Abstract
Many considerations, involving understanding and selection of multiple experimental parameters, are required to perform MicroPET studies properly. The large number of these parameters/variables and their complicated interdependence make their optimal choice nontrivial. We have a developed kinetic imaging system (KIS), an integrated software system, to assist the planning, design, and data analysis of MicroPET studies. The system serves multiple functions–education, virtual experimentation, experimental design, and image analysis of simulated/experimental data–and consists of four main functional modules–“Dictionary,” “Virtual Experimentation,” “Image Analysis,” and “Model Fitting.” The “Dictionary” module provides didactic information on tracer kinetics, pharmacokinetic, MicroPET imaging, and relevant biological/pharmacological information. The “Virtual Experimentation” module allows users to examine via computer simulations the effect of biochemical/pharmacokinetic parameters on tissue tracer kinetics. It generates dynamic MicroPET images based on the user's assignment of kinetics or kinetic parameters to different tissue organs in a 3-D digital mouse phantom. Experimental parameters can be adjusted to investigate the design options of a MicroPET experiment. The “Image Analysis” module is a full-fledged image display/manipulation program. The “Model Fitting” module provides model-fitting capability for measured/simulated tissue kinetics. The system can be run either through the Web or as a stand-alone process. With KIS, radiotracer characteristics, administration method, dose level, imaging sequence, and image resolution-to-noise tradeoff can be evaluated using virtual experimentation. KIS is designed for biology/pharmaceutical scientists to make learning and applying tracer kinetics fun and easy.
Keywords: Tracer kinetics, MicroPET, Virtual experimentation, Molecular imaging
Introduction
The importance of molecular imaging and biomarkers is growing rapidly in health care, in biological studies, and in drug evaluation [1-6]. Molecular imaging can provide valuable information on the function of cells and tissues in living organisms that used to be completely inaccessible. Because of the complicated biological/physiological processes involved and the many variable parameters available in imaging, guidelines for the selection of imaging parameters and/or for the development of biomarkers are nontrivial [2, 6]. Appropriate strategies for probe selection, data acquisition, and image analysis, are thus unclear in many situations. A good understanding of the principles of tracer kinetics and the characteristics of imaging devices is essential for the proper design of molecular imaging and/or biomarker development studies. Although there are many available books and articles that one can turn to for specific information on biology, physiology, pharmacology, as well as tracer kinetics and biomedical imaging, the interplay among these different fields is not appreciated or emphasized, nor do they provide the means to learn by doing kinetics.
With the rapid development of computer technology, using high technology to fill the gap in linking knowledge between the fields of biology, physiology, and pharmacology, and tracer methods and biomedical imaging is a logical step. Various computer software systems are currently available that can assist users on the simulation and analysis of tracer kinetics (e.g., BLD [7], COMKAT [8], SIMPLE [9], and others). Not many of these tools, however, include biology, physiology, pharmacology, and imaging factors, and only a few incorporate an educational aspect to aid investigators to effectively design and perform molecular imaging studies. Thus we have developed a software system that integrates education, virtual experimentation, image analysis, and kinetic modeling to facilitate preparation of biological and pharmaceutical images for investigators who routinely perform molecular imaging. Kinetic imaging systems (KIS), a software that links tracer kinetics, imaging, pharmacology, and biology, is initially targeted toward MicroPET users who work with experimental mouse models either for developing biomarkers or for studying pharmacokinetics and biological responses [6]. The software system, however, is applicable to general imaging studies in tracer kinetics and pharmacokinetics in other experimental animals as well as in humans.
In this article, we report the design criteria, configuration, major functional components, and general operation of KIS. A couple of examples of how the system can be used are described. Some additional features of the system for future development are also discussed.
Guiding Concepts and Specifications of the System Design of KIS
As the main goal of trying to integrate various fields together is to help biological and pharmaceutical investigators perform molecular imaging, there is a clear need for an effective educational approach. We have adopted the traditional rule of “learning through experimentation” as the guiding principle in the design of KIS. Virtual experimentation, instead of real experiments, is chosen to provide maximal freedom in variable adjustments, to allow fast feedback, to incur minimal costs, and to exploit the capability of computational technology. To be successful, virtual experimentation also needs to closely parallel the real experimental conditions.
KIS needs to be user-friendly, simple, and yet the content needs to be stimulating, fun, and closely related to what the users are doing or are going to do experimentally. We also wanted to encourage as many people as possible to use the system, without necessarily overburdening our limited user-support capacity. This led us to decide to code the system completely in Java, and to make it an Internet-based application software. Such a system will be available to users anywhere there is Internet access. There will be only one “current” version of the software. As opposed to the support required for traditional software systems, there is no need to keep track of the version, the corresponding users, and their computer platforms. Furthermore, with this approach, the Internet browser (e.g., Explorer, Netscape, and Mozilla Firefox) can handle many program functions that are related to user interaction and data input/output (I/O). This not only helps reduce our coding efforts, but also allows the application software to be platform-independent, and automatically ensures the upward compatibility of the program with future computer developments (in hardware and software). This upward compatibility also bears the advantage of lengthening the product life of the software.
