Abstract
Previous studies suggest that deficits in neural synchronization and temporal integration are characteristic of schizophrenia. These phenomena have been rarely studied in SPD, which shares phenomenological and genetic similarities with schizophrenia. Event-related potentials (ERPs) were obtained using an auditory oddball task from 21 patients with schizophrenia, 19 subjects with SPD and 19 healthy control subjects. Inter-trial coherence (ITC) and event-related spectral perturbation (ERSP) were measured across trials to target tones using time-frequency analysis. ITC measures phase locking or consistency, while ERSP measures changes in power relative to baseline activity. P300 latency and amplitude were also measured from the averaged ERP to target tones. In the time-frequency analysis, subjects with SPD showed intact power but a deficit in the ITC in delta and theta frequencies compared to control subjects. Patients with schizophrenia showed deficits for both ERSP and ITC in the delta and theta frequencies. While patients with schizophrenia showed reduced P300 amplitude and delayed latency for averaged ERPs, subjects with SPD did not differ from either group. Synchronization or timing abnormalities may represent a biomarker for schizophrenia spectrum disorders, and contribute to aberrant perceptual and cognitive integration.
Keywords: Schizophrenia, Schizotypal Personality Disorder, auditory event-related potentials, synchronization, time frequency analysis
1. Introduction
One of the most robust neurobiological findings in schizophrenia is reduction in the amplitude of the P300 component of auditory event-related potential (ERP) (Ford et al., 1999; Jeon and Polich, 2003; Roth et al., 1980). The P300 component, a positive deflection in the electroencephalogram (EEG) elicited by infrequent target stimuli interspersed in a series of more frequent stimuli, has been linked to a variety of cognitive processes involved in stimulus discrimination, including selective attention, expectancies, and contextual updating (Donchin and Coles, 1988; Polich and Kok, 1995; Walhovd and Fjell, 2003). Twin and family studies indicate that P300 amplitude has a heritability of about 60% (van Beijsterveldt and van Baal, 2002) and first degree relatives of probands with schizophrenia have been reported to show reduced P300 amplitude as well (Turetsky et al., 2007). These findings suggest that the auditory P300 has potential as a neurophysiological endophenotypic of schizophrenia (Turetsky et al., 2007).
While the auditory P300 component is consistently affected in schizophrenia, only a few studies have examined P300 in Schizotypal Personality Disorder (SPD). SPD shares common symptoms and genetic liability with schizophrenia (Kendler et al., 1993; Webb and Levinson, 1993). However, individuals with SPD seldom suffer the severe psychosocial impairment, hospitalization and long term anti-psychotic medication exposure that accompany schizophrenia. ERP studies of SPD have shown moderate (Mannan et al., 2001), mild (Niznikiewicz et al., 2000) or trend level reduction of auditory P300 amplitude (Salisbury et al., 1996; Trestman et al., 1996).
One limitation of previous studies of P300 in SPD is the reliance on P300 measurements from an ERP averaged across a series of trials. The averaged ERP isolates EEG activity that is phase-locked to a stimulus and is therefore insensitive to temporal variations in the response which fluctuate across trials (Jung et al., 2001). However, experimental studies using behavioral and physiological measures suggest that schizophrenia is associated with disturbances of timing, synchronization and temporal integration (Brown et al., 2005; Carroll et al., 2008; Kwon et al., 1999). Several ERP studies have shown that increased ERP variability among individual trials may be characteristic of schizophrenia (Anderson et al., 1995; Ford et al., 1994; Patterson et al., 2000). In an elegant study comparing reaction time (RT) and RT variability, Vinogradov et al. (1998) concluded that RT and RT variability represented separate aspects of symptomatic and cognitive dysfunction in schizophrenia. A behavioral study (Carroll et al., 2008) indicated that time duration judgments are also more variable in schizophrenia.
Time-frequency signal analysis techniques are well suited to characterize inter-trial variability of ERP responses. This approach can differentiate of the amplitude or power of EEG activity from phase consistency or coherence across trials at specific frequencies (Makeig et al., 2002). Inter-trial coherence (ITC), or the phase locking factor, estimates the consistency of the phase of EEG signal across trials at different frequencies. Event-related spectral perturbation (ERSP), on the other hand, is a measure of change of power from baseline associated with a stimulus presentation, and includes both phase locked and non-phase locked activity. Recent studies using time frequency measures indicate that both phase locked and non-phase locked auditory ERP target responses are reduced in patients with schizophrenia in the delta and theta frequency ranges (Doege et al., 2009; Ford et al., 2008). Additionally, Ford et al. (2008) found that stronger gamma synchrony was associated with larger P300 amplitude values in control subjects, but not in patients with schizophrenia. The purpose of this study was to use time-frequency measures to test whether phase coherence and power of the auditory ERP were similarly affected in SPD and schizophrenia. Measures of RT and RT variability were also obtained. We predicted reductions of ITC and ERSP in the delta and theta responses to target stimuli, increased RT variability in both schizophrenia and SPD, and a weakened correlation between gamma activity and P300 amplitude in schizophrenia.
2. Methods
2.1 Subjects
Nineteen subjects with SPD, 21 patients with schizophrenia, and 19 control subjects were assessed (Table 1). Patients with schizophrenia were recruited from clinics affiliated with the Indiana University School of Medicine and diagnosed using Structured Clinical Interview for the DSM IV (SCID-I) (First, 1997) supplemented by clinical records. Current symptoms were assessed using the Positive and Negative Syndrome Scale (PANSS) (Kay et al., 1987). The subjects with SPD and control subjects were recruited through newspaper and on-line advertisements. Subjects with SPD were diagnosed using SCID-II. The SCID-I was also used to exclude SPD patients who had an Axis I disorder. All control participants were interviewed using the SCID-NP and the Schizoid, Paranoid and Schizotypal Personality Disorder modules of the SCID II to exclude psychiatric disorders. Exclusion criteria for all subjects included history of cardiovascular disease, history of neurological disease, history of a head injury with loss of consciousness, and a current or previous diagnosis of substance dependence or alcohol dependence. The Schizotypal Personality Questionnaire (SPQ) (Raine, 1991) was obtained from all participants with the exception of two patients with schizophrenia. Tests from Wechsler Adult Intelligence Scale - III (Wechsler, 1997) including Similarities, Picture Completion, Digit Symbol and Digit Span were used to assess current intellectual function. Oral and written informed consent was obtained from all participants. Study procedures were approved by the Indiana University's Human Subjects Institutional Review Board.
Table 1.
