Abstract
In the present event-related potential (ERP) study we investigated the neural and temporal dynamics of motor imagery in participants with right-sided hemiparetic cerebral palsy (HCP; n = 10) and in left-handed control participants (n = 10). A mental rotation task was used in which participants were required to judge the laterality of hand pictures. At a behavioral level participants with HCP were slower in making hand laterality judgments compared to control subjects, especially when presented with pictures representing the affected hand. At a neural level, individuals with HCP were characterized by a reduced rotation-related negativity (RRN) over parietal areas, that was delayed in onset with respect to control participants. Interestingly, participants that were relatively mildly impaired showed a stronger RRN for the rotation of right-hand stimuli than participants that were more strongly impaired in their motor function, suggesting a direct relation between the motor imagery process and the biomechanical constraints of the participant. Together, the results provide new insights in the relation between motor imagery and motor capabilities and indicate that participants with HCP may be characterized by a compromised ability to use motor imagery.
Keywords: hemiparetic cerebral palsy, motor imagery, event-related brain potentials, rotation-related negativity, posterior parietal cortex
Introduction
In our daily lives we often perform actions, such as drinking from a cup or grasping a cup to catch a fly, without giving the underlying processes involved much further thought. Only in the case of action planning deficits the need to anticipate the consequences of our actions becomes unmistakably clear (Steenbergen et al., 2009). For instance, grasping a cup to catch a fly requires the selection of a different handgrip than grasping a cup to drink (i.e., a failure to select the appropriate inverted handgrip would result in smashing the fly, rather than catching it). As the example illustrates we often plan our actions ahead, based on the desired outcome or the end-state of the action (Rosenbaum et al., 1992).
Recent studies indicate that individuals with hemiparetic cerebral palsy (HCP), in addition to having problems with motor execution (Bax et al., 2005), are characterized by action planning deficits as well (Steenbergen and Gordon, 2006; Steenbergen et al., 2009). For instance, in contrast to controls who adapted the start posture of their hand to the upcoming movement (Rosenbaum et al., 1992), individuals with HCP had a preference to use a comfortable start position, also when this resulted in an awkward end posture (Mutsaarts et al., 2005; Craje et al., 2010). In addition, it has been found that participants with HCP often failed to anticipate the fingertip forces required for smoothly grasping an object (Duff and Gordon, 2003). Other studies indicate that in people with HCP grasping kinematics toward the target object were – in contrast to control participants – not influenced by later task demands (Steenbergen et al., 2004; Chen and Yang, 2007). Several studies have indicated that action planning deficits are especially apparent in right-sided HCP (i.e., impairment of the right side of the body; Mutsaarts et al., 2007; Craje et al., 2009), in line with the proposed role of the left hemisphere in action planning (Haaland and Harrington, 1996; Vingerhoets, 2008).
Problems with action planning may be related to difficulties with motor imagery, i.e., the ability to use an internal simulation of bodily movements without over motor execution to predict the consequences of an upcoming action (Jeannerod and Frak, 1999; Johnson, 2000). As the example with the cup illustrates, we often plan our actions ahead based on the desired outcome of the action. Anticipatory action planning likely requires the use of a forward model of the upcoming action that takes into account the final goal of the action and the movement constraints of the body. The involvement of motor imagery in action planning is corroborated by several studies showing that motor imagery activates comparable brain areas as used during action planning and execution (e.g., Zacks, 2008). In addition, computational approaches have underlined the importance of internal forward models for motor control as well (Wolpert, 1997). Accordingly, action planning deficits in individuals with HCP may be related to an impaired ability to use motor imagery.
Motor imagery is often studied by using Parson's classical hand rotation paradigm in which participants judge the laterality of pictures, representing left or right hands in different rotation angles (Parsons, 1994). Typically, reaction times and error rates increase as a function of the rotation angle of the stimulus, suggesting that participants engage in a cognitive process of mental rotation. In line with the notion of a compromised motor imagery ability in individuals with HCP, Mutsaarts et al. (2007) showed a linear increase in reaction times as a function of rotation angle in participants with left HCP but not in participants with right HCP, suggesting that motor imagery may be specifically impaired in right HCP. However, other studies have failed to replicate these findings, showing a linear reaction time increase for both control and HCP participants (Steenbergen et al., 2007; Martins et al., 2009). In sum, the evidence that action planning deficits in CP arise due to problems with motor imagery is still inconclusive.
One possible explanation for the apparent discrepancy between the different studies may be that reaction times only reflect the outcome of the cognitive process of performing the task (identifying whether a stimulus represents a left or a right hand) rather than the motor imagery process itself. Because overall participants with HCP respond relatively slow, it is unclear at what stage the motor imagery process is disrupted (e.g., initial recognition of hand orientation, mental rotation of hands, or response selection). Because of its high temporal resolution, electroencephalogram (EEG) provides an excellent opportunity to capture the time course of motor imagery and can thereby provide better insight in the functional and neural dynamics of motor imagery in CP. Previous studies have indicated that the mental hand rotation task implicitly triggers a motor imagery process, as evidenced by the sensitivity of reaction times to postural constraints (Parsons, 1994; de Lange et al., 2006) and by neuroimaging studies showing increased activation in parietal and supplementary motor areas during execution of this task (for review, see de Lange et al., 2008b). A robust finding in EEG studies on the mental hand rotation task is the rotation-related negativity (RRN), which is a parietal negative slow wave that is stronger for rotated compared to upright stimuli (for review, see Heil, 2002). Typically, the stronger parietal negativity for rotated compared to upright hand stimuli is interpreted as a neural correlate of the actual mental rotation process, likely reflecting a stronger activation of the parietal cortex for rotated stimuli (Wijers et al., 1989; Heil, 2002; Thayer and Johnson, 2006; Riecansky and Jagla, 2008). Accordingly, impaired motor imagery of participants with right-sided HCP may be reflected in a reduced and/or delayed RRN.