Major Functional Modules of KIS
The developed KIS has four inter-connected functional modules–“Dictionary,” “Virtual Experimentation,” “Image Analysis,” and “Model Fitting.” The user can start any one of these four modules directly from the opening panel of KIS, as shown in Fig. 1. The functional connection and data flows among the modules are shown in Fig. 2. All user actions are activated by point-and-click, and drop-down menus are used throughout. The major functions and features of the modules are briefly described separately below.
Fig. 1.
A screen capture of the opening window of KIS. It shows the four major modules–“Dictionary,” “Virtual Experimentation,” “Image Analysis,” and “Model Fitting”–of KIS that a user can start directly. The “Virtual Experimentation” module has two halves. Clicking on the left half of the module will start the regional kinetics simulation (Fig. 4a), whereas clicking on the right side of the module will start the whole-body kinetics simulation (Fig. 4b). A forum is also set up to allow user feedback and discussion of all aspects of the software system.
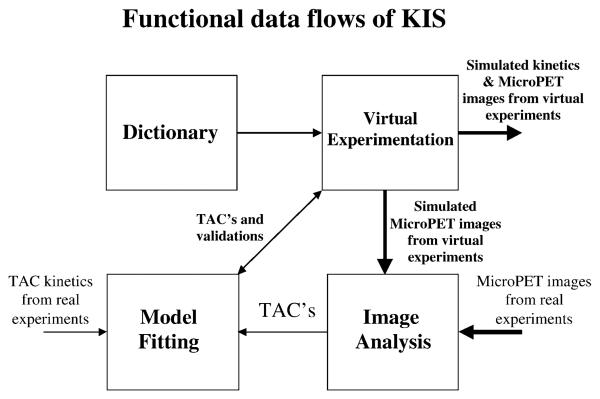
Fig. 2.
A schematic diagram showing the functional data flow and interaction of the four modules of KIS. Because of the modularity design of the system, intermediate data/ images from each module can be exported to other systems, and data/images from other sources (e.g., real PET) can be directly imported into KIS.
Dictionary Module
The “Dictionary” module provides didactic information on tracer kinetics, image quantitation, and mathematical modeling. It contains a description of the model configurations for many commonly used PET tracers (see Table 1) and a glossary of terms used in tracer kinetics. General step-by-step instructions for operation of the three other functional modules are also provided in this module. In addition, it contains two coronal cryosection images of a mouse as well as a 3-D rotating rendering of the internal organs of a whole mouse (Fig. 3) to help users familiarize themselves with the anatomical location of various organs relative to each other.
Table 1.
List of built-in tracer models in KIS
| 2-Deoxy-2-[F-18]fluory-D-glucose (FDG) |
| 6-[F-18]Fluoro-L-DOPA (FDOPA) |
| [F-18]Fluoro-hydroxymethyl-butyl-guanine (FHBG) |
| [F-18]Fluorothymidine (FLT) |
| [F-18]Carbomethoxy-fluoromethylphenyl-tropanes (FWIN) |
| [F-18]fluoro-ethyl-spiperone (FESP) |
| Ga-68 EDTA (a two-compartment model) |
| O-15 Water (a single-compartment model) |
| O-15 Oxygen |
| Sum of exponentials |
This table illustrates the flexibility of the default models that the system currently provides. More models including those for C-11 labeled biomarkers can be easily added to the list in the future to satisfy the need of general users.
Fig. 3.
Two window frames in the “Dictionary” module. (a) Images of a cryosectioned mouse, based on which the 3-D digital mouse phantom used in KIS was constructed. (b) A 3-D rendering view of the constructed 3-D digital mouse phantom.
Virtual Experimentation Module
A unique characteristic of PET is its ability to measure quantitatively the kinetics of a PET tracer (a biomarker or a labeled drug) throughout the body to give a full set of dynamic images. With analysis of the dynamic images [e.g., selecting region of interest (ROI)], one can obtain time activity curves (TACs) of the tracer in selected tissue regions and estimate a biological or pharmacological function in the tissue (see Model Fitting Module section below).
The “Virtual Experimentation” module provides the capability to simulate tissue tracer kinetic curves as well as realistic 3-D dynamic mouse MicroPET images according to user-selected characteristics of the tracer. The module consists of two parallel submodules–regional kinetics and whole-body kinetics simulations. The regional kinetics submodule allows the user to specify the kinetics in each organ tissue separately, but it requires an input function (e.g., plasma TAC of biomarker) [10] to be specified. The whole-body kinetics submodule simulates the kinetics of all organ tissues (including that in the blood) at the same time. The user can specify the administration schedule of the biomarker. When the regional kinetics simulation is chosen, a window with five related panels–Model, Input Function, Input Parameters, Tissue Kinetics, and Kinetic Image–like the one in Fig. 4a, will show up.