Characteristics of study population
| Variables | Control | SPD | Schizophrenia |
|---|---|---|---|
| Age | 36.4±10.5 | 39.2±11.9 | 36.8±12.8 |
| Sex (male/female) | 14/5 | 10/9 | 17/4 |
| Education (years) | 14.2±2.2 | 13.1±1.8 | 11.1±1.3 a |
| WAIS-R subtasks | |||
| Picture completion | 11.3±5.9 | 9.2±4.1 | 6.9±2.8 b |
| Similarities | 10.5±5.0 | 9.4±3.8 | 7.4±2.1 c |
| Digit symbol | 9.3±3.6 | 9.1±3.0 | 5.4±2.4 a |
| Digit span | 10.4±3.0 | 9.1±3.4 | 7.0±3.0 b |
| SPQ total score | 17.1±11.7 | 36.8±12.6 b | 33.9±22.1 d |
| Duration of illness (years) |
15.0 ±12.1 | ||
| PANSS total score | 59.5±13.3 | ||
| Positive score | 17.5±6.0 | ||
| Negative score | 14.6±3.8 | ||
| General score | 27.4±6.8 |
Values represent means ± SDs. SPQ: Schizotypal Personality Questionnaire; PANSS: Positive and Negative Syndrome Scale. ANOVA followed by Tukey’s test was used to test for group differences.
p < 0.001: compared to healthy control and p<0.005 compared to SPD.
p < 0.005: compared to healthy control.
p < 0.05: compared to healthy control.
p < 0.01: compared to healthy control.
2.2 Recording Procedures and Data Processing
Subjects were seated comfortably in a sound-attenuated room during EEG recording. Participants listened to 86 db SPL tone pips (50 ms duration) presented through Etymotic insert earphones during an auditory discrimination task. Subjects responded with a button press to infrequent (P = .15) 1500 Hz target tones randomly interspersed amid a series of 1000 Hz tones. The inter-stimulus interval was 1200 ms. A total of 75 target tones were presented in an invariant pseudorandom sequence. Hand of response was counterbalanced across subjects. During task performance, EEG data were acquired from 29 electrode sites using an electrode cap (Falk Minnow Service, Munich, Germany). Vertical and horizontal electrooculograms were recorded for offline eye blink correction and artifact rejection. Data were sampled at 1000 Hz using a band-pass filter of 0.1 to 200 Hz using a Neuroscan SYNAMPS recording system (Neuroscan Inc, El Paso, TX). Cortical leads were referenced to the tip of the nose and all impedances were maintained below 10 kΩ.
In off-line analysis, a band-pass filter of 0.5 to 55 Hz was applied to EEG data followed by ERP averaging and time-frequency analysis. Eye artifacts were corrected using regression-based weighting procedure described by Gratton, Coles and Donchin (Gratton et al., 1983). Artifact rejection for each trial and channel used an amplitude threshold of 150 µV. The analyses were performed on the two second epochs (−800 to 1200 ms) with a correct response. For measurement of P300 latency and amplitude, epochs were additionally filtered with a low pass of 25 Hz and averaged across the infrequent target ERP trials. A 500 ms pre-stimulus period was used for baseline correction. P300 latency and amplitude was measured as the largest positive value in the latency range of 260 to 600 ms in each of 5 channels (Fz, Cz, Pz, T7 and T8).
2.3 Time frequency analysis
Event-related spectral perturbation (ERSP) and inter-trial coherence (ITC) across trials were calculated using algorithms from EEGLAB (Delorme and Makeig, 2004). The 2 s event-related epoch in a single trial was processed using sub-windows of 512 ms sliding in 10 ms steps. The signal windows (512 points) were zero-padded with a pad-ratio of 2 to complete 1024 points, resulting in an interpolated frequency resolution of about 1 Hz per frequency bin. Each sub-window was subjected to short time Fourier transforms (sFFT) with Hanning window tapering, which has relatively good resolution for low frequency activity (Barry, 2009). The output power spectrum had a frequency range of 1.95 Hz to 49.80 Hz. The ERSP was computed by obtaining power for each trial and time point, subtracting the mean value of the baseline period (−800 ms to 0 ms) over the entire trial duration and then averaging across trials. ITC is an estimate of mean normalized phase across trials. A phasor (or the normalized complex number) is obtained from the complex output of the frequency transformation by dividing by its complex norm for each trial. The phasor is then averaged across trials and a complex norm is taken to obtain the ITC. The ITC values can range from 0 (absence of synchronization) to 1 (perfect synchronization, or phase reproducibility across trials at a given latency), providing a measure of across-trial phase coherence.
2.4 Statistical Analysis
Differences in demographic and physiological characteristics among the three groups (control subjects, patients with schizophrenia and SPD patients) were assessed using one-way analysis of variance (ANOVA) for continuous variables and chi-square tests for categorical variables. For group comparisons of P300 amplitude and latency, ANOVA with the factors of Group (3) and Channel (5: Fz, Cz, Pz, T7 and T8) was used. When appropriate, the Greenhouse-Geisser correction was applied to significance testing. The Tukey HSD test was used to characterize significant main effects and interactions.
For time-frequency analysis, mean ERSP and ITC were calculated in five frequency bands: delta (2–4 Hz), theta (5–7 Hz), alpha (8–12 Hz), beta (13–30 Hz), and gamma (31–50 Hz). To examine the spatial distribution of ERSP and ITC in each frequency band, topographies of ERSP and ITC were obtained by interpolating the averaged ERSP and ITC values from 0 ms to 400 ms between channels. In all channels, ANOVAs with the between groups factor of Diagnosis (3) were applied to the averaged ERSP and ITC values during the time period of interest (0–400 ms). By interpolating the value of −log10 (P value) between channels, statistical topographic maps were obtained to illustrate the brain regions showing group differences in ERSP and ITC at different scalp locations. A false discovery rate (FDR) corrected q-value threshold of 0.05 was used to adjust for the multiple comparisons among channels (Benjamini and Hochberg, 1995). Second, to compare ERSP and ITC at the channels which showed the largest effect sizes, the ERSP and ITC values were averaged within 100 ms intervals from 0 to 400 ms. A Group (3) × Time (4) repeated measures ANOVAs with time as within-group factors was carried out on the ERSP and ITC values. The Tukey HSD test was used to detect significant differences between groups.
3. Results
3.1 Demographic Clinical and Intellectual Assessment
Table 1 shows the demographic and clinical characteristics of study participants. There was no significant difference in the proportion of sex among groups (Pearson Chi-Square = 4.0, df=2, P = 0.135). The patients with schizophrenia had less education than SPD or control subjects (F(2,56) = 15.87, P < 0.001). The total SPQ scores of SPD and schizophrenia groups were higher than those of the control group (F(2,56)=8.37, P < 0.005). All Wechsler subtest scores of the schizophrenia group were lower than those of the control group, while the SPD group did not differ from the control group.
