Abstract
The link between in utero and neonatal exposure to environmental toxicants, such as endocrine-disrupting chemicals (EDCs) and adult female reproductive disorders is well established in both epidemiological and animal studies. Recent studies examining the epigenetic mechanisms involved in mediating the effects of EDCs on female reproduction are gathering momentum. In this review, we describe the developmental processes that are susceptible to EDC exposures in female reproductive system, with a special emphasis on the ovary. We discuss studies with select EDCs that have been shown to have physiological and correlated epigenetic effects in the ovary, neuroendocrine system, and uterus. Importantly, EDCs that can directly target the ovary can alter epigenetic mechanisms in the oocyte, leading to transgenerational epigenetic effects. The potential mechanisms involved in such effects are also discussed.
Keywords: epigenetics, techniques, DNA methylation, ovary, transgenerational epigenetic effects, EDC, DES, BPA, genistein, methoxychlor
1. Introduction
Female fertility disorders are becoming increasingly prevalent and an emerging women’s health concern [1; 2; 3]. These disease outcomes reflect the interplay between individuals genetic composition and the environment. Since the genetic composition at the population level is relatively slow to change, the significant role of our dramatically changed environment, in the last century, has to be considered. The impact of the environment, especially during development is highly relevant to adult health and is highlighted by the Barker hypothesis, also known as the developmental origins of health and disease (DoHAD) [4; 5; 6]. Although the Barker hypothesis was originally based on nutritional deprivation in utero and cardiovascular or metabolic disorders in adulthood, its perspective can be applied to developmental exposure to environmental endocrine-disrupting chemicals (EDCs) and fertility disorders in females during adulthood [3].
EDCs are synthetic and natural compounds in the environment that interfere with (i.e., mimic and/or antagonize) the actions of endogenous hormones and disrupt the functions of the endocrine system. The United States Environmental Protection Agency (EPA) has identified hundreds of EDCs that have estrogenic, anti-estrogenic, or anti-androgenic activities [2; 7; 8]. Potentially there are many compounds that either already exist in the environment or are newly introduced, whose effects have not yet been studied in the female reproductive system. EDCs include several groups of chemicals such as insecticides, herbicides and fungicides; plastics and plasticizers; surfactants; industrial chemicals such as polychloro biphenyls (PCBs), polybrominated biphenyls (PBBs), and dioxins; flame retardants; pharmaceuticals; and phytoestrogens such as genistein and coumestrol. There are numerous ways in which exposures to EDCs can occur. While a few compounds are for medicinal use, exposures to the vast majority of these EDCs are through unintended exposure. For example, DDT, which was banned in 1972, was intended for use as a pesticide against disease-bearing insects. It was heavily used for many years and eventually it was found that wildlife, especially bird populations were drastically diminished due to consumption of DDT tainted fish [9].
The overall fertility rate of women aged 15–44 years in the United States dropped 44% between 1960 and 2002 [10]. Lifestyle choices may have been a major contributor of this decline as this study included all women in this age group. However, according to the data from the National Survey for Family Growth, the ‘impaired fecundity rate’ was increased from 11% to 15% between 1982 and 2002 [11]. Furthermore, the incidence of female reproductive disorders such as early puberty, irregular menstrual cycles, endometriosis, premature ovarian failure, and polycystic ovarian disorder is increasing in parallel with the increasing number of EDCs in the environment [1].
Human epidemiological studies suggest that there is a clear association between developmental EDC exposure and adverse health outcomes in females. For example, high maternal serum concentration of p′p′-DDT significantly reduces the probability of pregnancy for their daughters [12]. In addition, there is an association between potential exposure to other EDCs, such as bisphenol A (BPA) and genistein and female reproductive problems. Levels of BPA in blood are associated with a variety of conditions in women including endometrial hyperplasia, recurrent miscarriages, sterility, and polycystic ovary syndrome (PCOS) [13; 14; 15]. An example is that of the inverse correlation between the levels of BPA in urine of women undergoing infertility treatment with the number of eggs recovered in in vitro fertilization (IVF) clinics and serum estradiol levels [16]. High BPA exposure is also associated with chromosomal abnormalities [17]. More alarming recent evidence suggests that there is an association between intrauterine and early life exposure to soy formula and uterine fibroids in adulthood [18]. The same study also showed a similar association between in utero diethylstilbestrol (DES) exposure and fibroids.
Direct evidence from unintentional human exposure to EDCs and observations with wildlife species or lab animals shows that developmental exposure to EDCs singly or in mixtures can cause similar types of consequences as described above [19; 20; 21; 22; 23; 24]. Further evidence from lab animal studies for the effects of developmental exposure to EDCs on the female reproductive system is provided in section 4. The importance of epigenetics (vs genetics) in disease susceptibility and phenotypic differences are becoming very clear [25; 26; 27; 28]. Epigenetic mechanisms that play a role in the delayed effects of developmental exposure to EDCs are also presented in section 4 and Table 1. From a public health perspective, the most serious concern emerging from these studies is that developmental exposure to adverse environmental EDCs may lead to effects in subsequent generation(s) via epigenetic mechanisms, broadly termed transgenerational epigenetic effects (see Section 5 for more details).
Table 1.