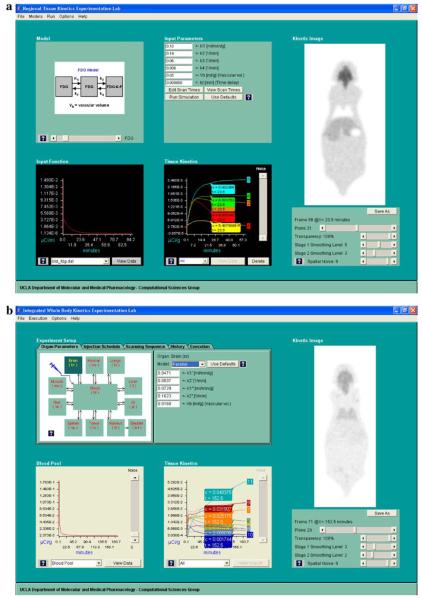
Fig. 4.
(a) Screen capture of the regional kinetics simulation window of the “Virtual Experimentation” module that allows user selection of model configuration (including values of the model parameters), input function, and imaging parameters. The simulated kinetics shown in the lower middle panel can be assigned to different organs of the digital mouse phantom to give a set of dynamic mouse PET images (shown in the kinetic images panel). (b) Screen capture of the whole-body kinetics simulation window of the “Virtual Experimentation” module, after a virtual experiment has been executed. The resulting blood TAC is shown in lower left; the TACs of other organ tissues are in the lower middle panel. The corresponding dynamic MicroPET image are shown on the right-hand side of the figure.
The “Model” panel shows the tracer kinetic model being selected to simulate kinetics. Currently, nine built-in model configurations (see Table 1), including that of 2-deoxy-2-[F-18]fluoro-D-glucose (FDG) [11, 12], can be selected on the panel. By clicking on the “?” button, the user can obtain a more detailed description of that model and its parameters. More model configurations will be added in the future to meet users' needs (see Discussion section).
The “Input Function” panel displays the input function [10] (e.g., plasma TAC of biomarker) to be used for a particular kinetic simulation. A default input function would be selected and plotted in this panel for any particular model selected in the “Model” panel, but the user can also select from a built-in list of input functions or read from a text file. The numerical values of the input functions can also be displayed and edited interactively.
The “Input Parameters” panel shows the parameter values of the selected model. Any desirable parameters can be typed in. On this panel, one can modify from a default imaging frame sequence to specify a version that the user wants to test. Clicking on the “run simulation” button on this panel will generate a tissue TAC that is based on the model, the input function, and the parameter values, specified in the “Models,” “Input Function,” and “Input Parameters” panels, respectively. The generated tissue TAC will be automatically added to the TAC plot on the “Tissue Kinetics” panel.
On the “Tissue Kinetics” panel, multiple tissue TACs can be displayed, separately or together. New ones can be added through the simulation procedure, and any existing ones can be removed. Also, measurement noise corresponding to PET imaging [13, 14] can be added to the simulated tissue TACs to show what a realistic tissue TAC would be like. A “pop-up” scan box is also displayed along with each TAC on the plot that shows the scan time and the TAC value at the scan frame indicated. The “pop-up” scan box can be moved along the TAC to any desired time point on the TAC plot, via a click-and-hold action with the computer mouse.
The “Kinetic Image” panel contains a digital phantom of a whole mouse. The digital phantom contains 64 coronal planes of 98 × 256 (W × H) pixels each. The phantom at this time has eight color-coded organ tissues. Clicking the pointer in an organ region will assign to this organ the tissue TAC currently selected in the “Tissue kinetics” panel. By selecting different tissue TACs in the “Tissue kinetics” panel and assigning them to different organ tissues, the user can simulate a dynamic mouse MicroPET image overlaying the digital phantom. By adjusting the tool bar that controls the relative transparency of the two overlaid sets of images, the simulated image (in gray-level) could become more or less visible over the color-coded digital atlas. The image of any other coronal plane can be selected similarly by a tool bar. The image of any scan frame number can be selected by moving the “pop-up” scan box on the “Tissue Kinetics” panel. Various noise levels corresponding to realistic PET imaging [13, 14] can be added to the simulated images. Two separate image-smoothing operations can be performed. The first one is applied before the image noise is added (to simulate the intrinsic spatial resolution of a MicroPET scanner), and the second one is applied after image noise is added (to simulate smoothing due to filtering in the image reconstruction or in the postreconstruction processing). With these operations, the simulated dynamic images can be realistic (Fig. 4a and b). These images can be saved in data files in the user's computer disk, exactly like those obtained from real mouse experiments with MicroPET scanners.