3.2 Behavioral Performance
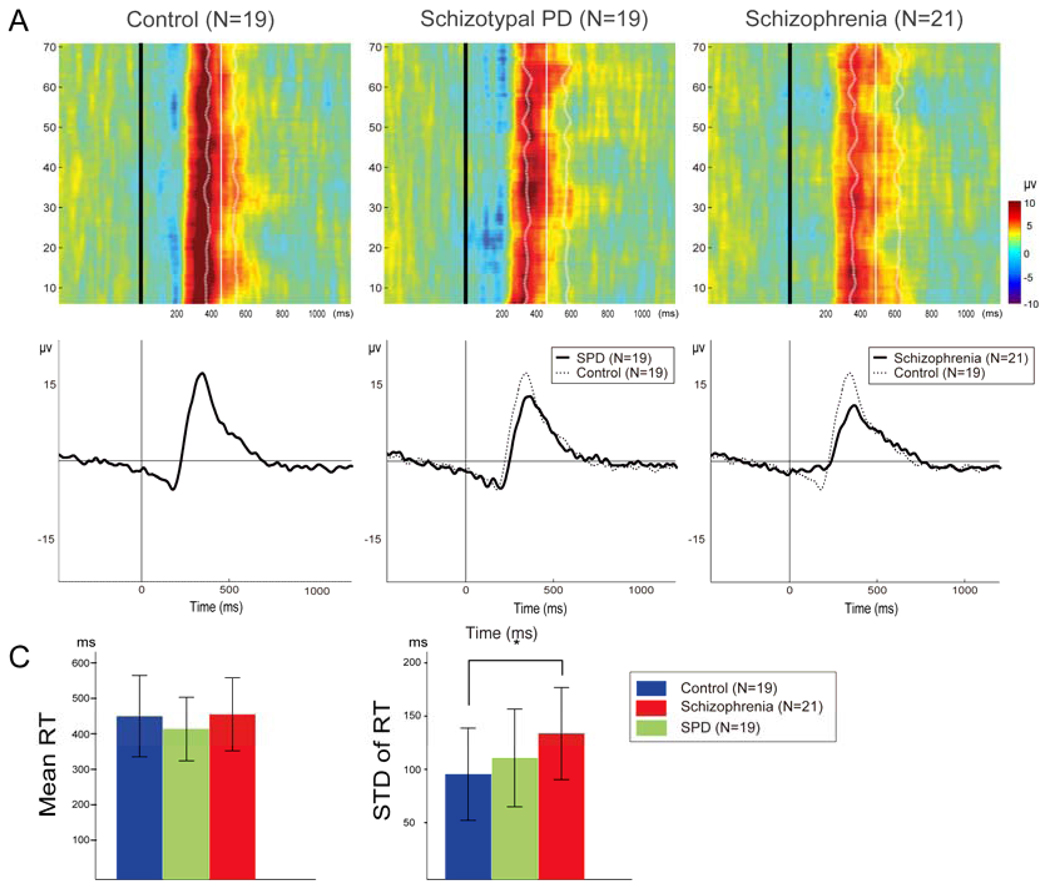
There was no group difference for commission errors (F(2,58)=1.47, P = 0.239) and a trend for a group effect for omissions (F(2,58)=3.11, P = 0.052). This trend was associated with an increased omissions rate in the SPD group (8.11 ± 10.75) compared to the control group (1.64 ± 3.76 %, P = 0.059) due to three SPD subjects with omission rates above 30%. Only trials with correct responses were used for subsequent behavioral and EEG analysis. Mean reaction time (RT) did not differ among the three groups (F(2,58)=0.94, P = 0.399): 449.69±114.55 ms for control subjects, 413.62±89.53 ms for SPD patients, and 454.98±102.52 ms for schizophrenia patients. The variation of RT, measured by standard deviation of RT across trials within subjects showed group differences (F(2,58)=3.82, P = 0.028): 95.36±43.28 ms for control subjects, 110.63±45.85 ms for SPD patients, and 133.57±43.27 ms for schizophrenia patients (Figure 1c). The Tukey test revealed that the patients with schizophrenia showed increased standard deviation of RT across trials compared to control subjects (P = 0.022).
Figure 1.
A) Group averaged event-related potential (ERP) trial by trial responses recorded at Pz from each diagnostic group. Each colored horizontal trace represents a 2 second single-trial group ERP response whose voltage variations are color-coded (see right color scale). The black vertical line represents the onset of target stimuli and the white vertical line represents group-averaged mean reaction time (RT) across trials. The dotted line represents the group-averaged RT variability as measured by difference between mean RT across trials and RT in each trial in each subject. B) Averaged ERP to target tones for each group recorded from Pz. C) Mean RT and the variation of RT as measured by standard deviation of RT across trials within subject.
3.3 P300 amplitude and latency
P300 amplitude was reduced and latency was prolonged in patients with schizophrenia compared to control subjects, while the SPD group did not differ from either group (Figure 1b). The repeated measure ANOVA showed a significant group effect on P300 amplitude (F(2,56)=4.08, P = 0.022). Tukey tests indicated that P300 amplitude was reduced in the patients with schizophrenia compared to control subjects (6.8±3.4 versus 9.9±3.4 µV, P = 0.018). The ANOVA on P300 latency revealed a main effect of group (F(2,56)=7.05, P = 0.002). P300 latency was prolonged in the patients with schizophrenia (385.2±38.6 ms) compared to control subjects (339.7 ± 38.6 ms, P = 0.001). The number of epochs used in the analysis was 73.4± 2.7 in control, 67.1±10.7 in SPD and 70.0±7.1 in SRP. The number of epochs in SPD was lower than control (P = 0.036).
3.4 Time-Frequency Analysis
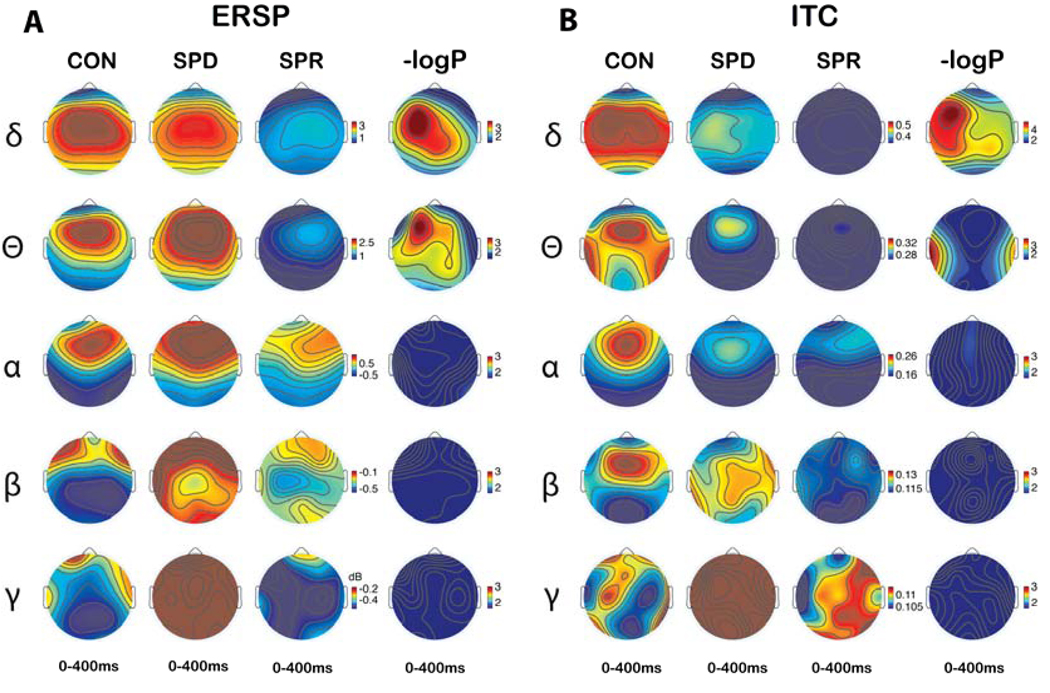
Figure 1a shows mean ERP values for each diagnostic group to the target tones for each target trial, providing a visual display of the group variability in the amplitude and latency of event-evoked responses among trials (Makeig et al., 2002). Mean RT and RT variability across trials are also displayed. Figure 2 shows topographic maps illustrating the distribution of mean ERSP and ITC from 0 to 400 ms. Figure 2 also show the statistical topographies of −log10(P) values for comparisons of averaged ERSP and ITC values during the 0 to 400 ms period among the three groups. Topographies were produced with the threshold of −log10(P) in which P corresponds to FDR corrected q=0.05). In Figure 2, dark blue indicates non-significant P values; all other colors indicate significant values at the FDR corrected q = 0.05 level. Delta ERSP and ITC differed among groups at most electrode sites, with the largest effects at left frontal sites. Theta ERSP also showed pervasive deficits with a left frontal maximum effect. The bilateral temporal area showed group differences for ITC in the theta frequency band.