Materials and Methods
Participants
A total of 20 participants participated in the study. The group consisted of 10 participants diagnosed with right HCP (left brain damage, three females, mean age = 18.3 years, SD = 1.2 years) and 10 neurologically healthy control participants (two males, mean age = 19.7 years, SD = 2.2 years). Participants with HCP were recruited via their school (a school for special education Mariëndael in Arnhem, the Netherlands), or via the Dutch society of parents of physically disabled children (“BOSK”). Only limited individual information on the individual neuropathology and the brain areas affected was available. To obtain insight in the clinical picture of each participant, relevant tests were performed related to hand function. Hand function was tested using the Box and Blocks test (gross dexterity; Mathiowetz et al., 1985) and the Purdue Pegboard test (fine dexterity; Tiffin, 1985) for both the impaired and unimpaired hand. The severity of the paresis was estimated by calculating the ratio between the score of the impaired and the unimpaired hand. Accordingly, a score near 1 indicates that hand function among both hands is comparable (i.e., mild paresis), whereas a score near 0 indicates a strong difference between the impaired and unimpaired hand (i.e., severe paresis). Control participants were students from the Radboud University Nijmegen, who participated for course credits or an experimental fee. All participants were left-handed, as assessed by an online inventory for handedness. All participants gave informed consent prior to the experiment. The study was approved by the local ethics committee, in accordance with the declaration of Helsinki.
Stimuli
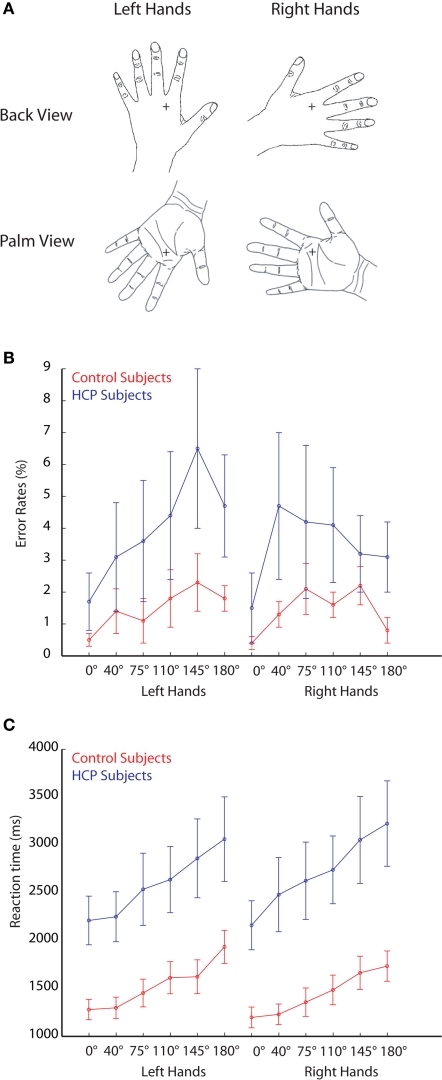
Stimuli consisted of line drawings from left and right hands viewed from the palm and from the back with different rotation angles (0°, 40°, 75°, 110°, 145°, 180°, 215°, 250°, 285°, 320°; see example stimuli in Figure 1). The stimuli were displayed on a 19′ computer screen in front of the subject, resulting in a visual angle of approximately 2°.
Figure 1.
Stimuli and behavioral performance. (A) Example stimuli used in the experiment representing left and right hands. (B) Error rates during the mental rotation of hand stimuli for control participants (red lines) and HCP participants (blue lines). (C) Reaction times during the mental rotation of hand stimuli for control participants (red lines) and HCP participants (blue lines). Error bars represent standard errors.
Experimental procedure
Participants were seated in an electrically and sound-shielded room in front of a computer screen and the participants’ left hand rested on a button box with two response keys. At the beginning of each trial a fixation cross was presented for 2–3 s, followed by the presentation of a line drawing of a left or a right hand in varying degrees of rotation. Participants were instructed to indicate whether the picture on the screen represented a left or a right hand, by pressing the left or the right response key of the button box with their left hand. After the participant responded, the picture was replaced by a fixation cross, indicating the start of the next trial. At the beginning of the experiment we checked carefully if each participant correctly understood the task, by presenting several example stimuli and asking the participant to verbally report the laterality of the hand observed. Laterality of the hand, view of the hand and rotation of the hand was varied randomly from trial to trial. Participants completed two experimental blocks, which consisted of 200 trials. Between blocks the participant rested. In total each subject performed 400 trials (2 hands × 2 views × 10 rotation angles × 10 repetitions) which took about 60 min. The experiment was controlled by a computer running Presentation 12.2.09 (Neurobehavioral Systems, Albany, USA) and markers for stimulus onset and the participant's responses were sent to a separate computer used for EEG recording.