If the whole-body kinetics simulation is selected, a window display as shown in Fig. 4b will appear. The panel on the upper left corner shows the configuration of the whole-body model used. For each organ, there corresponds a two-compartment model, which is arranged either concatenated or in parallel and can be reduced to a single-compartment model. The model parameters for each organ tissue can be set by the user. The imaging frame sequence can also be set or adjusted by the user similar to the case for regional kinetics simulation. Furthermore, the user may also define the administration schedule of the biomarker. When the execution tab is selected, and the execution button is clicked, the kinetics in all organ tissues (including that in blood) are generated by solving the differential equation shown in the Appendix. All the generated kinetics are automatically mapped to the digital mouse phantom to form dynamic mouse MicroPET images, the resolution and noise levels of which can be adjusted just like in the case of regional kinetics simulation described earlier. To help users keep a good record of what had been done so far, all the parameters used for each executed experiment can be saved in the users' local files, which can be reloaded any time in the future to be reexecuted to give identical and repeatable results.
Image Analysis Module
The “Image Analysis” module is a full-fledged image display and manipulation program, which contains many features such as image windowing, cine-display, ROI drawing, TAC calculation, reslicing, and 3-D orthogonal viewing, that assists users to analyze dynamic images (see Fig. 5). It is adapted from the Web-based JANUS program [15, 16] that we have previously developed and has been in use for viewing and manipulating biomedical images remotely since 1997. Features have been expanded to read and write images and data from the user's local disk storage (see technical notes below), and it can read/write images of multiple formats (including CTI6 or CTI7 formats, DICOM, Concorde, Analyze, UCLA PACS, Interfile, ACR-NEMA, and raw data format).
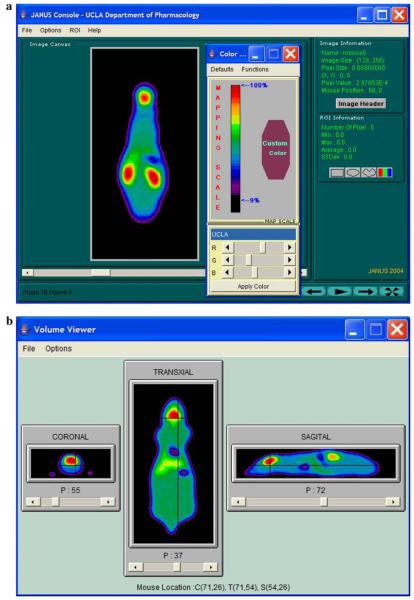
Fig. 5.
Two screen captures of the “Image Analysis” module, showing several functional features of the module–color windowing and ROI value display in (a) and orthogonal volume viewing in (b) of a mouse FDG PET image generated by virtual experimentation.
Model Fitting Module
The “Model Fitting” module provides model-fitting capability that allows the user to estimate biological and pharmacological parameters from measured (or simulated) tissue kinetics, based on a kinetic model and an input function. Figure 6 shows the window display of the “Model Fitting” module. Both the tissue kinetics and the input function can be read from text files. All the built-in model configurations in the Virtual Experimentation Module can be selected to fit the tissue kinetics. One can also define the model as a sum of exponential components (to determine the macroparameters [17]). In addition, one can perform Patlak plot [18] or Logan plot [19] of the tissue kinetics, or simply fit the tissue kinetics with a multiexponential function (up to four exponentials) or with a polynomial (up to order 5). The model fitting minimizes the weighted square of the differences between the tissue kinetics and the model predicted curve based on a set of current parameter values. The weighting for each scan frame of the tissue kinetics as well as the initial values of the parameters can be selected from built-in default options or set by the user. In addition, the user can define the specific parameters to be adjusted in the model fitting process. A Marquardt–Levenberg algorithm for nonlinear regression [20, 21] is used for the model fitting. Convergence is considered to have been achieved when the maximal change among all the parameter values at each iteration is less than a preselected threshold value (default: <0.01%) or the maximal number of iterations exceeds a preselected limit. The fitting results as well as the residuals of the fit are plotted on a panel in the “Output” panel. A report of the resulted model parameters along with their standard errors of the estimate (SEE) and some statistics (e.g., F-statistics) that indicate how well the model fits the data are available for viewing, printing, or to be saved in files.
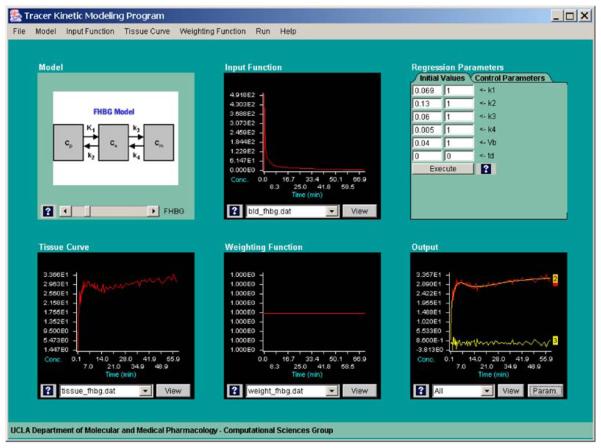
Fig. 6.
A screen capture of the “Model Fitting” module. The user can evaluate a particular model to fit a measured/simulated tissue TAC with a corresponding input function. Data weighting, parameters to be adjusted, and initial parameter values can be selected by the user, and the quality of the fitting results (including the residuals of the fit) is statistically evaluated and graphically displayed.