Figure 2.
Time frequency spectrogram topography of the averaged event-related spectral perturbation (ERSP) (A) and inter-trial coherence (ITC) (B) during 0 to 400 ms. The 4th and 8th Columns represent statistical topographies of −log10(P) values for comparisons among three groups of averaged ERSP and ITC during 0–400ms, respectively. The −log10(P) value in which P corresponds to FDR corrected q=0.05 was used for the threshold of the color maps. A lack of a significant difference on the FDR test is indicated by dark blue on the maps. All other colors indicate significant differences.
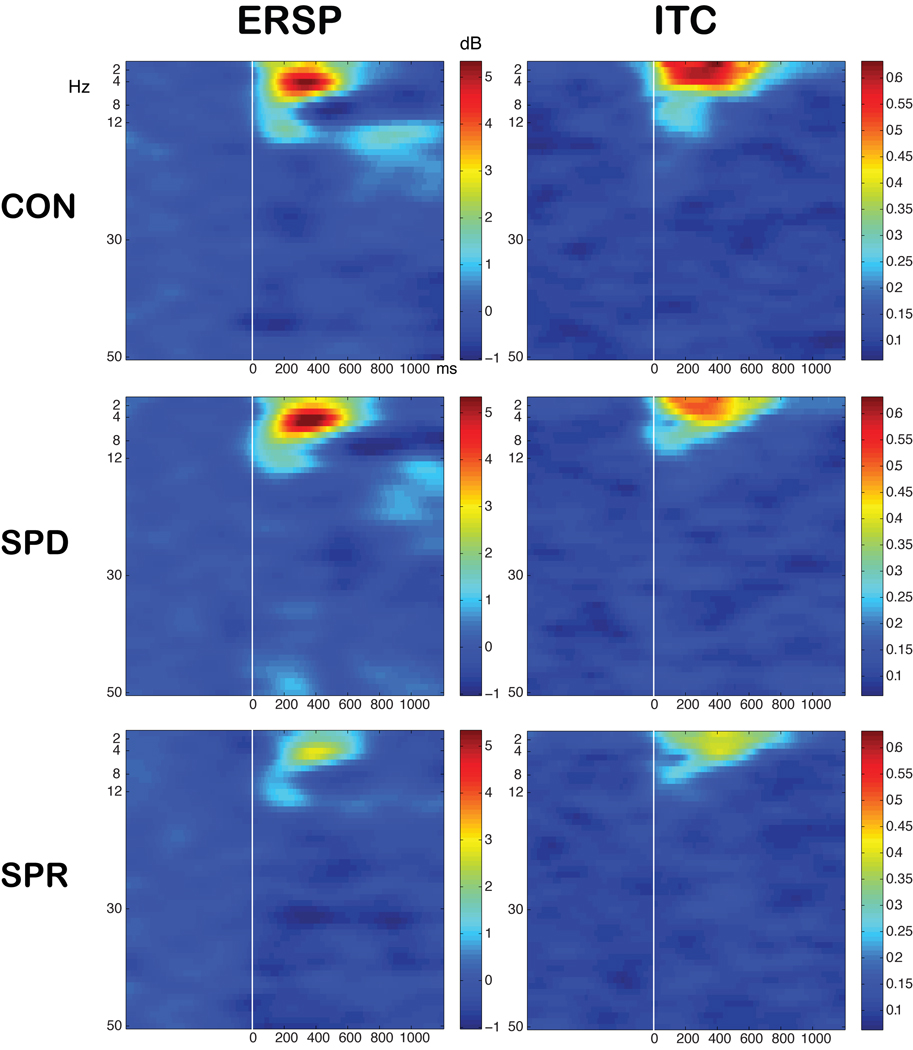
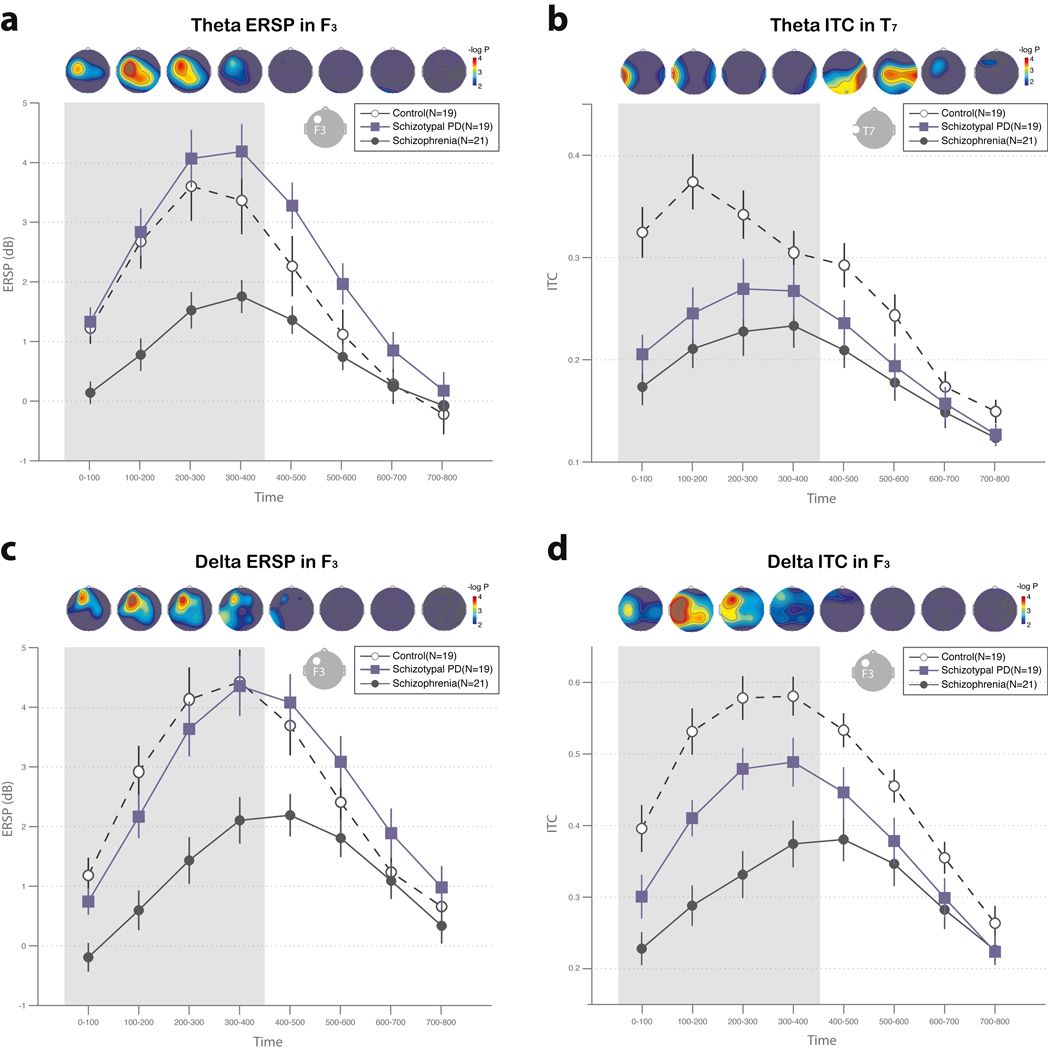
Delta and theta ERSP was reduced in the schizophrenia group, but not in the SPD group, compared to control subjects. Figure 3 shows time-frequency spectrogram at F3. The time courses of these group differences are shown in Figure 4 for delta ERSP, theta ERSP and delta ITC at F3 and theta ITC at T7. A repeated measures ANOVA with time as within-group factors on delta ERSP in F3 showed a group effect (F(2,56)=9.76, P < 0.001). Tukey tests revealed decreased delta ERSP in schizophrenia compared to control (mean ERSP of schizophrenia across time series = 1.00±1.67 dB versus mean ERSP of control= 3.20±1.67 dB, P < 0.001) and SPD (1.00±1.67 dB versus 2.75±1.67 dB, P = 0.005). The analysis on theta ERSP in F3 revealed a group effect (F(2,56)=9.43, P < 0.001). Post-hoc analysis showed decreased theta ERSP in schizophrenia compared to control (1.05±1.59 dB versus 2.71±1.60 dB, P = 0.005) and SPD (1.05±1.59 dB versus 3.10±1.60 dB, P < 0.001). There was no difference between SPD and control subjects on these measures.
Figure 3.
Time frequency spectrogram of the averaged event-related spectral perturbation (ERSP) and inter-trial coherence (ITC) values across the epoch in the channel F3.
Figure 4.
The time series of delta ERSP (a) and ITC (b) in the channel F3, theta ERSP in the channel F3, (c) and theta ITC in the channel T7 (d) The topographies represents the −log10(P) values with P representing the significance level in the comparisons of corresponding averaged ERSP or ITC during each time interval. The −log10(P) values of 2 (p=0.01) was used for the threshold of color map. The post-hoc group comparison was done during the time interval from 0 ms to 400ms (shaded area). The vertical line denotes standard error.