Studies conducting epigenetic analyses of effects of EDC exposures
| Compound (primary action) | Dose | Species | Treatment period | Age and tissue examined for epigenetic alterations | Loci analyzed | Techniques used | Observed epigenetic effect | Gene expression changes | Reference(s) |
|---|---|---|---|---|---|---|---|---|---|
| MXC (estrogenic, anti- estrogenic and anti- androgenic) | 20 μg/kg/day or 100 mg/kg/day | Rat | E 19 – PND 7 | PND 60, ovary | Whole genome, ERα and ERβ | AP-PCR, bisulfite sequencing | Hypermethylation of multiple loci, promoter region of ERβ, no alterations in methylation of ERα | Reduced expression of ERβ, no change in ERα | [143] |
| DES (estrogenic) | 1 mg/kg/day | Rat | PND 5 and PND 10-12 | 1, 6, 12 hrs after treatment, uterus | EZH2* | Immunoprecipitations, immunohistochemistry | Increase in PI3K/Akt signaling and EZH2 levels | Reduced H3K27Me3 trimethylation | [56] |
| 1 or 1000 μg/kg/day | Mouse | PND 1-5 | PND 19 and 6 and 18 months, uterus | Whole genome, Nsbp1* | MSRF, Bisulfite sequencing | Nsbp1 hypermethylation at puberty, hypomethylation in DES-exposed older animals | Reduced expression of Nsbp1 at puberty, persistent overexpression in DES- exposed older animals | [204] | |
| 2 μg/pup/day | Mouse | PND 1-5 | PND 17, 21, 30, uterus and DES- induced uterine tumors at 6 months | lactoferrin | Bisulfite sequencing | Hypomethylation of promoter | Persistent overexpression of lactoferrin | [260] | |
| 0.1–100 μg/kg/day | Mouse | E 12-18 | 7–8 months, uterus | Whole genome | DMH | Hypermethylation of ribosomal DNA | Increase in uterine weight | [266] | |
| 2 μg/pup/day | Mouse | PND 1-5 | PND 17- 60, uterus | c-fos | Bisulfite sequencing | Hypomethylation of exon-4 | Persistent overexpression of c-fos | [261] | |
| 3 μg/pup/day | Mouse | PND 1-5 | PND 5, 14, 30 | Dnmts | QPCR, RLGS | Lower levels of Dnmts at PND 5 and 14, aberrant DNA methylation at PND 30 | Both hypo and hyper methylation observed in 10 genomic loci | [262] | |
| 2 μg/pup/day | Mouse | PND 1-5 | PND 5, 30 and 18 months, uterus |
Hoxa-10 Hoxa-11 |
Bisulfite sequencing | No change at PND 5 or 30. Significant hypermethylation only at 18 months in DES-induced uterine carcinomas | Repression of Hoxa-10 and Hoxa-11 expression | [265] | |
| 10 μg/kg/day | Mouse | E 9- 16 | PND 14 | Hoxa-10 | Bisulfite sequencing | Regionalized hypermethylation in the uterus | Decreased expression in the cranial uterus and increase in the caudal region | [264] | |
| Genistein (estrogenic) | 50 mg/kg/day | Mouse | PND 1-5 | PND 19 and 6 and 18 months, uterus | Whole genome, Nsbp1* | MSRF, Bisulfite sequencing | Nsbp1 hypermethylation at puberty, hypomethylation in genistein- exposed older animals, reversal by ovariectomy | Reduced expression of Nsbp1 at puberty, persistent overexpression in genistein- exposed older animals | [204] |
| BPA (estrogenic. anti- estrogenic) | 0.05 mg/kg/day, 20 mg/kg/day | Mouse | PND 1, 3, 5, and 7 | PND 60 | Hoxa-10 | Bisulfite sequencing | No change | Decreased Hoxa-10 expression and increased SMRT* | [307] |
PND = postnatal day, E = embryonic,
EZH2- histone methyl transferase enhancer of Zeste homolog 2; Nsbp1- nucleosomal binding protein 1; SMRT- silencing mediator for retinoic acid and thyroid hormone receptor. See Figure 1 for detailed abbreviations of techniques used.
2. Epigenetics
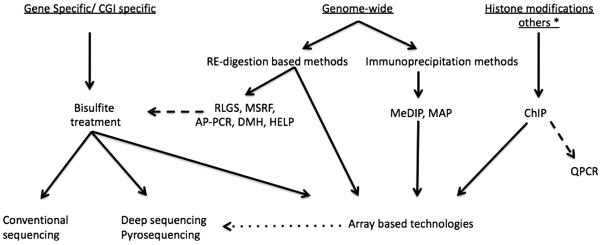
The term “epigenetics” was first introduced in the 1940s by Conrad H. Waddington to describe the developmental program where genes determine the individual’s phenotype and internal and external environmental cues are also taken into consideration [29; 30]. Although the word epigenetic was originally derived from an older terminology “epigenesis” – referring to an embryological concept, it literally means “beyond or above genetics” [31]. However, the current use of the term is somewhat restricted and describes the study of mitotically and meiotically heritable changes in the gene function (i.e., expression) without changing the DNA sequence [32; 33]. These changes are effected by several molecular mechanisms now commonly known as epigenetic mechanisms. These include DNA methylation and post-translational modifications of histone proteins, which bring about changes in chromatin structure and function. The important role of non-coding RNAs as an additional epigenetic mechanism in this process is being recognized. Figure 1 describes the variety of techniques that can be employed to study epigenetic mechanisms. For further details on this topic refer to Supplemental Material.
Figure 1. Techniques available to examine epigenetic mechanisms.
Bisulfite treatment and conventional sequencing is the most common approach to obtain gene-specific CpG methylation status. Genome-wide analyses are necessary for identification of networks/pathways that are potentially altered. Combinations of restriction enzyme (RE) digestions at methylation sensitive sites or the immunoprecipitation of methyl cytosine residues followed by genomic arrays or high throughput sequencing alongside chromatin immunoprecipitation (ChIP) assays have given rise to a wide range of choices. See Supplemental Material online for details.
Abbreviations: RLGS, restriction landmark genomic scanning; MSRF, methylation sensitive restriction fingerprinting; AP-PCR, methylation sensitive arbitrarily-primed PCR; HELP, HpaII tiny fragment enrichment by ligation-mediated PCR; DMH, differential methylation hybridization; MedIP, methylated DNA immunoprecipitation; MAP, methyl binding domain affinity purification, and QPCR, quantitative real-time PCR. * See [357] for details of techniques used in microRNA analysis.
Validation methods: broken lines; Optional steps: dotted line.
2.1. Epigenetic mechanisms
2.1.1. DNA methylation
Methylation of DNA occurs at cytosine residues in CpG dinucleotides and was first implicated in the control of gene regulation and X-chromosome inactivation in a heritable manner, in the mid-1970s [34; 35]. Since then it has been well established that DNA methylation is essential for processes such as genomic imprinting, suppression of retrotransposons, and X-chromosome inactivation [36]. CpGs are underrepresented in the genome except in segments of DNA called CpG islands (CGIs), where the CG content is markedly increased compared to the AT content [37]. Approximately 60–70% of human genes contain CGIs in their promoter regions [38]. CpG sites that are not located in CGIs, such as in repeat sequences and retrotransposons, are usually methylated [39] as are those involved in X-chromosome inactivation and genomic imprinting processes (see Section 6, [40]). In general, DNA methylation is associated with the interference of transcription factor binding, resulting in a down-regulation of gene expression [41], while demethylation allows access of transcription factors and upregulation of gene expression [42; 43]. The methylation status of CpGs that are not part of CGIs, but are associated with the promoter region of the genes, does not usually affect the gene regulation. Such genes are primarily regulated by availability of transcription machinery such as TATA binding protein (TBP) and by histone modifications (see below, [37; 44]). Increased CGI methylation is normally associated with aging and can silence genes. Furthermore, the silencing of tumor suppressor genes can lead to cancer and other serious pathologies [39; 40; 44]. Exposure to EDCs can also induce such changes in CGIs, which can turn on/off critical genes in various organs, leading to organ dysfunction (see section 4).