Details of all features and functions cannot be fully described here, but are included in the help menu and help buttons in all the modules and panels. With the major functions and features briefly described above, KIS allows the users to easily simulate dynamic mouse MicroPET images based on user selection of physical, biological, pharmacologic, radiotracer, and experimental parameters, and to perform image and kinetic analysis to evaluate the effects of any specific factors.
System Structure and Programming Components of KIS
KIS can run on any computer platform that has an Internet browser and an Internet connection/access. There is no special client software, plug-ins, or special setup required. Recommended memory size of the computer is 512 MB, although it also works for less memory with a slight compromise in speed. The website of the software is currently at http://dragon.nuc.ucla.edu/kis/index.html. The user needs to request an access account in advance in order to use it. There are a few technical features of KIS that are worth mentioning. KIS has the capability to access (read and write) data/images in local disk storage, even when it is executed through the Internet (i.e., running the Applet) on platforms with Windows or MacOS X. This capability may require the browser to be authorized according to automatically prompted instructions when such an access is requested. This capability plus the ability to read/ write images of different image formats are important for the success of an Internet-based program designed to generate and process user-specific images/data of various formats.
It is also worth mentioning that the programming structure of KIS is highly modular, designed to allow future expansions (see Discussion section for some of them) to be added with minimal coding changes. An important consideration throughout the design and implementation stage is the performance of KIS in response time (e.g., the generation of all tissue kinetics of the whole-body kinetics simulation and the creation of the dynamic MicroPET images take only a couple of seconds). Technical solutions in many cases are not trivial, because many tasks are computationally intensive and involve very large volumes of data. The goal to achieve a truly server-based software system posted an additional constraint on the size of the Applet bi-code to be downloaded for each user-activated action. Fortunately, structure modularity and good system response are usually not conflicting requirements. However, a number of trade-offs among response time, speed, coding complexity, and memory requirement have been made to achieve the performance level that meets the needs of the users. This, we anticipate, will continue to be a challenge for future expansion of KIS.
Discussion
In the effort to integrate biology, pharmacology, tracer kinetics, and imaging science, we have set for KIS to achieve three integrated objectives–education, virtual experimentation, and a tool for image analysis. While these three objectives can be defined and evaluated individually, they are not separable. Successful achievement in any one area would depend on success in the other two. The current version of KIS, we believe, has achieved well in all three objectives. We envision the possible use of KIS in a wide range of situations. A few examples of how KIS can be used are described below.
As a tool for education and self-learning, the dictionary module provides a summary of concise descriptions of various related subtopics about tracer kinetics and molecular imaging. The user can have a “hands-on” experience of these topics by using the virtual experimentation module to try out different scenarios. For example, after learning what tracer transport, clearance, reactions binding to targets, etc., are, the operator can use this module to experiment how they could affect the kinetics. In doing the simulation, he can find out how tissue kinetics is likewise influenced by the input function, and could go back to look up what input function is and how it is related to the uptake, clearance, and metabolism of the tracer in other organ tissues. After reviewing the 3-D mouse atlas in the dictionary module, he can examine how different organ tissues might appear on a set of multiplane MicroPET images of a particular tracer (e.g., FDG) by using the virtual experimentation module. He could further use the volume viewer in the image analysis module to explore the relative locations of various organs in orthogonal view displays. In going through the process, he may observed that a specific organ is not clearly visible, and could go back to the Dictionary and Virtual Experimentation modules to learn how to adjust the various factors to enhance the visibility of that particular organ. In fact, this is the learning path that we go through in school as students. First, we listen to lectures in a classroom setting. Then we go to the laboratory to try out the new knowledge that we have acquired in lectures. The laboratory experiments provide new observations that in turn stimulate our curiosity to explore some more (through further studies/lectures). The process is repeated over and over. Even after leaving school, when we apply what we have learned to real problems, we use the tools that we have learned, and continue to go through the same studying and experimenting cycle. This is the way we envision how users will use KIS as an educational tool.
Investigators who are planning to perform MicroPET imaging experiments may use KIS to determine the proper imaging protocol. The investigator may already have a general idea of the kinetic rates of the tracer in various organ tissues. By adjusting the imaging time, the scan duration, the image noise level, and the spatial resolution, the user can easily find out the set of parameter combinations that would yield acceptable image results. If there is some uncertainty on how the tracer should be administered, the user can use whole-body kinetics simulation to examine how the shape of the input function as well as those of the kinetics in various tissues would be affected and then make the proper decision.
For those who plan to run dynamic imaging experiments to determine the values of biological parameters in the transport, binding, and clearance processes, the selection of the proper imaging protocol to use is rarely very apparent. Again, one can use the “Virtual Experimentation” module of KIS to try different scanning sequences to generate the expected tissue kinetics. One can then use the “Model Fitting” module to estimate the parameter values. Through this process, one can determine the proper scanning sequence to use and subsequently be confident of the reliability of the expected results. The information obtained could be used in experimental design to determine the number of animal experiments needed to provide statistically significant results. In all cases, there is a rich set of information in watching and measuring how a tracer or drug moves throughout the various organ systems over time, as well as the progressive changes in how well they enter, exit, and participate in subsequent biological processes in tissues of the body.