ITC was reduced in both the SPD and schizophrenia groups in the delta and theta frequency ranges. The ANOVA on delta ITC at F3 showed a group effect (F(2,56)=17.13, P < 0.001). Post-hoc analysis revealed decreased delta ITC in the schizophrenia compared to control group (0.31±0.11 versus 0.52±0.12, P < 0.001) and SPD (0.31±0.11 versus 0.42±0.12, P = 0.008). SPD also showed decreased delta ITC compared to the control group at F3 (0.42±0.12 versus 0.52±0.12, P = 0.02). The analysis on theta ITC at T7 revealed a group effect as well (F(2,56)=10.46, P < 0.001). Post-hoc analysis revealed decreased theta ITC in schizophrenia compared to control (0.21±0.09 versus 0.34±0.09, P < 0.001). The SPD group also showed decreased theta ITC compared to the control group at T7 (0.25±0.09 versus 0.34±0.09, P = 0.02).
To facilitate comparison with other studies focusing on activity concurrent with peak P300 amplitude (e.g., Ford et al., 2008), a secondary analysis was conducted on ERSP or ITC for the delta and frequency bands in the 10 ms time interval overlapping with peak P300 latency at Pz (340 ms). The ANOVA revealed significant main effect of group for delta ERSP (F(2,56)=6.20, P = 0.004) showing lower power in the schizophrenia group compared to the other two groups, and for delta ITC (F(2,56)=7.24, P = 0.002) showing lower amplitude in schizophrenia than control subjects. Thus, the schizophrenia group, but not the SPD group, showed delta deficits at Pz near the P300 peak.
A second exploratory analysis evaluated the 0 to 400 ms ERSP and ITC values to standard (non-target) stimuli using identical methods to the analysis of the target responses for delta, theta, alpha, beta and gamma activity. None of these measures differed among groups at any site using FDR analysis.
3.5 Correlation and Regression Analyses
To address the relationship between activity in different frequency measures and P300 peak amplitude, we first used multiple regression analysis in the entire sample. The set of ERSP and ITC values for each frequency of interest (delta, theta, beta and gamma values at the P300 peak latency) was used to predict P300 amplitude for the entire sample at Pz. A stepwise procedure was used to enter (P = .05) and remove (P = .10) predictors from the set of time frequency variables. The time frequency measures predicted P300 amplitude at Pz with an overall R2 value of .32 (P < .001). The only significant coefficient in the model was delta ITC (beta = .57, P < .001). Separate multiple regression analyses using the same set of time frequency measures to predict P300 amplitude were computed for each diagnostic group. For control subjects, R2 =.26 (P = .025), delta ERSP was the sole significant predictor (beta = .51, P = .03). For patients with schizophrenia, R2 =.37 (P = .02), with the predictors delta ERSP (beta = .68, p = .005) and theta ERSP (beta = −.47, P = .04). For SPD subjects, R2 =.41 (P = .003), with delta ITC the sole predictor (beta = .64, P = .002). These analyses suggest that delta activity is most consistently related to P300 amplitude among the time frequency measures.