Normal mammalian development requires the action of DNA methyltransferases (DNMTs) for the establishment (DNMT3A and 3B) and maintenance (DNMT1) of DNA methylation within the genome [39; 45]. DNMT3L (DNMT3-like) is similar in sequence to DNMT3A and B but lacks enzymatic activity and functions as a regulator of DNMT3A and B. DNMT1o is oocyte specific, accumulates in oocytes, and maintains the imprinted genes in the embryo [45]. Deletion of any of these enzymes leads to DNA methylation abnormalities and causes mutant mice to die either embryonically [Dnmt1, Dnmt3b, and Dnmt3a and Dnmt3b double knock out (KO) mice] or in the early postnatal period (Dnmt3a). When Dnmt3l or Dnmt1o are deleted, the mutant animals are viable but their heterozygous mutant offspring die embryonically [45; 46]. The function of DNMT2 is not known since the mice lacking Dnmt2 are normal [39; 45]. The expression level of these enzymes is highly regulated and peaks during the establishment of imprinting during postnatal oocyte development [47; 48; 49; 50]. In male germ cells, the levels are upregulated before birth [embryonic day (E) 15.5-18.5] roughly corresponding to the time of establishment of imprinting [47; 51].
2.1.2. Histone modifications
Histones are proteins of the nucleosome core. The core consists of two copies of each of the following four histones: H2A, H2B, H3 and H4. The DNA is wrapped around this core complex [52]. The function of the nucleosome core proteins goes beyond the packaging of DNA and includes the highly elaborate post-translational modifications of the amino acids at N termini, which play a significant role in gene expression and is named the “histone code” [37; 53]. The modifications are on specific amino acid residues and include acetylation of lysine, methylation of lysine and arginine, and phosphorylation of serine and threonine on the various histone proteins.
In general, acetylation of lysine residues, which takes place in the N terminal of all four histone proteins, is associated with relaxation of chromatin, allowing access to transcription factors and active transcription – euchromatin. Similarly, methylation of lysine 4 of histone 3 (H3K4) and H3K79 is also associated with euchromatin [37; 54; 55]. In contrast, along with deacetylation, various levels of methylation (one to three) of H3K9, H3K27, and H4K20 leads to compaction of the chromatin, disallowing the access of transcription factors and causing the silencing of the loci (heterochromatin). This silencing can be reversible or irreversible, depending on further modification [37; 54; 55; 56]. For example, some histone methylation events (e.g., H3K9me3) work in conjunction with DNA methylation to stably silence genes [39].
The enzymes that add acetyl and methyl groups to lysine residues include various histone acetyl transferases (HAT) and lysine methyltransferases (HMT), respectively. In contrast, the enzymes that remove acetyl and methyl groups are histone deacetylase (HDAC) and various lysine demethylases [39; 57]. The effects of environmental EDCs on histone modifications are relatively less known, but evidence is accumulating regarding histone modifications that are altered by EDC exposures in the reproductive tract [56]. It is expected that more research will be directed to understanding the effects of EDCs on histone modifications.
2.1.3. Non-coding RNA
The importance of non-coding RNAs (ncRNA) in biological processes has been recognized more recently [58]. Non-coding RNAs are transcripts without a clear open reading frame. Their size can range from 15-21 nucleotides (nt; miRNAs), 100–200 nt (small RNAs), or over 10,000 nt (large RNAs) [59]. The major role of ncRNAs is in gene silencing. The varying mechanisms include X-chromosome inactivation (e.g., Xist and Tsix), chromatin structure regulation (e.g., RNA-induced initiation of transcriptional silencing and RNAi mediated heterochromatin formation), genomic imprinting (e.g., H19 and Air), and RNA directed DNA methylation. ncRNAs alter many cellular activities related to differentiation and development, abnormalities of which can lead to cancer, neurological diseases, and imprinting disorders (e.g., Beckwith-Wiedmann syndrome and Prader-Willi/Angelman syndrome) [60; 61]. It is likely that EDCs can affect these important epigenetic regulators, but little data is available at this time.
2.2. Interaction between different epigenetic mechanisms
The three epigenetic mechanisms described above work in an interactive cooperation during the establishment and maintenance of epigenetic marks on the DNA, to regulate cellular development, differentiation, and function [46; 62; 63]. In general, during the establishment of the marks, histone modifications lead to DNA methylation, while in maintenance or inheritance of the marks, DNA methylation plays a primary role. For example, the protection of CGIs from methylation during development is primarily mediated by histone modifications. Specifically, RNA polymerase II is recruited to CpG islands in a sequence-specific manner, which in turn recruits specific methyltransferases that methylate H3K4. Methylated H3K4 inhibits the contact of de novo DNMTs with DNA, leaving the CpG islands unmethylated while the rest of the CpG sites are methylated [37; 64]. Similarly, during the targeted de novo methylation of the CpG islands during later stages of development, histone modifications guide the de novo DNMTs to the CpG islands following recruitment of the repressors to these sites [30; 37; 64]. In contrast, for inheritance of epigenetic marks following cell division, maintenance of DNA methylation by DNMT1 provides the initial silencing conferring mitotic heritability, which is further enhanced by a series of histone modifications that are conducive to gene silencing [30]. Specifically, methylated DNA (i) recruits a methyl cytosine binding protein (e.g., MECP2 or MDB2) that in turn recruits a HDAC to deacetylate DNA, (ii) directs H3K9 methylation, and (iii) inhibits H3K4 methylation [41; 64; 65]. In other cases, the sites that are actively regulated have bimodal modifications including trimethylation of H3K4 and H3K27 at CGIs in promoter regions, which can lead to silencing or activation depending on the other factors affecting gene regulation [56; 57; 64]}. There is also clear but emerging evidence that an ncRNA is a part of these interactions [66]. In plants, ncRNA-directed chromatin modifications such as histone modifications, and DNA methylation, are well known [67]. In addition, ncRNAs, histone modification and DNA methylation work cooperatively in X-chromosome inactivation [68], allele-specific gene silencing [59; 69], and silencing of retrotransposons [70].
3. Development of female reproductive system
Successful female reproductive function requires three components that work in close communication with each other: the ovary, the neuroendocrine system or hypothalamus pituitary gonadal (HPG) axis, and the reproductive tract, including the uterus and oviduct. Therefore, EDC exposure that can affect any one these components can impact the others as well. A recent review provides in-depth information on ovarian development and function and on the factors that play a role in these processes [71]. In this section, we summarize ovarian development and emphasize the stages that are likely to be affected by EDCs (see Figure 2). We reviewed the actions of steroid hormones (e.g., estrogens, androgens), including their receptors as well as select transcription and paracrine factors. Even though relatively little is known of the epigenetic regulation of these factors, we focused on those that are regulated by steroid hormones. In the last part of this section, the most vulnerable processes in neuroendocrine and uterine development are highlighted. We refer primarily to research studies using mice and rats because data from a large body of developmental studies with transgenic mice and extensive toxicological studies with these species are available.