In the evaluation of biomarkers, a radiochemist or pharmaceutical scientist could use the “Virtual Experimentation” module to test how the modification of the functional property of a molecule (e.g., hydrophobicity or binding affinity) or its removal from circulation (via kidney excretion or liver metabolism) would affect the success of the molecule as an imaging agent or drug, because the former would affect the transport/binding rate to tissues and the latter affects the input function and background activity (e.g., vascular activity and/or accumulations in kidney and bladder). The modeling module can also be used to determine the image analysis procedure (e.g., Patlak [18] or Logan analysis [19, 22], or reference tissue methods [23, 24]) that one can use to quantitate the receptor density (or binding potential), transport rate, or enzymatic characteristics in tissue. Use of virtual experimentation considerably facilitates the process of trial and error, and substantially reduces the number and thus the cost of real animal experiments that are needed.
One does not need much in-depth knowledge to perform molecular imaging, because, with KIS, one can quickly learn through the repeated process of virtual experimentation and modeling analysis. For those who are already familiar with various aspects of molecular imaging, which we envision what most KIS users would be after using it for a while, KIS would continue to be a useful tool for analysis and kinetic modeling of real experimental data/images. With the integrated virtual experimentation capability in KIS, these users can easily compare real observations with simulations to test various hypotheses with regard to the mechanisms of tracer or drug uptake and clearance in body organs.
Future Enhancements/Expansions
Although it can serve the needs of many users, we do not claim that the current version of KIS can satisfy all the possible needs of a wide variety of potential users. In fact, there are numerous expansions and feature enhancements that one can think of. We plan to start an international and multi-institutional academic forum, as indicated in the opening window of KIS (Fig. 1), to evaluate the system and to collect suggestions on how to enrich the features of the system to make it more suitable to users. Some feature expansions are already on our plans for future versions of KIS. For example, we plan to continue to enhance its virtual experimentation capability. More detail structures (e.g., large vasculature) as well as the cardiac/respiratory motion can be added to the mouse digital phantom [25]. The number of digital phantoms could be expanded to include other animal species or strains and for specific organs (e.g., brain, chest, or heart). Another area of expansion is the number of built-in kinetic models. Flexibility that would allow users to define their specific model configurations is being implemented. Inclusion of more physical factors of the imaging process is also possible, so that users can also evaluate the effect of scatter, random coincidence, image reconstruction algorithms, as well as the effectiveness of various data processing methods. Because of the modular design of the system, these expansions and additions can be performed easily without requiring a large team of programmers for system maintenance.
To further enhance its educational value, we plan to expand the dictionary module to include a tutorial on tracer kinetics to guide the learning process and improve its effectiveness. Guided operational procedures of the other modules of KIS will be most likely integrated into such a tutorial as “lab” sessions.
In terms of image analysis capability, there are also many possible feature additions. For example, the inclusion of factor analysis for extraction of blood time activity curves (input function) [26, 27] could facilitate the modeling and quantitative analysis. It could also simplify the experimental procedure by removing the requirement for blood sampling [26-28]. The use of elastic image warping [29] and standardized atlas could allow tissue time activities to be obtained automatically, without the labor-intensive procedure of ROI drawing [30]. However, methods to achieve these goals are still under development. When these methods are fully developed and well characterized, it will not be difficult to incorporate them into KIS, because the programming framework of KIS has anticipated future feature expansions.
Programming-wise, KIS is a true Internet-based program. It is currently installed on a SUN server (Ultra Enterprise 450) and is connected to the Internet using a Netscape web server. There is no special client software, plug-ins, or special setup required, and it incurs minimal effort in terms of software maintenance and user support. We first reported the successful development of KIS in the annual conference of Academy of Molecular Imaging (AMI) in March 2004. Many users have requested access accounts since then and there has been a growing number of access through the Internet. Users, however, have not experienced any performance degradation despite our growing user load. The online system has the same performance (user-experienced) as when the system is run as a stand-alone program (as a Java Application program). The number of KIS users will probably have to increase by 1 order of magnitude before users can notice any effect on the program response. It is a very stable system, and we plan to use it as a framework for future addition of new functions and features to continue serving the needs of users in molecular imaging.
In summary, KIS performs all the functions that it was designed for and it achieves all the technical objectives that we had set at the beginning. By providing a user-friendly environment for virtual experimentation, image analysis, and tracer modeling in molecular imaging, KIS integrates tracer kinetics, biology, and imaging. It is an educational as well as a practical tool in molecular imaging for learning, for experimental design, and for actual data analysis. Further expansions in function and features are ongoing. We expect KIS to help the continual growth of molecular imaging, especially in facilitating user learning and in quantifying images in terms of biological interpretation, and also to expand user access to the value derived from the “kinetics” of molecular interactions within the biological systems of the body.