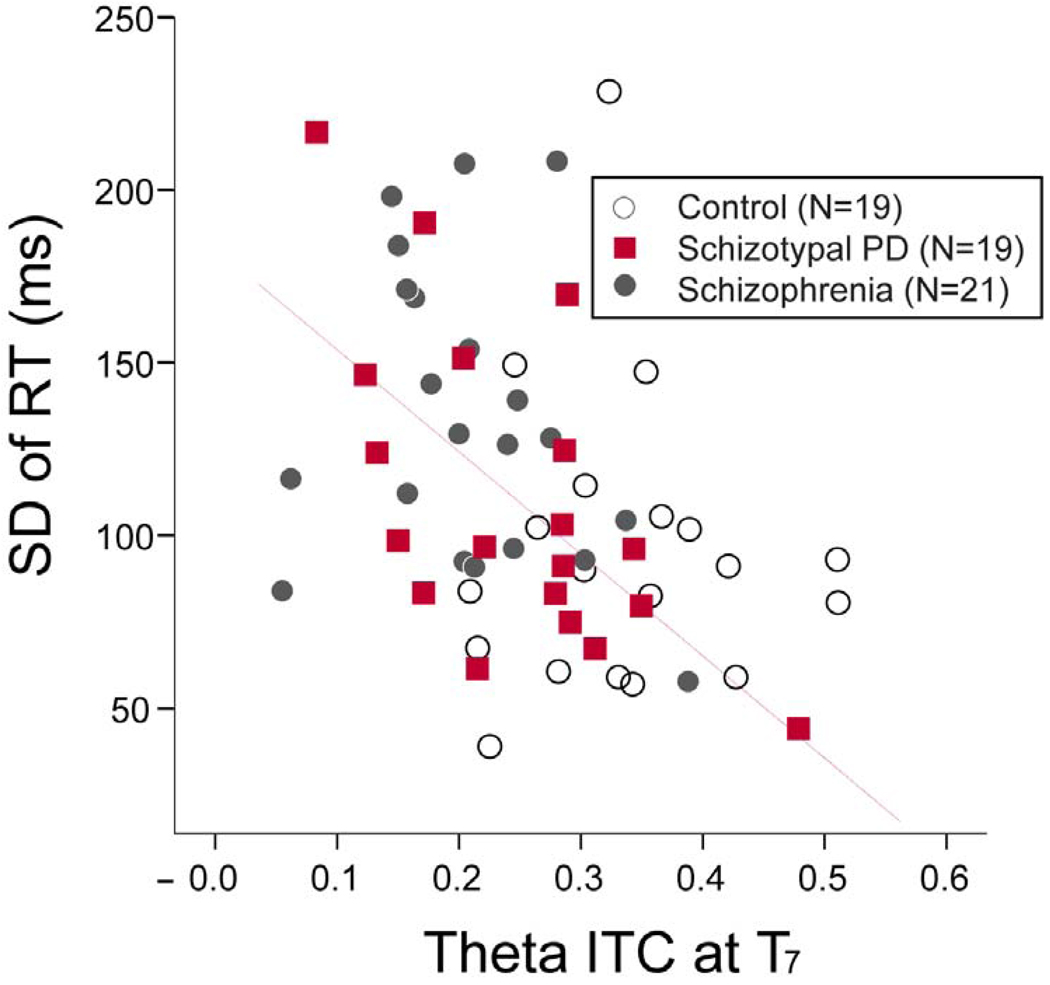
The relationship between mean ITC in the channels showing group differences and the standard deviation (SD) of RT was assessed using Pearson correlation coefficients. The mean theta ITC in T7 (0–400ms) showed negative correlations with the SD of RT in the group of SPD subjects (r = −0.62, P = 0.005) and total population (r = −0.41, P = 0.001) (Figure 5). However, the correlation coefficient was not significant in the schizophrenia (r = −0.249, P = 0.28) or control groups (r = 0.00, P = 1.00).
Figure 5.
Scatterplot showing the correlation between inter-trial coherence (ITC) at channel T7 and standard deviation (STD) of reaction time (RT). The correlation coefficient was significant within the schizotypal personality disorder (SPD) group (r2=0.26, p<0.05).
4. Discussion
The present study compared auditory ERPs in schizophrenia, SPD and control subjects using both conventional averaged measures as well as time-frequency analyses of power and phase consistency. While P300 amplitude and latency were abnormal in schizophrenia, neither measure was affected in SPD. In contrast, time frequency analysis revealed that measures of power (ERSP) and phase consistency (ITC) were differentially sensitive to schizophrenia and SPD. ERSP in the delta and theta bands was decreased in schizophrenia but was unaffected in SPD. However, ITC in the delta and theta bands was reduced in both the schizophrenia and SPD groups, indicative of increased temporal variability in neural responses in both disorders. RT variability was higher in the patients with schizophrenia compared to control subjects, and RT and ITC were correlated in SPD subjects.
The present results are similar to other studies of target ERPs in schizophrenia using measures of delta and theta phase locking or power. Ford et al. (2008) found reduced ERSP and ITC to target stimuli in schizophrenia using auditory oddball paradigm, Doege et al. (2009) reported reductions in evoked as well as induced power in schizophrenia, and Röschke and Fell (1997) found decreased delta amplification in schizophrenia. In the visual modality, Ergen et al. (2008) found a reduction in evoked delta power but not in total delta power in schizophrenia, suggestive of a disturbance in phase synchronization.
Delta and theta activity to target stimuli, but not non-target stimuli, were reduced in schizophrenia. In contrast, Doege et al. (2009) found that delta and theta activity were reduced to both target and non-target stimuli. This could be related to the longer interval between stimuli in the oddball protocol in Doege et al., as well as differences in analytic strategies. While event-related delta and theta activity is reduced in auditory oddball paradigms, Winterer et al. (2000) found increased background delta and theta activity in unmedicated patients with schizophrenia. In their analysis of the early auditory ERP response (50 to 200 ms) in an equiprobable discrimination paradigm, reduced event-related phase-locking and bitemporal coherence appeared to result in low signal to noise ratio in patients with schizophrenia.
The relationship of the P300 amplitude measured from the averaged ERP in the time domain to ITC and ERSP was characterized using multiple regression analyses. Delta ERSP or ITC were the most consistent predictors of P300 amplitude across diagnostic groups. This relationship is in agreement with univariate correlations by Doege et al. (2009), and is concordant with the topographic distribution of delta activity and P300 amplitude. We did not replicate the finding by Ford et al. (2008) that gamma phase locking factor predicted P300 amplitude in control subjects, but not in patients with schizophrenia.
Correlational analysis does not address the issue of whether P300, or correlated activity in a given frequency band, is an evoked response within a neural network unrelated to baseline activity, or instead is due to the phase resetting of ongoing activity which then emerges in the averaged ERP or in the ITC measure (Sauseng et al., 2007). It is possible that the ITC deficit in schizophrenia and SPD represents a failure of phase resetting in response to a stimulus, although definitive criteria for this effect remain controversial (Sauseng et al., 2007). Decreased ERSP in schizophrenia, on the other hand, captures non-phase locked activity induced by a stimulus. This suggests a failure of neural activation in response to stimulation in schizophrenia.
Since synchronized neural activity is thought to be an important mechanism in integration of neural ensembles, disturbed timing and synchronization could contribute to cognitive disturbances in schizophrenia (Uhlhaas and Singer, 2006). Although models of oscillatory disturbances have usually emphasized gamma and beta range abnormalities in schizophrenia (Krishnan et al., 2009; Spencer et al., 2008), deficits in lower frequencies encompassing delta and theta frequencies have also been reported (Basar-Eroglu et al., 2008). Both animal studies (Huxter et al., 2003; Lee et al., 2005) and human studies (Jacobs et al., 2007) suggest that oscillations in these lower frequencies may coordinate the timing of neuronal spiking. In animal models, NMDA-R antagonists such as phencyclidine (PCP) or DOI (1-[2,5-dimethoxy-4-iodophenyl-2-aminopropane) disrupt slow wave oscillations (Celada et al., 2008; Kargieman et al., 2007).