EEG measurements
The EEG was recorded using 61 active electrodes that were placed in an actiCAP (Brain Products, Munich, Germany). Electrode positions were based on the M-10 Equidistant 61-Channel-Arrangement, with an inter-electrode distance of 37 ± 3 mm (given a head circumference of 58 cm). All electrodes were referenced to the left mastoid online and re-referenced offline to the linked mastoids. The impedance of the electrodes was kept below 10 kOhm. EEG and EOG signals were amplified using two 32-channel BrainAmp DC EEG amplifiers. The signal was sampled at 500 Hz and filtered online with an 80-Hz high cut-off filter and a 10-s time constant.
Data analysis
Trials with incorrect responses or trials in which the reaction time exceeded the participant's mean by more than three standard deviations were excluded from analysis. Reaction times were analyzed using an ANOVA repeated measures analysis with Hand (Left, Right) and Rotation angle (0°, 40°, 75°, 110°, 145°, 180°)1 as within-subjects factor and Group (HCP participants, control participants) as between-subjects factor.
Event-related potentials (ERPs) were calculated relative to the onset of the picture from −200 to 800 ms (using a 100-ms pre-stimulus baseline). Trials with movement artifacts were excluded from analysis on the basis of careful visual inspection of the raw data. Ocular artifacts were corrected using a semi-automatic correction procedure based on the algorithm of Gratton et al. (1983). To increase the signal-to-noise ratio for the ERP analysis, stimuli with different rotation angles were collapsed in two different categories: stimuli with a small rotation angle (0°, 40°, 75°, 285°, and 320°) and stimuli with a large rotation angle (110°, 145°, 180°, 215°, and 250°). In line with previous neuroimaging studies on mental rotation, data was pooled over stimuli viewed from the backside and from the palm side (cf. Helmich et al., 2007, 2009; de Lange et al., 2008a). For each individual subject ERPs were calculated separately for left- and right-hand stimuli and for stimuli with a small and a large rotation angle, resulting in a total of 100 repetitions per condition. Grand average ERPs were obtained by averaging the data across participants for the individual stimuli.
To test for statistical significance, individual ERPs were exported over the time-interval and electrodes of interest. ERPs were analyzed using a repeated measures ANOVA with the factors Hand (Left, Right), Rotation angle (Small: 0°, 40°, 75°, 320°, and 285°; Large: 110°, 145°, 180°, 215°, and 250°) and Hemisphere (Left, Right) as within-subjects factors and Group (HCP participants, control participants) as a between-subjects factor.
Results
Behavioral results
Participant information is provided in Table 1. As can be seen, for three HCP participants performance of the affected hand approached or even outperformed the unaffected hand. One possibility is that these participants, that were relatively mildly impaired, compensated for their motor deficits by using an alternative strategy (e.g., moving the trunk instead of the arm; Michaelsen et al., 2004). Compensatory motor strategies in HCP patients are typically reflected in a different kinematic profile (Michaelsen et al., 2004; Raghavan et al., 2010). Although the present task was probably too insensitive to pick up these subtle differences, it still provided a general measure to differentiate HCP patients according to the severity of their motor impairments.
Table 1.
Participant information for participants with HCP.
| Part | Gen | Age | Box and Blocks | Purdue Pegboard | ||||
|---|---|---|---|---|---|---|---|---|
| UH | IH | Ratio | UH | IH | Ratio | |||
| 1 | M | 18.8 | 69 | 20 | 0.29 | 28 | 4 | 0.14 |
| 2 | F | 19.3 | 76 | 11 | 0.15 | 31 | 0 | 0 |
| 3 | M | 15.7 | 47 | 56 | 1.19 | 22 | 21 | 0.96 |
| 4 | M | 17.5 | 63 | 9 | 0.14 | 20 | 0 | 0.0 |
| 5 | M | 18.1 | 49 | 33 | 0.67 | 23 | 2 | 0.09 |
| 6 | M | 20.1 | 49 | 26 | 0.53 | 30 | 0 | 0 |
| 7 | F | 18.4 | 47 | 18 | 0.38 | 32 | 0 | 0.0 |
| 8 | M | 21.1 | 57 | 60 | 1.05 | 22 | 23 | 1.05 |
| 9 | M | 17.8 | 56 | 60 | 1.07 | 27 | 20 | 0.74 |
| 10 | F | 19.3 | 49 | 16 | 0.33 | 28 | 0 | 0.0 |
Gen, gender; IH, impaired hand; UH, unimpaired hand; Ratio, score (impaired hand)/score (unimpaired hand).
During the experiment, participants were required to judge whether the presented picture was a left or a right hand, irrespective of its rotation angle or whether the palm or the back of the hand was shown. Analysis of the error rates showed that participants made more errors with increased rotation angle, F(5,14) = 4.9, p < 0.01 (see Figure 1). Participants were equally proficient in judging rotated images of left hands (error rate: 4.0 ± 1.4%) and right hands (error rate: 4.1 ± 1.5%). No difference was found in error rates between control participants (error rate: 4.3 ± 1.0%) and HCP participants (error rate: 11.2 ± 4.9%).