Acknowledgments
The authors would like to acknowledge the contribution of Dr. David Stout and Dr. Richard Leahy for constructing the mouse phantom used in KIS, Mr. David Vu for system and computer programming assistance, and Dr. Osman Ratib for designing the KIS logo (shown on lower left corner of Fig. 1). They would also like to thank Dr. Wolfgang Weber for his feedback on improving the software system. This work was partly supported by DOE contract DE-FC03-02ER63420 and NIH Grants R01-EB001943, P50-CA086306 and R24-CA 92865.
Appendix
Mathematical Equation for Generation of TACs in All Organ Tissues in the Whole-Body Kinetics Simulation Sub-module
The mathematical equation used for the simulation of the whole-body kinetics of biomarkers is a set of linear ordinary differential equations shown below with a driving function, u(t), that is the administration schedule of the biomarker into the blood pool.
where the capital letter C denotes the concentrations of the biomarker in blood (subscript b), kidney (k), bladder (d), and other major organs, respectively (i = 1, …, n for brain, myocardium, lung, GI, liver, spleen, skeleton, muscle; the second subscript f and b denote, respectively, the free and bound compartments for each tissue). The dot above the concentrations denotes time derivative of the concentrations. The ki's are the transport rate constants in local tissue in organ i (the second subscript for the k's corresponds to the convention used for the rate constant in a two-compartment model, like the FDG model [11, 12], except that, for kidney and bladder compartments, kk3 is for transport from kidney to bladder and kd4 is for transport from bladder out of the body), and νi's are the spatial volumes of the different organs, which are preset to be equal to the volumes of these organs in the digital mouse phantom that the generated kinetics are mapped to. The volume of blood pool, νb, is set to 10% of total body volume. In the present implementation, the above set of differential equations is solved numerically using a fourth-order Runge–Kutta method [31]. After the concentration curves in the equation above are generated, a vascular component, equal to the tissue vascular volume (an organ-dependent and user-set value) times Cb, is added to each concentration curve to obtain the TAC for each of the major organ tissues.
References
- 1.Phelps ME. The merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–681. [PubMed] [Google Scholar]
- 2.Phelps M. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci USA. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gambhir S, Herschman HR, Cherry SR, Barrio JR, Satyamurthy N, Toyokuni T, Phelps ME, Larson SM, Balatoni J, Finn R, Tjuvajev J, Blasberg R. Imaging transgene expression with radionuclide imaging technologies. Neoplasia. 2000;2:118–138. doi: 10.1038/sj.neo.7900083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu C-D, Kerppola TK. Simultaneous visualization of multiple protein interactions in living cells using multicolor fluorescence complementation analysis. Nat Biotechnol. 2003;21:539–545. doi: 10.1038/nbt816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iyer M, Barrio JR, Namavari M, Bauer E, Satyamurthy N, Nguyen K, Toyokuni T, Phelps ME, Herschman HR, Gambhir SS. 8-[F-18]fluoropenciclovir: An improved reporter probe for imaging HSV1-tk reporter gene expression in vivo using PET. J Nucl Med. 2001;42:96–105. [PubMed] [Google Scholar]
- 6.Phelps ME. PET: Molecular imaging and its biological applications. Springer; Berlin Heidelberg New York: 2004. [Google Scholar]
- 7.Carson R, Huang SC, Phelps ME. BLD: A software system for physiological data handling and model analysis; Proceedings of the 5th Annual Symposium on Computer Application in Medical Care; 1981. pp. 562–565. [Google Scholar]
- 8.Muzic RF, Cornelius S. COMKAT: Compartment model kinetic analysis tool. J Nucl Med. 2001;42:636–645. [PubMed] [Google Scholar]
- 9.Gambhir S, Mahoney DK, Turner MS, Wong ATC, Rosenqvist G, Huang SC, Phelps ME. Symbolic interactive modeling package and learning environment (SIMPLE): A new compartmental modeling tool. In: Anderson JG, Katzper M, editors. Simulation in the medical sciences. The Society for Computer Simulation; Phoenix: 1995. pp. 173–186. [Google Scholar]
- 10.Huang SC, Phelps ME. Principles of tracer kinetic modeling in positron emission tomography and autoradiography. In: Phelps ME, Mazziotta J, Schelbert HR, editors. Positron emission tomography and autoradiography. Raven Press; New York: 1985. pp. 287–346. [Google Scholar]