From a cognitive perspective, the correlates of stimulus evoked delta and theta oscillations have been of increasing experimental and theoretical interest. Yordanova et al. (2000) found that delta and theta activity could be differentiated on the basis of reactivity to manipulations of probability and task demands in an auditory oddball paradigm. Yordanova et al. concluded that delta activity in the 312.5 to 437.5 ms window most closely approximated the response characteristics of the P300 component. More generally, theta oscillations have been linked to working memory operations in both animal and human studies (Uhlhaas et al., 2008) as well as attentional processes (Doege et al., 2009). Delta activity has also been implicated in memory operations and integration. Schroeder and Lakatos (2009) argue that periodic or rhythmic streams of stimuli entrain delta rhythm to optimize the alignment of task relevant stimuli with neuronal excitability. Lower frequency synchronization may also be important in integration across broadly distributed brain regions (Uhlhaas et al., 2008). Consequently, delta and theta range disturbances may contribute to alterations in attention, memory operations and long range integration in schizophrenia.
In conclusion, theoretical models and experimental evidence suggest that neural synchrony in the delta and theta ranges have an important role in facilitating sensory and cognitive performance in animals and humans. Increased phase variability of delta and theta activity in schizophrenia and SPD may impair information processing, possibly secondary to NMDA dysregulation. Increased neurophysiological variability may therefore represent a biomarker which provides a link to cellular models, pharmacological manipulations and genetic risk factors in schizophrenia spectrum disorders.
Acknowledgments
We are grateful to Colleen Merrill, Ashlee Steffen and Jennifer Boggs, who assisted in data collection, and to the participants in the study.
Footnotes
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
All authors declare that they have no conflicts of interest.
Contributors
Drs. Shin, O’Donnell, Hetrick and Brenner designed this study and contributed to the writing of this manuscript. Dr. Krishnan provided consultation on the time frequency analysis procedures. Dr. Shekhar contributed to the interpretation of psychopharmacological issues, assessment and diagnostic procedures, and relevant cellular neuroscience. Dr. Malloy assisted with neuropsychological assessment procedures, training and analysis.
References
- Anderson J, Gordon E, Barry RJ, Rennie C, Beumont PJ, Meares R. Maximum variance of late component event related potentials (190–240 ms) in unmedicated schizophrenic patients. Psychiatry Res. 1995;56:229–236. doi: 10.1016/0165-1781(95)02556-c. [DOI] [PubMed] [Google Scholar]
- Barry RJ. Evoked activity and EEG phase resetting in the genesis of auditory Go/NoGo ERPs. Biol Psychol. 2009;80:292–299. doi: 10.1016/j.biopsycho.2008.10.009. [DOI] [PubMed] [Google Scholar]
- Basar-Eroglu C, Schmiedt-Fehr C, Mathes B, Zimmermann J, Brand A. Are oscillatory brain responses generally reduced in schizophrenia during long sustained attentional processing? Int J Psychophysiol. 2008 doi: 10.1016/j.ijpsycho.2008.07.004. [DOI] [PubMed] [Google Scholar]
- Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methodological) 1995;57:289–300. [Google Scholar]
- Brown SM, Kieffaber PD, Carroll CA, Vohs JL, Tracy JA, Shekhar A, et al. Eyeblink conditioning deficits indicate timing and cerebellar abnormalities in schizophrenia. Brain Cogn. 2005;58:94–108. doi: 10.1016/j.bandc.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Carroll CA, Boggs J, O'Donnell BF, Shekhar A, Hetrick WP. Temporal processing dysfunction in schizophrenia. Brain Cogn. 2008;67:150–161. doi: 10.1016/j.bandc.2007.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celada P, Puig MV, Diaz-Mataix L, Artigas F. The hallucinogen DOI reduces low-frequency oscillations in rat prefrontal cortex: reversal by antipsychotic drugs. Biol Psychiatry. 2008;64:392–400. doi: 10.1016/j.biopsych.2008.03.013. [DOI] [PubMed] [Google Scholar]
- Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. doi: 10.1016/j.jneumeth.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Doege K, Bates AT, White TP, Das D, Boks MP, Liddle PF. Reduced event-related low frequency EEG activity in schizophrenia during an auditory oddball task. Psychophysiology. 2009;46:566–577. doi: 10.1111/j.1469-8986.2009.00785.x. [DOI] [PubMed] [Google Scholar]
- Donchin E, Coles MG. Is the P300 component a manifestation of context updating? Behavioral and Brain Sciences. 1988;11:357–373. [Google Scholar]
- Ergen M, Marbach S, Brand A, Basar-Eroglu C, Demiralp T. P3 and delta band responses in visual oddball paradigm in schizophrenia. Neurosci Lett. 2008;440:304–308. doi: 10.1016/j.neulet.2008.05.054. [DOI] [PubMed] [Google Scholar]
- First MB. Structured clinical interview for DSM-IV axis I disorders : SCID - I: clinician version : administration booklet. Washington, D.C: American Psychiatric Press; 1997. [Google Scholar]
- Ford JM, Mathalon DH, Marsh L, Faustman WO, Harris D, Hoff AL, et al. P300 amplitude is related to clinical state in severely and moderately ill patients with schizophrenia. Biol Psychiatry. 1999;46:94–101. doi: 10.1016/s0006-3223(98)00290-x. [DOI] [PubMed] [Google Scholar]
- Ford JM, Roach BJ, Hoffman RS, Mathalon DH. The dependence of P300 amplitude on gamma synchrony breaks down in schizophrenia. Brain Res. 2008;1235:133–142. doi: 10.1016/j.brainres.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford JM, White P, Lim KO, Pfefferbaum A. Schizophrenics have fewer and smaller P300s: a single-trial analysis. Biol Psychiatry. 1994;35:96–103. doi: 10.1016/0006-3223(94)91198-3. [DOI] [PubMed] [Google Scholar]
- Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55:468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- Huxter J, Burgess N, O'Keefe J. Independent rate and temporal coding in hippocampal pyramidal cells. Nature. 2003;425:828–832. doi: 10.1038/nature02058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Kahana MJ, Ekstrom AD, Fried I. Brain oscillations control timing of single-neuron activity in humans. J Neurosci. 2007;27:3839–3844. doi: 10.1523/JNEUROSCI.4636-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon YW, Polich J. Meta-analysis of P300 and schizophrenia: patients, paradigms, and practical implications. Psychophysiology. 2003;40:684–701. doi: 10.1111/1469-8986.00070. [DOI] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Westerfield M, Townsend J, Courchesne E, Sejnowski TJ. Analysis and visualization of single-trial event-related potentials. Hum Brain Mapp. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kargieman L, Santana N, Mengod G, Celada P, Artigas F. Antipsychotic drugs reverse the disruption in prefrontal cortex function produced by NMDA receptor blockade with phencyclidine. Proc Natl Acad Sci U S A. 2007;104:14843–14848. doi: 10.1073/pnas.0704848104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull. 1987;13:261–276. doi: 10.1093/schbul/13.2.261. [DOI] [PubMed] [Google Scholar]