Reaction times increased with increasing rotation angle, F(5,14) = 10.4, p < 0.001 (see Figure 1). No significant difference was found in reaction times between the rotation of left and right hands [mean RT left hands: 2061 ms, mean RT right hands: 2077 ms; F(1,18) < 1]. An interaction between hand and group reflected that control participants were relatively faster in responding to right hand compared to left hand stimuli, whereas HCP participants were relatively faster in responding to left hand compared to right-hand stimuli, F(1,18) = 6.0, p < 0.05 (see Figure 1). Overall, participants with HCP responded slower (mean RT = 2651 ms) than control participants (1487 ms), F(1,18) = 9.6, p < 0.01.
Event-related potentials
The onset of the stimulus resulted in a pattern of visual components and slow waves. P1 (peak at 90 ms), N1 (peak at 130 ms), and N2 (peak at 280 ms) were found at bilateral occipital sites. P2 (peak at 180 ms) was found over frontal sites. From about 300 ms onward a positive slow wave developed that was found maximal above parietal cortex.
ERP effects of stimulus rotation
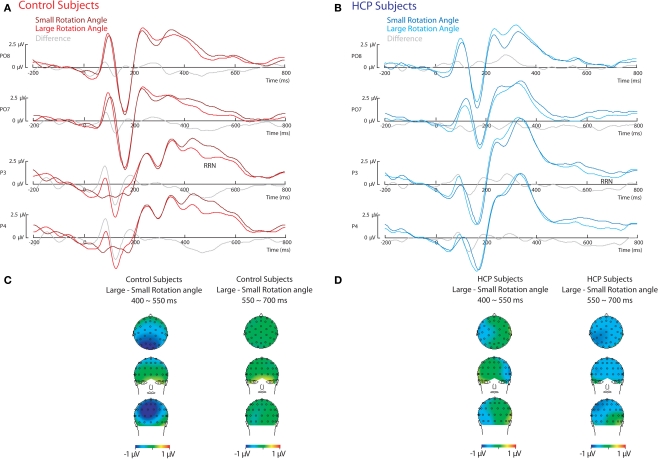
As can be seen in Figure 2, for both control and HCP participants, effects of stimulus rotation became apparent in a stronger parietal negativity for stimuli with a large compared to a small rotation angle (RRN). However, for control participants the RRN was observed from about 400 to 550 ms, whereas for hemiparetic participants the RRN was observed from about 550 to 700 ms. Because of these differences in timing two separate ANOVAs were conducted.
Figure 2.
Event-related potential (ERP) effects of rotation angle. ERPs relative to stimulus onset during the mental rotation of hands for control participants (A) and HCP participants (B). Scalp topography of the difference between stimuli with a large and a small rotation angle for control participants (C) and HCP participants (D).
First, from 400 to 550 ms effects of stimulus rotation became apparent in a stronger RRN over parietal sites for stimuli with a large compared to a small rotation, F(1,18) = 9.5, p < 0.01. An interaction between Group and Rotation indicated that the RRN was stronger for control compared to HCP participants in this early time-window, F(1,18) = 4.8, p < 0.05 (see Figure 2).
Second, from 550 to 700 ms, effects of stimulus rotation also became apparent in a RRN over parietal sites for stimuli with a large compared to a small rotation angle, F(1,18) = 6.9, p < 0.05. An interaction between Group and Hemisphere reflected that control participants showed a stronger negativity over the right hemisphere, whereas for HCP participants no lateralization was observed, F(1,18) = 10.9, p < 0.01. No interaction between Group and Rotation was observed in this later time-window.
To directly compare the RRN between both groups, in an additional analysis the early RRN (400–550 ms) for control participants was compared with the late RRN (550–700 ms) for participants with HCP. A main effect of Group, F(1,18) = 9.4, p < 0.01, indicated that the early RRN for control participants was stronger than the late RRN for participants with HCP.
Correlation analysis
To investigate the relation between the severity of motor impairments and the ERP effects of motor imagery, a correlation analysis was conducted. Correlations were calculated between the RRN for left- and right-hand stimuli and hemiparetic participants’ scores on the Box and Blocks test and the Purdue Pegboard task. Only significant correlations will be reported.
As can be seen in Table 2, significant correlations were observed between the score on the Box and Blocks test and the early RRN for right-hand stimuli, for both the left hemisphere, r = 0.712, p < 0.05 and the right hemisphere, r = 0.691, p < 0.05. In addition, significant correlations were observed between the score on the Purdue Pegboard task and the early RRN for right-hand stimuli for the left hemisphere, r = 0.646, p < 0.05 and a marginally significant effect for the right hemisphere, r = 0.601, p = 0.066. These correlations reflect that participants that were only mildly impaired with their right hand showed a stronger early RRN for right-hand stimuli than participants that were more strongly impaired.
Table 2.
