular transport of secretory proteins in the pancreatic exocrine cell. IV. Metabolic requirements. J Cell Biol. 1968 Dec;39(3):589–603. doi: 10.1083/jcb.39.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser C. A., Schekman R. Distinct sets of SEC genes govern transport vesicle formation and fusion early in the secretory pathway. Cell. 1990 May 18;61(4):723–733. doi: 10.1016/0092-8674(90)90483-u. [DOI] [PubMed] [Google Scholar]
- Klausner R. D., Donaldson J. G., Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992 Mar;116(5):1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Donaldson J. G., Schweizer A., Berger E. G., Hauri H. P., Yuan L. C., Klausner R. D. Microtubule-dependent retrograde transport of proteins into the ER in the presence of brefeldin A suggests an ER recycling pathway. Cell. 1990 Mar 9;60(5):821–836. doi: 10.1016/0092-8674(90)90096-w. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Merisko E. M., Farquhar M. G., Palade G. E. Redistribution of clathrin heavy and light chains in anoxic pancreatic acinar cells. Pancreas. 1986;1(2):110–123. doi: 10.1097/00006676-198603000-00002. [DOI] [PubMed] [Google Scholar]
- Merisko E. M., Fletcher M., Palade G. E. The reorganization of the Golgi complex in anoxic pancreatic acinar cells. Pancreas. 1986;1(2):95–109. doi: 10.1097/00006676-198603000-00001. [DOI] [PubMed] [Google Scholar]
- Misumi Y., Misumi Y., Miki K., Takatsuki A., Tamura G., Ikehara Y. Novel blockade by brefeldin A of intracellular transport of secretory proteins in cultured rat hepatocytes. J Biol Chem. 1986 Aug 25;261(24):11398–11403. [PubMed] [Google Scholar]
- Oda K., Fujiwara T., Ikehara Y. Brefeldin A arrests the intracellular transport of viral envelope proteins in primary cultured rat hepatocytes and HepG2 cells. Biochem J. 1990 Jan 1;265(1):161–167. doi: 10.1042/bj2650161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda K., Nishimura Y. Brefeldin A inhibits the targeting of cathepsin D and cathepsin H to lysosomes in rat hepatocytes. Biochem Biophys Res Commun. 1989 Aug 30;163(1):220–225. doi: 10.1016/0006-291x(89)92124-4. [DOI] [PubMed] [Google Scholar]
- Orci L., Ravazzola M., Meda P., Holcomb C., Moore H. P., Hicke L., Schekman R. Mammalian Sec23p homologue is restricted to the endoplasmic reticulum transitional cytoplasm. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8611–8615. doi: 10.1073/pnas.88.19.8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. Intracellular aspects of the process of protein synthesis. Science. 1975 Aug 1;189(4200):347–358. doi: 10.1126/science.1096303. [DOI] [PubMed] [Google Scholar]
- Robinson M. S., Kreis T. E. Recruitment of coat proteins onto Golgi membranes in intact and permeabilized cells: effects of brefeldin A and G protein activators. Cell. 1992 Apr 3;69(1):129–138. doi: 10.1016/0092-8674(92)90124-u. [DOI] [PubMed] [Google Scholar]
- Saraste J., Palade G. E., Farquhar M. G. Antibodies to rat pancreas Golgi subfractions: identification of a 58-kD cis-Golgi protein. J Cell Biol. 1987 Nov;105(5):2021–2029. doi: 10.1083/jcb.105.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheele G. Pancreatic lobules in the in vitro study of pancreatic acinar cell function. Methods Enzymol. 1983;98:17–28. doi: 10.1016/0076-6879(83)98135-1. [DOI] [PubMed] [Google Scholar]
- Serafini T., Stenbeck G., Brecht A., Lottspeich F., Orci L., Rothman J. E., Wieland F. T. A coat subunit of Golgi-derived non-clathrin-coated vesicles with homology to the clathrin-coated vesicle coat protein beta-adaptin. Nature. 1991 Jan 17;349(6306):215–220. doi: 10.1038/349215a0. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Sex, maps, and imprinting. Cell. 1991 Jan 11;64(1):1–3. doi: 10.1016/0092-8674(91)90199-9. [DOI] [PubMed] [Google Scholar]
- Tokuyasu K. T. Use of poly(vinylpyrrolidone) and poly(vinyl alcohol) for cryoultramicrotomy. Histochem J. 1989 Mar;21(3):163–171. doi: 10.1007/BF01007491. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Effects of Brefeldin A on the Golgi complex, endoplasmic reticulum and viral envelope glycoproteins in murine erythroleukemia cells. Eur J Cell Biol. 1991 Feb;54(1):38–54. [PubMed] [Google Scholar]
- Waters M. G., Serafini T., Rothman J. E. 'Coatomer': a cytosolic protein complex containing subunits of non-clathrin-coated Golgi transport vesicles. Nature. 1991 Jan 17;349(6306):248–251. doi: 10.1038/349248a0. [DOI] [PubMed] [Google Scholar]