- Kendler KS, McGuire M, Gruenberg AM, O'Hare A, Spellman M, Walsh D. The Roscommon Family Study. III. Schizophrenia-related personality disorders in relatives. Arch Gen Psychiatry. 1993;50:781–788. doi: 10.1001/archpsyc.1993.01820220033004. [DOI] [PubMed] [Google Scholar]
- Krishnan GP, Hetrick WP, Brenner CA, Shekhar A, Steffen AN, O'Donnell BF. Steady state and induced auditory gamma deficits in schizophrenia. Neuroimage. 2009 doi: 10.1016/j.neuroimage.2009.03.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon JS, O'Donnell BF, Wallenstein GV, Greene RW, Hirayasu Y, Nestor PG, et al. Gamma frequency-range abnormalities to auditory stimulation in schizophrenia. Arch Gen Psychiatry. 1999;56:1001–1005. doi: 10.1001/archpsyc.56.11.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Simpson GV, Logothetis NK, Rainer G. Phase locking of single neuron activity to theta oscillations during working memory in monkey extrastriate visual cortex. Neuron. 2005;45:147–156. doi: 10.1016/j.neuron.2004.12.025. [DOI] [PubMed] [Google Scholar]
- Makeig S, Westerfield M, Jung TP, Enghoff S, Townsend J, Courchesne E, et al. Dynamic brain sources of visual evoked responses. Science. 2002;295:690–694. doi: 10.1126/science.1066168. [DOI] [PubMed] [Google Scholar]
- Mannan MR, Hiramatsu KI, Hokama H, Ohta H. Abnormalities of auditory event-related potentials in students with schizotypal personality disorder. Psychiatry Clin Neurosci. 2001;55:451–457. doi: 10.1046/j.1440-1819.2001.00889.x. [DOI] [PubMed] [Google Scholar]
- Niznikiewicz MA, Voglmaier MM, Shenton ME, Dickey CC, Seidman LJ, Teh E, et al. Lateralized P3 deficit in schizotypal personality disorder. Biol Psychiatry. 2000;48:702–705. doi: 10.1016/s0006-3223(00)00938-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patterson JV, Jin Y, Gierczak M, Hetrick WP, Potkin S, Bunney WE, Jr, et al. Effects of temporal variability on p50 and the gating ratio in schizophrenia: a frequency domain adaptive filter single-trial analysis. Arch Gen Psychiatry. 2000;57:57–64. doi: 10.1001/archpsyc.57.1.57. [DOI] [PubMed] [Google Scholar]
- Polich J, Kok A. Cognitive and biological determinants of P300: an integrative review. Biol Psychol. 1995;41:103–146. doi: 10.1016/0301-0511(95)05130-9. [DOI] [PubMed] [Google Scholar]
- Raine A. The SPQ: a scale for the assessment of schizotypal personality based on DSM-III-R criteria. Schizophr Bull. 1991;17:555–564. doi: 10.1093/schbul/17.4.555. [DOI] [PubMed] [Google Scholar]
- Roschke J, Fell J. Spectral analysis of P300 generation in depression and schizophrenia. Neuropsychobiology. 1997;35:108–114. doi: 10.1159/000119400. [DOI] [PubMed] [Google Scholar]
- Roth WT, Pfefferbaum A, Horvath TB, Berger PA, Kopell BS. P3 reduction in auditory evoked potentials of schizophrenics. Electroencephalogr Clin Neurophysiol. 1980;49:497–505. doi: 10.1016/0013-4694(80)90392-2. [DOI] [PubMed] [Google Scholar]
- Salisbury DF, Voglmaier MM, Seidman LJ, McCarley RW. Topographic abnormalities of P3 in schizotypal personality disorder. Biol Psychiatry. 1996;40:165–172. doi: 10.1016/0006-3223(95)00373-8. [DOI] [PubMed] [Google Scholar]
- Sauseng P, Klimesch W, Gruber WR, Hanslmayr S, Freunberger R, Doppelmayr M. Are event-related potential components generated by phase resetting of brain oscillations? A critical discussion. Neuroscience. 2007;146:1435–1444. doi: 10.1016/j.neuroscience.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Schroeder CE, Lakatos P. Low-frequency neuronal oscillations as instruments of sensory selection. Trends Neurosci. 2009;32:9–18. doi: 10.1016/j.tins.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer KM, Niznikiewicz MA, Shenton ME, McCarley RW. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol Psychiatry. 2008;63:744–747. doi: 10.1016/j.biopsych.2007.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trestman RL, Horvath T, Kalus O, Peterson AE, Coccaro E, Mitropoulou V, et al. Event-related potentials in schizotypal personality disorder. J Neuropsychiatry Clin Neurosci. 1996;8:33–40. doi: 10.1176/jnp.8.1.33. [DOI] [PubMed] [Google Scholar]
- Turetsky BI, Calkins ME, Light GA, Olincy A, Radant AD, Swerdlow NR. Neurophysiological endophenotypes of schizophrenia: the viability of selected candidate measures. Schizophr Bull. 2007;33:69–94. doi: 10.1093/schbul/sbl060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Haenschel C, Nikolic D, Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas PJ, Singer W. Neural synchrony in brain disorders: relevance for cognitive dysfunctions and pathophysiology. Neuron. 2006;52:155–168. doi: 10.1016/j.neuron.2006.09.020. [DOI] [PubMed] [Google Scholar]
- van Beijsterveldt CE, van Baal GC. Twin and family studies of the human electroencephalogram: a review and a meta-analysis. Biol Psychol. 2002;61:111–138. doi: 10.1016/s0301-0511(02)00055-8. [DOI] [PubMed] [Google Scholar]
- Vinogradov S, Poole JH, Willis-Shore J, Ober BA, Shenaut GK. Slower and more variable reaction times in schizophrenia: what do they signify? Schizophr Res. 1998;32:183–190. doi: 10.1016/s0920-9964(98)00043-7. [DOI] [PubMed] [Google Scholar]
- Walhovd KB, Fjell AM. The relationship between P3 and neuropsychological function in an adult life span sample. Biol Psychol. 2003;62:65–87. doi: 10.1016/s0301-0511(02)00093-5. [DOI] [PubMed] [Google Scholar]
- Webb CT, Levinson DF. Schizotypal and paranoid personality disorder in the relatives of patients with schizophrenia and affective disorders: a review. Schizophr Res. 1993;11:81–92. doi: 10.1016/0920-9964(93)90041-g. [DOI] [PubMed] [Google Scholar]
- Wechsler D. San Antonio: The Psychological Corporation; 1997. The WAIS-III and WMS-III technical manual. [Google Scholar]
- Winterer G, Ziller M, Dorn H, Frick K, Mulert C, Wuebben Y, et al. Schizophrenia: reduced signal-to-noise ratio and impaired phase-locking during information processing. Clin Neurophysiol. 2000;111:837–849. doi: 10.1016/s1388-2457(99)00322-3. [DOI] [PubMed] [Google Scholar]
- Yordanova J, Devrim M, Kolev V, Ademoglu A, Demiralp T. Multiple time-frequency components account for the complex functional reactivity of P300. Neuroreport. 2000;11:1097–1103. doi: 10.1097/00001756-200004070-00038. [DOI] [PubMed] [Google Scholar]