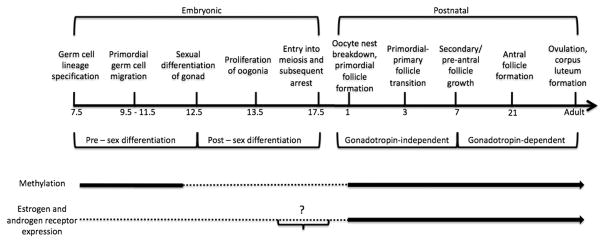
Figure 2. Timeline of ovarian development in mice.
The main stages of ovary development and function are shown. See text for details. After early embryonic lineage specification, germ cells proliferate and become sexually differentiated in mid-gestation. During these stages, the germ cell genome is methylated (thick line), with demethylation (dotted line) occuring after migration. Epigenetic reprogramming is sex-specific and remethylation events (thick line) occur postnatally in the ovary. The expression of estrogen and androgen receptors is at low levels or absent (dotted line) embryonically and increases (thick line) from early postnatal stages.
? - further studies are needed. Timeline is not to scale.
3.1. Ovary development and function
3.1.1. Primordial germ cells and gonadal differentiation
The mammalian ovary consists of two general cell populations: somatic cells and germ cells. The sexually undifferentiated primordial germ cells originate from extragonadal tissues at around embryonic day (E) 7.0-7.5 [72] and begin their migration into the indifferent gonad at E9.0-9.5 [73], where they rapidly proliferate. In the male (XY) gonad, proliferating germ cells are enclosed in the seminiferous cord and enter mitotic arrest at approximately E14 [74]. In the female (XX) gonad, proliferating germ cells form oocyte nests, enter meiosis, and arrest at meiosis I by E17.5 [75].
The indifferent gonad is derived from the cells of the genital ridge and gives rise to the embryonic testis or ovary. The key factor that initiates testis differentiation is SRY (sex-determining region of the Y chromosome). When Sry/SRY gene is deleted or mutates, an ovary forms in an XY embryo instead of a testis [76]. Conversely, if Sry is expressed in an XX embryo, a testis forms instead of an ovary [77]. Progression of the gonadal differentiation farther along the male pathway requires multiple additional factors that are downstream of SRY (see for review [78; 79; 80]).
Since testis development requires SRY and in its absence, an indifferent gonad differentiates into an ovary, ovarian developmental pathway had initially been considered to be a “default” or “passive” pathway [80]. However, accumulating experimental evidence, suggests the contrary. Many genes are differentially upregulated in the ovary during the period of gonadal differentiation [81; 82; 83; 84]. Some of these factors are essential in ovary differentiation, and in their absence an XX gonad displays testis-like features such as coelemic vessel formation (reviewed elsewhere [85]).
One of the major differences between male and female germ cell differentiation pathways is the timing of the establishment of genomic imprinting, or epigenetic programming through DNA methylation (see Figure 2, [86]), a process that is considered to be vulnerable to the effects of EDCs [87; 88]. In rodents, the germ cell genome that is normally methylated prior to migrating to the genital ridge [86] is unmethylated, including at imprinted loci, after the migration. Subsequently, re-methylation, which is mediated through an interaction between somatic and germ cells, occurs in a sex-specific manner at different developmental stages [89]. Therefore, the effects of EDCs on this event also can be sex-specific. In mice, while male germ cell re-methylation starts at E14 and is mostly completed by E16-17 [51; 90], female germ cell re-methylation is initiated during the postnatal period PND 1-5 and continues throughout oocyte growth until the preantral follicle stage [91; 92].
3.1.2. Oocyte nest breakdown and early folliculogenesis
Meiotic female germ cells, which are referred to as oocytes, are arrested at the early diplotene phase of meiotic prophase I and enclosed in oocyte nests that are surrounded by somatic pregranulosa cells. Starting at E16.5, most of these germ cells are eliminated via apoptosis [93]. Accordingly, deletion of the pro-apoptotic molecule Bax increases the number of primordial follicles in the ovary [94]. The remaining oocytes are surrounded by a single layer of flattened pregranulosa cells and form the primordial follicles. Primordial follicles start appearing postnatally, and this process is mostly completed by PND3-4. Most of the primordial follicles remain quiescent, but some begin growing and transition to the next stage, primary follicles. Both of these early processes, primordial follicle formation and primordial to primary follicle transition are tightly regulated by interactions between paracrine factors, transcription factors, and steroid hormones. However, importantly, since these processes determine the success of female reproduction their disturbances can lead to early depletion of follicles and therefore may result in early reproductive senescence [95].
3.1.3. Primordial follicle formation
The oocyte plays a central role in primordial follicle formation, supported by the data that in the absence of oocytes, primordial follicles fail to form [96]. One such oocyte-derived factor is the basic helix-loop-helix family member factor in germline-alpha (FIGLA). Figla-deficient female mice do not form primordial follicles, thus highlighting its critical role in that process [97]. Several growth factors and cytokines including tumor necrosis factor-α (TNFα), neurotrophins (NTs), inhibins, and activins have been shown to play roles in primordial follicle formation [98; 99; 100; 101].
In both mice and rats, progesterone has been shown to inhibit primordial follicle formation by inhibiting apoptosis [102; 103]. Later it was shown that the inhibitory action of progesterone could be reversed by pro-apototic TNFα [98; 104]. In mice, estradiol also blocks primordial follicle formation [103]. The inhibitory action of estradiol and other estrogenic compounds (e.g., DES) may involve the inhibition of pro-apoptotic molecules, such as Fas ligand [105]. Inhibition of apoptosis by progesterone or estradiol leads to multioocyte follicles (MOFs) [103].
Activins have a stimulatory role on primordial follicle formation. Neonatal activin treatment increased the number of postnatal primordial follicle by 30% in mice. However, the excessive number of follicles was not maintained at puberty or beyond [101]. There is interplay between the inhibitory activity of estradiol and the stimulatory role of activins in follicular formation. Neonatal estradiol or DES induces MOFs but also inhibits activin levels in the ovary [106]. These results suggest that the paracrine systems that control the primordial follicle formation process can be influenced by estrogenic EDCs.