Correlation analysis.
| Pearson΄s r | p | |
|---|---|---|
| Ratio Box and Blocks × RRN left hands LH | −0.401 | 0.251 |
| Ratio Box and Blocks × RRN left hands RH | −0.142 | 0.695 |
| Ratio Box and Blocks × RRN right hands LH | 0.712 | 0.021 |
| Ratio Box and Blocks × RRN right hands RH | 0.691 | 0.027 |
| Ratio Purdue Pegboard × RRN left hands LH | −0.351 | 0.320 |
| Ratio Purdue Pegboard × RRN left hands RH | −0.092 | 0.801 |
| Ratio Purdue Pegboard × RRN right hands LH | 0.646 | 0.044 |
| Ratio Purdue Pegboard × RRN right hands RH | 0.601 | 0.066 |
Correlations between the score on the Box and Blocks test and the Purdue Pegboard test and the rotation-related negativity (RRN) for left and right-hand stimuli. Correlations were calculated separately for electrodes overlying the left hemisphere (LH) and the right hemisphere (RH).
Discussion
In the present study we investigated the functional and neural dynamics of motor imagery in participants with right-sided HCP. Our findings support the notion that participants with HCP may be characterized by an impaired motor imagery process. First, participants with HCP responded slower to stimuli representing the affected compared to the unaffected hand. Second, the ERP data indicated a reduced and delayed RRN for HCP compared to control participants. Finally, participants that were relatively mildly impaired showed a stronger RRN during the mental rotation of right hands, than participants that were more severely impaired.
The behavioral data showed a linear increase in reaction times for stimuli with an increased rotation angle for both control and HCP participants, thereby replicating previous findings (Steenbergen et al., 2007; Martins et al., 2009). Overall, participants with HCP responded slower than control participants. In addition, participants with HCP responded relatively slower to stimuli representing right hands compared to left hands. These findings suggest that the mental rotation process is partly affected by the biomechanical constraints of the subject, with slower responding to stimuli representing the affected hand (cf. Mutsaarts et al., 2007). Thereby the behavioral data suggest that participants with HCP may be characterized by a compromised process of motor imagery.
Interestingly, left-handed control subjects appeared to respond faster to stimuli representing right hands than to left hands. Whereas for right-handed subjects typically a reaction time advantage has been observed for the mental rotation of the dominant hand (cf. Parsons, 1987; Gentilucci et al., 1998; Ionta et al., 2007), left-handed subjects did not show this reaction time advantage for the dominant hand (cf. Gentilucci et al., 1998; Ionta and Blanke, 2009). One possibility is that because many tools and objects are specifically designed for right-handed people (e.g., video cameras, car keys etc.) left-handers are often forced to use their non-dominant hand, thereby possibly leading to greater bimanuality in left-handers.
At a neural level, both control and HCP participants showed a RRN during the mental rotation of hands. Interestingly, in participants with HCP the RRN was reduced in amplitude and was delayed in onset compared to control participants. The RRN is a classical finding in mental rotation studies and likely reflects a stronger activation of the posterior parietal cortex for stimuli with an increased rotation angle (Wijers et al., 1989; Heil, 2002; Riecansky and Jagla, 2008). Many studies have shown that the posterior parietal cortex is activated during motor imagery (for review, see Zacks, 2008). For instance, the intraparietal sulcus (IPS) was found activated during the mental rotation of hands and activation in this area increased linearly for stimuli with increased rotation angle (de Lange et al., 2005). Accordingly, the reduced and delayed RRN for HCP participants may reflect an impaired motor imagery process in participants with HCP.
This suggestion is further supported by the finding that participants that were relatively mildly impaired with their right hand showed a stronger RRN during the mental rotation of right hands than participants that were more strongly impaired. This finding suggests that the motor imagery process is partly affected by the biomechanical constraints of the participant (see also Williams et al., 2008). Whereas previous behavioral studies using the mental hand rotation paradigm have provided only inconclusive evidence (Mutsaarts et al., 2007; Steenbergen et al., 2007; Martins et al., 2009), the present study likely reflects a specific impairment in the actual motor imagery process, that is partly affected by the biomechanical constraints of the subject as well.
Previous studies have reported an RRN both for the rotation of hand stimuli (Heil, 2002; Thayer and Johnson, 2006) and for the rotation of letter or numeric stimuli (Wijers et al., 1989; Riecansky and Jagla, 2008) and accordingly, the RRN has been interpreted as a neural correlate of the actual mental rotation process. A long debated issue in cognitive science concerns the question whether mental rotation can be accomplished with or without modality-specific processing, i.e., recruitment of the same brain areas as used for perception and action (for review, see Pylyshyn, 2002). Interestingly, the finding that the RRN for the mental rotation of hands is related to one's motor capabilities suggests that participants engaged in a motor imagery process related to the rotation of their own hands. This finding suggests a direct connection between the motor imagery process and the biomechanical constraints of the participant and is in line with recent studies that have shown comparable effects of motor impairments on both motor imagery, action observation and recognition of body parts (for review, see Corradi-Dell'Acqua and Tessari, 2010). For instance, in a recent study it was found that hemiplegic subjects showed an impairment in the recognition of actions that were performed with the affected body part (Serino et al., 2010). Together these studies support the notion that motor imagery is an embodied process, that recruits neural resources related to the control and execution of real actions (for similar findings, see Parsons, 1994; de Lange et al., 2006; Helmich et al., 2009).
A critical question is whether participants with right-sided HCP and left-handed control participants are matched in terms of cerebral dominance. One could argue that HCP participants had a genetic predisposition to become right-handed prior to their prenatal brain injuries. However, although genetic factors play some role in determining handedness, hand preference is determined by environmental factors as well (for review, see Llaurens et al., 2009). Because HCP participants were forced to use their left hands from birth onward, they are well matched with left-handed control participants in terms of motor experience. In addition, a recent study underlines the plasticity of the human motor system, by showing a comparable left-hemispheric lateralization of the central sulcus for adult “converted” left-handers – who had been forced as children to write with their right hand – as right-handers (Kloppel et al., 2010). Together these findings suggest that the comparison between left-handed control participants and subjects with HCP is warranted.