- 11.Huang SC, Phelps ME, Hoffman EJ, Sideris K, Selin CJ, Kuhl DE. Noninvasive determination of local cerebral metabolic rate of glucose in man. Am J Physiol. 1980;238:E69–E82. doi: 10.1152/ajpendo.1980.238.1.E69. [DOI] [PubMed] [Google Scholar]
- 12.Phelps ME, Huang SC, Hoffman EJ, Selin CE, Kuhl DE. Tomographic measurement of regional cerebral glucose metabolic rate in man with (F-18) fluorodeoxyglucose: Validation of method. Ann Neurol. 1979;6:371–388. doi: 10.1002/ana.410060502. [DOI] [PubMed] [Google Scholar]
- 13.Huang SC, Feng D, Phelps ME. Model dependence and estimation reliability in measurement of cerebral oxygen utilization rate with O-15 oxygen and dynamic positron emission tomography. J Cereb Blood Flow Metab. 1985;6:105–119. doi: 10.1038/jcbfm.1986.13. [DOI] [PubMed] [Google Scholar]
- 14.Chen K, Huang SC, Yu DC. Effects of measurement errors in plasma radioactivity curve on parameter estimation in positron emission tomography. Phys Med Biol. 1991;36:1183–1200. doi: 10.1088/0031-9155/36/9/003. [DOI] [PubMed] [Google Scholar]
- 15.Truong D, Huang SC. Image analysis through the world wide web. J Nucl Med. 1997;38(suppl):309P. [Google Scholar]
- 16.Truong D, Huang SC, Hoh C, Vu D, Gambhir SS, Phelps ME. A Java/Internet-based platform independent system for nuclear medicine computing. J Nucl Med. 1998;39(suppl):278P. [Google Scholar]
- 17.DiStefano JJ, Landaw EM. Multiexponential, multicompartmental, and noncompartmental modeling. I. Methodological limitations and physiological interpretations. Am J Physiol. 1984;246:R651–R664. doi: 10.1152/ajpregu.1984.246.5.R651. [DOI] [PubMed] [Google Scholar]
- 18.Patlak C, Blasberg RG. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J Cereb Blood Flow Metab. 1983;3:1–7. doi: 10.1038/jcbfm.1983.1. [DOI] [PubMed] [Google Scholar]
- 19.Logan J, Fowler J, Volkow N, Wolf A, dewey S, Schlyer D, Macgregor R, Hitzmann R, Bendriem B, Gatley S, Christman D. Graphical analysis of reversible redioligand binding from timeYactivity measurements applied to [N-[C-11]-methyl-(−)-cocaine PET studies in human subjects. J Cereb Blood Flow Metab. 1990;10:740–747. doi: 10.1038/jcbfm.1990.127. [DOI] [PubMed] [Google Scholar]
- 20.Bard J. Nonlinear parameter estimation. Academic; New York: 1974. [Google Scholar]
- 21.Jenrich RI. An introduction to computational statistics: Regression analysis. Prentice-Hall; Inglewood Cliffs, NJ: 1995. [Google Scholar]
- 22.Logan J, Fowler JS, Volkow ND, Wang GJ, Ding YS, Alexoff DL. Distribution volume ratios without blood sampling from graphical analysis of PET data. J Cereb Blood Flow Metab. 1996;16:834–840. doi: 10.1097/00004647-199609000-00008. [DOI] [PubMed] [Google Scholar]
- 23.Lammertsma AA, Bench CJ, Hume SP, Osman S, Gunn K, Brooks DJ, Frackowiak RS. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 24.Lammertsma AA, Hume SP. Simplified reference tissue model for PET receptor studies. NeuroImage. 1996;4:153–158. doi: 10.1006/nimg.1996.0066. [DOI] [PubMed] [Google Scholar]
- 25.Segars WP, Tsui BMW, Frey EC, Johnson GA, Berr SS. Development of a 4D digital mouse phantom for molecular imaging research. Mol Imaging Biol. 2004;6:149–159. doi: 10.1016/j.mibio.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Hoh CK, Buxton DB, Schelbert HR, Choi Y, Hawkins RA, Phelps ME, Huang SC. Factor analysis for extraction of blood time activity curves in dynamic cardiac PET studies. J Nucl Med. 1995;36:1714–1722. [PubMed] [Google Scholar]
- 27.Wu H, Huang SC, Allada V, Wolfenden PJ, Schelbert HR, Phelps ME, Hoh CK. Factor analysis for derivation of input function from dynamic FDG PET studies in small hearts. J Nucl Med. 1996;37:1717–1722. [PubMed] [Google Scholar]
- 28.Huang SC, Wu HM, Shoghi-Jadid K, Stout D, Chatziioannou A, Schelbert HR, Barrio JR. Investigation of a new input function validation approach for dynamic mouse microPET studies. Mol Imaging Biol. 2004;6:34–46. doi: 10.1016/j.mibio.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 29.Liao W-H, Vese L, Huang SC, Bergsneider M, Osher S. Computational anatomy and implicit object representation: A level set approach. In: Gee J, Maintz J, Vannier M, editors. Biomedical image registration. Springer; Berlin Heidelberg New York: 2003. pp. 40–49. [Google Scholar]
- 30.Yu CL, Leow A, Kreissl M, Wu HM, Huang SC. Warping whole body mouse image to automate ROI value extraction using elastic matching technique. Mol Imaging Biol. 2005;7:165. [Google Scholar]
- 31.Press WH, Flannery BP, Teukolsky SA, Vetterling WT. Numerical recipes. Cambridge University Press; New York: 1986. pp. 550–554. [Google Scholar]