3.1.4. Primordial to primary follicle transition
Primordial to primary follicle transition is also described as “initial recruitment” (as opposed to “cyclic recruitment”, which takes place at the peri-antral stage during each cycle under the influence of gonadotropins after puberty [107]). Once follicular growth is initiated, folliculogenesis has to continue through one of two developmental pathways: ovulation or death by follicular atresia. Therefore, a close regulation of the initial recruitment is critical for reproductive success in order to not deplete the follicular reserve too rapidly.
The factors that are critical for this process include KIT ligand (KL) and its receptor, KIT; anti-Mullerian hormone (AMH); Forkhead box O3 (FOXO3) and FOXL2 transcription factors. Granulosa cells express KL, while oocytes and theca cells express its receptor KIT [108; 109]. KIT and KL are important in ovarian follicle development and are encoded by Steel (Sl) and white spotting (W) loci of the mouse, respectively. Initiation and maintenance of ovarian follicle development are adversely affected and follicles arrest at the primary follicle stage in mice homozygous for certain Sl alleles, such as Slpan and Slcon[110; 111]. Using in vitro culture systems, the stimulatory roles of KL in oocyte growth [112] and primordial to primary follicle transition were shown (reviewed in [95]). Interestingly, KL regulates trafficking of DNMTs between oocyte nucleus and cytoplasm during epigenetic reprogramming [113].
Another paracrine factor AMH, has an inhibitory effect on early follicle development, in contrast to KL. In Amh-null mice, primordial follicle recruitment is increased, so, the reserve is rapidly depleted [114]. In ovary organ culture experiments, AMH inhibits the initiation of primordial follicle growth, supporting the in vivo observation [115]. AMH starts being expressed in primary follicles, and remains detectable through the early antral stage. However, AMH expression is reduced in large antral follicles [116; 117]. It can thus be proposed that growing follicles produce AMH in order to down-regulate the number of follicles that are recruited during each cycle [116]. In addition, Amh has estrogen responsive elements in its promoter [118] suggesting that estrogenic or anti-estrogenic compounds, including EDCs can affect AMH expression and, in turn, the process of initial recruitment [117; 119; 120].
It is clear that estradiol and progesterone have inhibitory roles in the initial recruitment [102]. In a rat neonatal organ culture system, it was shown that the initial recruitment rate in the absence of estradiol is three times greater than the normal in vivo rate, suggesting an inhibitory role for estradiol in vivo. However, when estradiol was added to the ovary cultures, the rate of transition was dramatically reduced [102]. In vivo, when newborn rats were injected with estradiol benzoate (EB), the ovary had more primordial follicles [119]. It is interesting that the neonatal exposure to EB, while impairing follicular development, concomitantly elevates expression of inhibitory growth factor AMH [120]. In contrast, P450 aromatase KO (CYP19A1, ArKO) mice, which lack endogenous estradiol, have fewer primordial and primary follicles [121]. Collectively, these data suggest that while estradiol is needed for early folliculogenesis, excessive estradiol inhibits follicular progression.
The oocyte-derived FOXO3 was shown to be a major suppressor of primordial to primary follicle transition [122]. When it is deleted in mice, although the initial primordial follicle pool is established normally, primordial follicles are massively activated, leading to early elimination of follicular reserve and reproductive senescence. Androgens, which have dual roles in folliculogenesis, acting as a ligand for androgen receptor (AR) and as a substrate for aromatase enzyme may play a stimulatory role in the recruitment by inhibiting FOXO3. In neonatal mouse ovary organ culture, testosterone or dihydrotestosterone (non-aromatizable androgen) increased the number of primary follicles, but reduced secondary follicle numbers, suggesting that androgens stimulate primordial to primary follicle transition, but inhibit follicle growth beyond the primary stage [123]. Androgens may accomplish these roles by inhibiting FOXO3 activity and also suppressing the expression of growth differentiation factor-9 (GDF9), a well-known stimulator of follicle development beyond the primary stage (see Section 3.1.5). As a result, exposure to androgens causes an accumulation of preantral-stage follicles. Overall, estrogens may inhibit the initial recruitment by stimulating inhibitory paracrine factors (e.g., AMH) while androgens may stimulate the initial recruitment by inhibiting suppressive factors (e.g., FOXO3).
3.1.5. Preantral follicle development
Preantral follicle development includes the proliferation of granulosa cells to multiple layers, the growth of the oocyte in size, and the establishment of a second layer of somatic cells, the theca cells. However, the exact timing of this recruitment is debated and it may be species-specific [124]. Preantral growth is mediated primarily by oocyte-derived factors, most notably GDF9 and bone morphogenic protein-15 (BMP-15). Additional factors that are involved in the regulation of preantral follicle development include inhibins, KL, and NTs [95].
One of the essential paracrine factors that stimulate follicular growth beyond the primary follicle stage is GDF9, which is present in the oocyte from the primary follicle stage through ovulation [125; 126]. In addition, KL plays a role in theca cell recruitment [127; 128]. Evidence from studies with juvenile rats suggests that gonadotropins, luteinizing hormone (LH) and follicle stimulating hormone (FSH), have some role during preantral follicle growth [91; 129], and their receptors are expressed starting at PND7 [130]). Although gonadotropins stimulate growth and differentiation of preantral follicles, follicles can develop up to the antral stage in the absence of gonadotropins [131].
3.1.6. Follicle selection, antral follicle development, and ovulation
In monoovulators, one follicle within the recruited cohort is selected to complete the full course of folliculogenesis and ovulation. This selection takes place around the antral stage of folliculogenesis. The selected follicle has relatively high levels of estradiol secretion. Estradiol production is accomplished by cooperation of theca cells and granulosa cells under the regulation of LH and FSH, respectively, which forms the basis of the “two-cell two-gonadotropin” hypothesis (see [132; 133; 134] for details). Fine-tuning of this process is mediated by local growth factors such as insulin-like growth factors (IGF), activins, transforming growth factor (TGF) α and β, hepatocyte growth factor, and FGF7 [135].
IGFs are considered to be highly important for follicular maturation and therefore considered to be “co-gonadotropins” [136]. Igf1-deficient mice show a very similar ovarian phenotype as FSH-KO mice [71]. IGF-I stimulates cell proliferation and steroidogenesis in granulosa cells of various species [137; 138]. In contrast, IGF binding proteins (IGFBPs) can suppress FSH-induced follicular growth and differentiation, which leads to atresia, possibly by sequestering IGF-I protein and inhibiting its activity [139; 140]. FSH reduces IGFBP activity by stimulating proteolytic mechanisms that degrade IGFBPs. One such protease is pregnancy-associated plasma protein-A (PAPP-A), which degrades IGFBP and increases the bioavailability of IGFs for stimulation of the follicles. PAPP-A has been considered a marker of selected follicles [141]. Recently, it has been shown that Pappa KO mice have compromised fertility due to reduced ovulation and steroidogenic capacity [142]. Epigenetic regulation of PAPP-A by EDCs in the ovary was shown (see [143] and Section 4.1).