In the present study, only limited information on the individual neuropathology (i.e., onset and causes of brain injury; brain areas affected) of the HCP participants was available. Previous studies have shown large individual variability in terms of the brain areas affected in HCP, ranging from lesions in both gray and white matter, brain malformations to no detectable abnormalities at all (Wu et al., 2006; Korzeniewski et al., 2008). Still, the present study suggests that HCP participants – despite individual variability in neuropathology – are characterized by a functional deficit to use motor imagery. Moreover, the finding that the neural correlate of the motor imagery process (i.e., the RRN) was inversely related to the severity of the motor impairment opens interesting avenues for future studies that should address the relation between brain and behavior in more detail.
In sum, the main finding of the present study is that participants with HCP are characterized by a general motor impairment, accompanied by a compromised motor imagery process. More specifically, whereas previous studies using reaction time measures have provided only limited evidence for motor imagery impairments in HCP (Mutsaarts et al., 2007; Steenbergen et al., 2007; Martins et al., 2009), the present study provides better insight in the neural and temporal dynamics underlying motor imagery in HCP. The reduced RRN suggests a specific impairment in the actual motor imagery process (i.e., the mental rotation of hands), rather than in the initial recognition of hand stimuli (cf. Overney et al., 2005) or in the selection of a response (see however, van Elk et al., 2010). Thereby these findings extend previous studies that have suggested that action planning deficits in participants with HCP may be related to an impaired ability to use motor imagery (Steenbergen and Gordon, 2006; Mutsaarts et al., 2007).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
This study was supported by the Netherlands Organization for Scientific Research (grants 453-05-001 awarded to Harold Bekkering and grant 400-04-046 awarded to Celine Crajé). This paper was written during fellowships supported by The International Human Frontier Science Program Organization (grant ST000078/2009 to Michiel van Elk) and the Marie Curie Intra European Fellowship within the Seventh European Community Framework Program (IEF grant 252713 to Michiel van Elk).
Footnotes
1Please note that both clockwise and counter-clockwise rotations were included. Thus, for 40° stimuli were collapsed across 40° and 320°, for 75° stimuli were collapsed across 75° and 285° etc., resulting in 20 repetitions for 0° and 180° and 40 repetitions for the other rotation angles.
References
- Bax M., Goldstein M., Rosenbaum P., Leviton A., Paneth N., Dan B., Jacobsson B., Damiano D. (2005). Proposed definition and classification of cerebral palsy. Dev. Med. Child Neurol. 47, 571–576 10.1017/S001216220500112X [DOI] [PubMed] [Google Scholar]
- Chen Y. P., Yang T. F. (2007). Effect of task goals on the reaching patterns of children with cerebral palsy. J. Mot. Behav. 39, 317–324 10.3200/JMBR.39.4.317-325 [DOI] [PubMed] [Google Scholar]
- Corradi-Dell'Acqua C., Tessari A. (2010). Is the body in the eye of the beholder? Visual processing of bodies in individuals with anomalous anatomical sensory and motor features. Neuropsychologia 48, 689–702 10.1016/j.neuropsychologia.2009.11.029 [DOI] [PubMed] [Google Scholar]
- Craje C., van der Kamp J., Steenbergen B. (2009). Visual information for action planning in left and right congenital hemiparesis. Brain Res. 1261, 54–64 10.1016/j.brainres.2008.12.074 [DOI] [PubMed] [Google Scholar]
- Craje C., van Elk M., Beeren M., van Schie H. T., Bekkering H., Steenbergen B. (2010). Compromised motor planning and motor imagery in right hemiparetic cerebral palsy. Res. Dev. Disabil. 31, 1313–1322 10.1016/j.ridd.2010.07.010 [DOI] [PubMed] [Google Scholar]
- de Lange F. P., Hagoort P., Toni I. (2005). Neural topography and content of movement representations. J. Cogn. Neurosci. 17, 97–112 10.1162/0898929052880039 [DOI] [PubMed] [Google Scholar]
- de Lange F. P., Helmich R. C., Toni I. (2006). Posture influences motor imagery: an fMRI study. Neuroimage 33, 609–617 10.1016/j.neuroimage.2006.07.017 [DOI] [PubMed] [Google Scholar]