In the pre-ovulatory stage of folliculogenesis, estradiol production is markedly elevated, which exerts a positive-feedback effect on gonadotropin secretion. The rise in FSH and LH supports further increase in steroidogenesis by the selected follicle and initiates the luteinization, whereby granulosa cells switch from the almost exclusive production of estradiol to the production of both estradiol and progesterone. This process upregulates multiple genes, including the LH receptor (LHR) in the granulosa cell to prepare for upcoming ovulation. The feedback dynamics within the HPG axis continue and culminate with the pre-ovulatory LH that stimulates ovulation [144]. Elevated LH, and to a lesser extent FSH, stimulate the terminal differentiation of granulosa cells leading to their further luteinization. Multiple genes play roles in ovulation, including estrogen receptor (ER) β, progesterone receptor, proteases, epidermal growth factor-like proteins, and prostaglandin synthase-2 (see [144] for review). Following ovulation, the remnants of the ovulated follicle are stimulated by LH to terminally differentiate into the corpus luteum (CL). The CL, as a primary source of progesterone, is essential for enabling the initiation and maintenance of pregnancy (reviewed in [145; 146]).
As presented here, the ovary is a hormone-responsive tissue and contains follicles at every stage of their development and CL. The events described above are highly dynamic and require time- and cell-specific and stage-dependent regulation of numerous genes, which could be controlled by epigenetic mechanisms, and can be affected by the developmental exposure to EDCs. Therefore, the ovary provides a unique target for EDCs for epigenetic modulation.
3.2. Expression patterns and roles of ERs and androgen receptor in the ovary
Actions of EDCs in the ovary can be mediated by ERs (ERα and ERβ) and AR. ERα and ERβ are expressed during early folliculogenesis in the ovary in cell- and stage- specific manner in several species, including primates [147], cattle [148], rats [149](Zama and Uzumcu unpublished), and mice [150]. ERα is expressed primarily in theca cells, and ERβ is expressed in granulosa cells. Gene-deletion studies in mice show that ERβ is essential for FSH-directed granulosa cell differentiation as well as for LH responsiveness [151; 152]. Data also show that ERβ can facilitate mechanisms that promote follicle maturation from the early antral to the pre-ovulatory stages [153; 154]. ERβ may also play a major role in primordial follicle formation in the ovary [154; 155; 156; 157; 158]. In contrast, although ERα plays a role in the regulation of theca cell steroidogenesis in the ovary, its main function is to mediate estrogen-regulated feedback in the hypothalamus and pituitary [159; 160].
Androgen receptor is expressed in the ovaries of several species, including rats [161], marmosets [162], humans [163], and sheep [164]. The expression occurs in granulosa cell, oocyte, and to a lesser extent thecal/interstitial cells [165; 166]. The expression starts with primordial stage follicles and its level is elevated in preantral stage and starts declining as the folliculogenesis progresses [167]. Although androgens modulate follicular development, they may not be essential for female fertility since testicular feminized mice (Tfm), in which the AR is dysfunctional, remain fertile with a reduced fecundity [168]. Similarly, Ar-null (ARKO) mice, although initially fertile with a similar ovarian histology as the wild-type, reach reproductive senescence earlier due to progressive follicular loss [169]. Interestingly, estradiol regulates androgen production through ERα primarily expressed in theca cells [170].
3.3. The establishment of the HPG axis and the development of the reproductive tract
The main hormones of the HPG axis are the hypothalamus-released gonadotropin releasing hormone (GnRH), pituitary gonadotropins, and gonadal steroid hormones. The establishment of the hypothalamic neural networks composed of multiple neuronal inputs (nuclei, or cell types) is essential for the pulsatile release of GnRH and pre-ovulatory surge of GnRH/LH in females. The brain is sexually-dimorphic [171], in both morphology and function. In female embryos, androgen production is low, and the small amount of estrogen produced is sequestered by α-fetoprotein, thus making it unavailable in the brain. However, in male embryos, the T produced from the testis is not sequestered by α-fetoprotein and is aromatized to estrogens in the brain, which masculinizes the brain [172]. EDC actions can alter this process since estrogenic EDCs are normally not sequestered by the α-fetoprotein, thereby providing an early estrogenic activity in the brain that potentially alters the sexually dimorphic development of the brain [14; 173]. There are two main sexually dimorphic nuclei in the hypothalamic region of the brain that are established in early neonatal stages in rats: the anteroventral periventricular nucleus (AVPV) of the pre-optic area (POA) and the sexually dimorphic nucleus of the POA (SDN-POA). The AVPV is larger in females while the SDN-POA is larger in the males. The AVPV innervations into the GnRH perikarya in the hypothalamus are among the multiple neuron inputs necessary for the normal secretion of GnRH [174]. The SDN-POA has a known role in normal sexual behavior, but the complete gamut of its functions is not known. Both these nuclei express steroid hormone receptors in abundance (albeit in sex-specific amounts), thereby making them susceptible to EDC actions. Several classes of EDCs have been shown to affect the AVPV, SDN-POA volume, and cellular phenotype depending on the timing of exposure and the dosage [175; 176; 177; 178].
The uterus differentiates from the Mullerian ducts along with the oviduct, cervix, and upper vagina [179]. Most stages of differentiation of the uterus occur postnatally in rodents, starting at PND1-3 [180], which matches human uterine development at around gestational week 14 [181]. Mesenchymal cells in close proximity to the epithelium differentiate into the endometrium, while the distal cells differentiate into the myometrium [182]. Subsequently, the secretory glands develop (adenogenesis) from luminal epithelium [183]. This latter process is completed by PND7 and involves many endocrine and paracrine inputs in mice.
The uterus has varied roles depending on the reproductive stage: implantation, maintenance of pregnancy, and parturition. Estrogens can induce target gene expression in the uterus for cellular functions such as organ growth and proliferation. ERs are actively expressed during Mullerian duct development and are seen as early as E13 in the mesenchyme, while the uterine epithelium expresses ERs soon after birth [184]. The main ER receptor in the uterus is ERα; using KO studies, it has been demonstrated that ERα disruption cause hypoplastic uterine and vaginal tissues [151; 185].
4. EDCs and their effects on the female reproductive system
In this section, we focus on selected EDCs, methoxychlor (MXC), DES, genistein, and BPA that have been shown in recent animal studies to adversely affect the female reproductive system, especially through epigenetic mechanisms (Table 1). Some of these effects are transgenerational, as well (see Section 5). Each EDC section was organized in the following order: brief description of the EDC, human epidemiological studies, experimental animal studies, physiological effects on the ovary, HPG, and uterus, and any associated epigenetic studies discussed along side the physiological effects in the specific tissue.
4.1. Methoxychlor (MXC)
MXC is an organochlorine pesticide that is used as a replacement for DDT and is a well-studied EDC. MXC is an estrogenic compound that demonstrates low-affinity binding for the ER [186]. The major MXC metabolites, HPTE [2, 2-bis-(p-hydroxyphenyl)-1, 1, 1-trichloroethane] and mono-OH MXC can function as estrogenic, anti-estrogenic, or anti-androgenic compounds [187; 188], and therefore constitute model compounds for similar EDCs common in the environment [132; 186].
Human epidemiological studies have shown that there is a strong association between occupational or nutritional exposure to organochlorine pesticides and female fertility. These include a two to threefold increase in risk of prolonged time-to-pregnancy and spontaneous abortion, among female greenhouse workers [189; 190] and increased infertility in women with agricultural work histories [191]. Adverse effects that were observed in these association studies are reminiscent of the effects observed in experimental animals exposed to MXC during adulthood. Exposure to MXC (2500 or 5000 ppm) interfered with the normal estrous cycle, reduced mating rate and litter size [192]. However, when the exposure was withdrawn, these animals reverted to regular estrous cycles. In general, this observation applies to MXC exposure in adulthood and is probably applicable to most other estrogenic EDCs. Further studies demonstrated that adult mice or rats that were exposed to MXC showed persistent vaginal estrus [193], direct inhibition of embryonic growth, implantation failure [194], pregnancy loss [195], and ovarian atrophy due to inhibition of folliculogenesis leading to atretic follicles and reduced ovulation and decreased numbers of CL [193; 196; 197]. It was shown that exposure to MXC in adult mice selectively affects the antral follicles and induces atresia using the Bcl2/Bax signaling pathway, without affecting the HPG axis [198].
In contrast, when the exposure periods included in utero and early postnatal development period, the effects lasted into adulthood with more severe outcomes on reproductive parameters in rats. These included acceleration of the vaginal opening (a sign of puberty), acceleration of the onset of the first estrus, irregular cycles with persistent vaginal estrus, reduced pregnancy rate and litter size despite apparent mating, and early reproductive senescence [199; 200; 201]. Serum estradiol and progesterone levels were altered with increased FSH levels [200]. The effects on the ovary were dramatic, with both folliculogenesis and ovulation being inhibited.
In a more recent study, female rats were treated during fetal and neonatal development (E19-PND7) with a dose of MXC that is comparable to the dose used in the above studies (100 mg/kg/day) the exposed females displayed similar abnormalities in reproductive parameters as well as in ovarian morphology by adulthood [202]. A close examination of follicle composition showed that developmental MXC treatment did not affect the total number of follicles or follicles at primary and secondary stages in adult females. However, the number of preantral and early antral follicles was increased and the number of CL was reduced, with numerous large cystic follicles. Immunohistochemical staining and quantification of expression patterns of important regulators of ovarian functions revealed that while LHR, CYP11A1, and CYP19A1 levels were reduced, levels of AMH and AR were increased, and levels of StAR and ERα were unchanged [202]. Especially noteworthy was that ERβ level was unchanged in primary and secondary follicles, yet decreased dramatically in peri-antral stage follicles, which are responsive to gonadotropins. These observations suggest that hormone-responsive follicles are most affected by EDC exposure. These data are also supported by a recent in vitro study examining the effect of HPTE on global gene expression in immature rat granulosa cell culture [203]. Treatment with HPTE either upregulates or down-regulates approximately 3–10 times more genes in FSH-stimulated than in unstimulated granulosa cells.
Epigenetic analyses using bisulfite-sequencing PCR and methylation-specific PCR showed that MXC caused hyper-methylation in multiple CpGs in two CGIs in ERβ promoter sequences while it had no effect on DNA methylation levels in the ERα promoter at PND60 [143]. This finding correlates with the lack of significant effects on the levels of ERα protein in the adult ovary [143; 202]. Further analysis has shown that the DNA methylation levels in the promoter regions of these genes were unchanged in neonatal ovaries (PND7) immediately after the exposure (Zama and Uzumcu, unpublished). These data demonstrate the age-dependence/hormone responsiveness of the epigenetic changes, which has also been shown in other tissues (e.g., uteri) with other compounds (e.g., DES, genistein) (see Section 4.3 [204]). The global DNA methylation analysis using AP-PCR showed that there were multiple loci that were hypermethylated in MXC-treated ovaries [143]. The majority of candidates were those encoding transcription factors or ribosomal proteins. One candidate that was shown to be hypermethylated in multiple MXC-treated samples was an endopeptidase encoded by Pappa locus (see Section 3.1.6 [143]). Reduced Pappa activity due to increased methylation could limit its availability in follicles and thus increase IGFBP content and sequester IGF-1. This could lead to the observed defect in follicle selection and maturation [202]. Interestingly, in the same set of studies, exposure to a low dose of MXC (20 μg/kg/day) caused a significant increase in the expression of AMH [202] and multiple methylation events both in the ERβ promoter sequences and the Pappa locus [143]. There was a significant upregulation in ERβ expression in the granulosa cells of multiple stages of follicles at PND7, similar to high-dose MXC-treated follicles (Zama and Uzumcu, unpublished). While these epigenetic alterations did not cause any functional defects in the low dose-MXC treated females, the high dose-MXC treated animals had the characteristic ovarian dysfunction.
While the above-mentioned epigenetic analyses and additional in vivo studies showing reduced ovulation rate in response to exogenous gonadotropin stimulation in developmentally MXC-treated prepubertal females, suggesting that there are direct effects on the ovary [202], some effects on the HPG axis have also been demonstrated. For example, developmental exposure to MXC alters behaviors in hamster [205] and eliminates or reduces sexually-dimorphic behaviors in mice [206; 207]. Although some studies show that treatment with MXC alters GnRH mRNA levels in the POA in adult female rats [208], oral exposure to 1200 ppm MXC during fetal and neonatal periods, did not affect the size of the SDN-POA in the hypothalamus [209]. In contrast, similar oral developmental exposure caused an increase in PR in the POA of adult female rats [210] and altered the number of gonadotropes and lactotropes in the pituitary [211]. More recently, it was shown that MXC exposure during fetal and neonatal periods altered ERα gene expression in the POA in aged (17–18 months) female rats [212]. Importantly, these long- lasting effects suggest an epigenetic mechanism of regulation in the brain as well and warrant further investigation [212].