- de Lange F. P., Jensen O., Bauer M., Toni I. (2008a). Interactions between posterior gamma and frontal alpha/beta oscillations during imagined actions. Front. Hum. Neurosci. 2: 7. 10.3389/neuro.09.007.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange F. P., Roelofs K., Toni I. (2008b). Motor imagery: a window into the mechanisms and alterations of the motor system. Cortex 44, 494–506 10.1016/j.cortex.2007.09.002 [DOI] [PubMed] [Google Scholar]
- Duff S. V., Gordon A. M. (2003). Learning of grasp control in children with hemiplegic cerebral palsy. Dev. Med. Child Neurol. 45, 746–757 10.1111/j.1469-8749.2003.tb00884.x [DOI] [PubMed] [Google Scholar]
- Gentilucci M., Daprati E., Gangitano M. (1998). Right-handers and left-handers have different representations of their own hand. Brain Res. Cogn. Brain Res. 6, 185–192 10.1016/S0926-6410(97)00034-7 [DOI] [PubMed] [Google Scholar]
- Gratton G., Coles M. G., Donchin E. (1983). A new method for off-line removal of ocular artifact. Electroencephalogr. Clin. Neurophysiol. 55, 468–484 10.1016/0013-4694(83)90135-9 [DOI] [PubMed] [Google Scholar]
- Haaland K. Y., Harrington D. L. (1996). Hemispheric asymmetry of movement. Curr. Opin. Neurobiol. 6, 796–800 10.1016/S0959-4388(96)80030-4 [DOI] [PubMed] [Google Scholar]
- Heil M. (2002). The functional significance of ERP effects during mental rotation. Psychophysiology 39, 535–545 10.1017/S0048577202020449 [DOI] [PubMed] [Google Scholar]
- Helmich R. C., Aarts E., de Lange F. P., Bloem B. R., Toni I. (2009). Increased dependence of action selection on recent motor history in Parkinson's disease. J. Neurosci. 29, 6105–6113 10.1523/JNEUROSCI.0704-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmich R. C., de Lange F. P., Bloem B. R., Toni I. (2007). Cerebral compensation during motor imagery in Parkinson's disease. Neuropsychologia 45, 2201–2215 10.1016/j.neuropsychologia.2007.02.024 [DOI] [PubMed] [Google Scholar]
- Ionta S., Blanke O. (2009). Differential influence of hands posture on mental rotation of hands and feet in left and right handers. Exp. Brain Res. 195, 207–217 10.1007/s00221-009-1770-0 [DOI] [PubMed] [Google Scholar]
- Ionta S., Fourkas A. D., Fiorio M., Aglioti S. M. (2007). The influence of hands posture on mental rotation of hands and feet. Exp. Brain Res. 183, 1–7 10.1007/s00221-007-1020-2 [DOI] [PubMed] [Google Scholar]
- Jeannerod M., Frak V. (1999). Mental imaging of motor activity in humans. Curr. Opin. Neurobiol. 9, 735–739 10.1016/S0959-4388(99)00038-0 [DOI] [PubMed] [Google Scholar]
- Johnson S. H. (2000). Thinking ahead: the case for motor imagery in prospective judgements of prehension. Cognition 74, 33–70 10.1016/S0010-0277(99)00063-3 [DOI] [PubMed] [Google Scholar]
- Kloppel S., Mangin J. F., Vongerichten A., Frackowiak R. S., Siebner H. R. (2010). Nurture versus nature: long-term impact of forced right-handedness on structure of pericentral cortex and basal ganglia. J. Neurosci. 30, 3271–3275 10.1523/JNEUROSCI.4394-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korzeniewski S. J., Birbeck G., DeLano M. C., Potchen M. J., Paneth N. (2008). A systematic review of neuroimaging for cerebral palsy. J. Child Neurol. 23, 216–227 10.1177/0883073807307983 [DOI] [PubMed] [Google Scholar]
- Llaurens V., Raymond M., Faurie C. (2009). Why are some people left-handed? An evolutionary perspective. Philos. Trans. R. Soc. Lond., B, Biol. Sci. 364, 881–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martins I. C., de Olivieira A. M., Anorim M. A. (2009). My hands can't move like that: mental rotation of body parts in cerebral palsy subjects, in Paper Presented at the Cognitive Science 2009 Retrieved from http://csjarchive.cogsci.rpi.edu/Proceedings/2009/papers/163/index.html
- Mathiowetz V., Volland G., Kashman N., Weber K. (1985). Adult norms for the box and block test for manual dexterity. Am. J. Occup. Ther. 157, 162–173 [DOI] [PubMed] [Google Scholar]
- Michaelsen S. M., Jacobs S., Roby-Brami A., Levin M. F. (2004). Compensation for distal impairments of grasping in adults with hemiparesis. Exp. Brain Res. 157, 162–173 10.1007/s00221-004-1829-x [DOI] [PubMed] [Google Scholar]
- Mutsaarts M., Steenbergen B., Bekkering H. (2005). Anticipatory planning of movement sequences in hemiparetic cerebral palsy. Motor. Control. 9, 439–458 [DOI] [PubMed] [Google Scholar]
- Mutsaarts M., Steenbergen B., Bekkering H. (2007). Impaired motor imagery in right hemiparetic cerebral palsy. Neuropsychologia 45, 853–859 10.1016/j.neuropsychologia.2006.08.020 [DOI] [PubMed] [Google Scholar]
- Overney L. S., Michel C. M., Harris I. M., Pegna A. J. (2005). Cerebral processes in mental transformations of body parts: recognition prior to rotation. Brain Res. Cogn. Brain Res. 25, 722–734 10.1016/j.cogbrainres.2005.09.024 [DOI] [PubMed] [Google Scholar]
- Parsons L. M. (1987). Imagined spatial transformation of one's hands and feet. Cogn. Psychol. 19, 178–241 10.1016/0010-0285(87)90011-9 [DOI] [PubMed] [Google Scholar]
- Parsons L. M. (1994). Temporal and kinematic properties of motor behavior reflected in mentally simulated action. J. Exp. Psychol. Hum. Percept. Perform. 20, 709–730 10.1037/0096-1523.20.4.709 [DOI] [PubMed] [Google Scholar]
- Pylyshyn Z. W. (2002). Mental imagery: in search of a theory. Behav. Brain Sci. 25, 157–182; discussion 182–237. 10.1017/S0140525X02000043 [DOI] [PubMed] [Google Scholar]
- Raghavan P., Santello M., Gordon A. M., Krakauer J. W. (2010). Compensatory motor control after stroke: an alternative joint strategy for object-dependent shaping of hand posture. J. Neurophysiol. 103, 3034–3043 10.1152/jn.00936.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riecansky I., Jagla F. (2008). Linking performance with brain potentials: mental rotation-related negativity revisited. Neuropsychologia 46, 3069–3073 10.1016/j.neuropsychologia.2008.06.016 [DOI] [PubMed] [Google Scholar]
- Rosenbaum D. A., Vaughan J., Barnes H. J., Jorgensen M. J. (1992). Time course of movement planning: selection of handgrips for object manipulation. J. Exp. Psychol. Learn. Mem. Cogn. 18, 1058–1073 10.1037/0278-7393.18.5.1058 [DOI] [PubMed] [Google Scholar]
- Serino A., De Filippo L., Casavecchia C., Coccia M., Shiffrar M., Ladavas E. (2010). Lesions to the motor system affect action perception. J. Cogn. Neurosci. 22, 413–426 10.1162/jocn.2009.21206 [DOI] [PubMed] [Google Scholar]
- Steenbergen B., Craje C., Nilsen D. M., Gordon A. M. (2009). Motor imagery training in hemiplegic cerebral palsy: a potentially useful therapeutic tool for rehabilitation. Dev. Med. Child Neurol. 51, 690–696 10.1111/j.1469-8749.2009.03371.x [DOI] [PubMed] [Google Scholar]
- Steenbergen B., Gordon A. M. (2006). Activity limitation in hemiplegic cerebral palsy: evidence for disorders in motor planning. Dev. Med. Child Neurol. 48, 780–783 10.1017/S0012162206001666 [DOI] [PubMed] [Google Scholar]
- Steenbergen B., Meulenbroek R. G., Rosenbaum D. A. (2004). Constraints on grip selection in hemiparetic cerebral palsy: effects of lesional side, end-point accuracy, and context. Brain Res. Cogn. Brain Res. 19, 145–159 10.1016/j.cogbrainres.2003.11.008 [DOI] [PubMed] [Google Scholar]
- Steenbergen B., van Nimwegen M., Craje C. (2007). Solving a mental rotation task in congenital hemiparesis: motor imagery versus visual imagery. Neuropsychologia 45, 3324–3328 10.1016/j.neuropsychologia.2007.07.002 [DOI] [PubMed] [Google Scholar]
- Thayer Z. C., Johnson B. W. (2006). Cerebral processes during visuo-motor imagery of hands. Psychophysiology 43, 401–412 10.1111/j.1469-8986.2006.00404.x [DOI] [PubMed] [Google Scholar]
- Tiffin J. (1985). Purdue-Pegboard Examiner Manual. Chicago: Science Research Associates [Google Scholar]
- van Elk M., Craje C., Beeren M. E., Steenbergen B., van Schie H. T., Bekkering H. (2010). Neural evidence for impaired action selection in right hemiparetic cerebral palsy. Brain Res. 1349, 56–67 10.1016/j.brainres.2010.06.055 [DOI] [PubMed] [Google Scholar]
- Vingerhoets G. (2008). Knowing about tools: neural correlates of tool familiarity and experience. Neuroimage 40, 1380–1391 10.1016/j.neuroimage.2007.12.058 [DOI] [PubMed] [Google Scholar]
- Wijers A. A., Otten L. J., Feenstra S., Mulder G., Mulder L. J. (1989). Brain potentials during selective attention, memory search, and mental rotation. Psychophysiology 26, 452–467 10.1111/j.1469-8986.1989.tb01951.x [DOI] [PubMed] [Google Scholar]
- Williams J., Thomas P. R., Maruff P., Wilson P. H. (2008). The link between motor impairment level and motor imagery ability in children with developmental coordination disorder. Hum. Mov. Sci. 27, 270–285 10.1016/j.humov.2008.02.008 [DOI] [PubMed] [Google Scholar]
- Wolpert D. M. (1997). Computational approaches to motor control. Trends Cogn. Sci. 1, 209–216 10.1016/S1364-6613(97)01070-X [DOI] [PubMed] [Google Scholar]
- Wu Y. W., Lindan C. E., Henning L. H., Yoshida C. K., Fullerton H. J., Ferriero D. M., Barkovich A. J., Croen L. A. (2006). Neuroimaging abnormalities in infants with congenital hemiparesis. Pediatr. Neurol. 35, 191–196 10.1016/j.pediatrneurol.2006.03.002 [DOI] [PubMed] [Google Scholar]
- Zacks J. M. (2008). Neuroimaging studies of mental rotation: a meta-analysis and review. J. Cogn. Neurosci., 20, 1–19 10.1162/jocn.2008.20013 [DOI] [PubMed] [Google Scholar]