Uterotrophic effects of MXC are well established [213]. MXC increases uterine wet weight, proliferation and protein secretion [214; 215]; these effects have been attributed to its estrogenic actions [117; 194; 205; 216; 217; 218]. In some cases, MXC can interfere with or differ from the actions of estradiol [193]; this was also reported in other experimental systems [219; 220; 221]. More recently, it was shown that in vivo, neonatal MXC exposure inhibits Hoxa-10 expression in the adult uterus in mice and interferes with the binding of estradiol to ERE of Hoxa-10 [222]. Although a potential epigenetic mechanism was suggested, confirmation of this possibility awaits future studies [223].
4.2. Diethylstilbestrol (DES)
DES is a nonsteroidal synthetic estrogen that was prescribed to pregnant women at doses of 5–150 mg/day to prevent miscarriages from 1940s to 1970s [224]). Even though early on DES was shown to be an ineffective drug, it was continued in use till the 1970s [225]. Numerous abnormalities in the reproductive, cardiovascular, and immune systems have since been reported in both male and female offspring of women treated with DES, and validated in animal models [19; 226; 227]. There are limited reports that these effects are being observed in the granddaughters of DES-treated women as well [228]. While DES caused vaginal clear cell adenocarcinoma in only 0.1% of the female offspring, over 95 % reported reproductive tract dysfunction and poor pregnancy outcomes [229; 230]. Since there is evidence of multi-generational effects, epigenetic mechanisms could play an important role and were therefore investigated: see Section 5 [231; 232; 233; 234].
Mice injected with a single dose of 10 μg/kg DES on E15 and examined at 7 months of age had no CL and numerous atretic follicles [235]. They were also found to have vacuolated interstitial tissue with lipid droplet inclusions. Other studies with varying doses of DES (5 μg/kg to 100 μg/kg) administered either in utero (E9-E16) [236], or neonatally (PND1-PND5) [237], demonstrated that adult DES ovaries developed similar hypertrophy and vacuolation of interstitial tissue, hemorrhagic cysts and lack of corpora lutea. These animals also had high levels of testosterone [236]. There was a dose-dependent reduction in the number of the litters as well as the number of oocytes ovulated after stimulation with exogenous gonadotropins [238]. The oocytes derived from such treated ovaries and used in IVF showed lower levels of fertilizability, suggesting reduced oocyte quality [239; 240; 241]. However, 5 µg/day DES-treated ovaries transplanted into untreated ovariectomized host mice were able to give rise to normal female offspring that in turn gave birth to normal size litters and had normal uterine morphology, suggesting that the DES treatment effects were not mediated via germ cells [242]. However, the age at which these animals were sacrificed was 8–12 weeks, and other studies have shown that DES-treated animals do develop epithelial cancers of the uterus by 18 months of age [233].
DES can bind to both ERs with many fold higher affinity than estradiol [155]. Multiple studies from Iguchi and colleagues showed that in utero (E15–18) and neonatally (PND1-5) DES-treated mice had ovaries containing excessive number of MOFs by adulthood [243; 244]. MOFs were also observed in ovaries that were treated in vitro at PND1-5, following their transplantation to untreated mice, suggesting a direct effect of DES in the ovary [244]. Recent studies showed that neonatal exposure to 3 μg/kg DES induced MOFs, a process mediated by ERβ and not ERα [158]. DES exposure was shown to reduce oocyte apoptosis (potentially suppressing oocyte nest breakdown) via ERβ signaling mechanisms. Furthermore, it was hypothesized that such alterations in the germ cell and somatic cell populations may affect the invasion of pregranulosa cells and basement membrane remodeling during primordial follicle formation [105]. Interestingly, the incidence of MOFs has been reported with other EDC exposures as well (see below, [157]).
The effects of DES on the sexual dimorphism of the brain have been documented. In utero and postnatal exposures increased the size of SDN-POA in females thereby defeminizing the region [245]. It was also found that PND1-10 treatment led to a significant reduction in the levels of LH secreted although a similar effect was not found when the exposure was prenatal (E16-20), highlighting the importance of DES actions on the neuroendocrine circuits [246].
It is well known that DES caused T-shaped uteri and clear cell adenocarcinoma of the uterus, cervix, and vagina in women whose mothers were exposed to DES during pregnancy [227]. There are numerous animal studies validating these human reports. For example, progeny of DES-treated mice have shown malformations of the uterus, squamous metaplasia of the luminar and glandular epithelium, endometrial hyperplasia and leiomyomas, and oviductal proliferative lesions [247; 248]. Ovariectomized animals when supplemented with estradiol are able to respond by a transient increase in gene expression and concomitant uterine proliferation and growth [249; 250; 251]. When such a stimulus is removed, the uterus returns to its unstimulated state. However, when DES or estradiol is administered during neonatal development, expression of immediate early genes such as lactoferrin, EGF, and proto-oncogenes such as c-fos, c-jun, and c-myc is upregulated even into adulthood [249; 252; 253]. Inversely, expression of genes that are necessary for uterine development, such as the Abdominal B (AbdB) Hox gene, Hoxa-10, (known to be controlled by estradiol and progesterone, [254]), Wnt7a as well as Msx2 are repressed leading to structural abnormalities of the reproductive tract [255; 256; 257; 258]. Numerous studies have been conducted to assess the methylation patterns of promoters of several of these estrogen-responsive genes associated with uterine development (see Table 1 for details).
Neonatal DES exposure in mice caused nearly 90% incidence of epithelial cancers of the uterus by 18 months of age [259]. In mice similarly treated with DES, the promoter region of the lactoferrin gene was found to be hypomethylated in the adult uterus. However, if the animals were exposed for the same length of time during adulthood, no such methylation or expression defects were observed [260]. Subsequently, it was also found that exon 4 of the c-fos gene was extensively hypomethylated while the promoter region and intron 1 was unaffected, thereby potentially allowing for the upregulation of c-fos expression [261]. QPCR studies performed by Sato and colleagues examining the expression of Dnmts in neonatally DES exposed C57BL/6 mice, revealed that expression of Dnmt1 and Dnmt3b was decreased at PND5 in DES-treated mice, and the pattern continued until PND14 [262]. Interestingly, it was found that human leiomyoma samples had alterations in the levels of Dnmts as well, with concomitant global hypomethylation [263].
As mentioned above, DES down-regulates Hoxa gene expression. These effects are akin to those associated with uterine abnormalities found in Hoxa KO mice. The predominant phenotype is the loss of boundary between the oviduct and uterus. It has been shown that the anterior to posterior specific pattern of Hoxa-9 is essential for the normal development and function of the uterus and that DES causes a posterior shift of Hoxa-9 and Hoxa-10 expression and homeotic anterior transformations [255]. A recent report by Bromer and colleagues has shown that after in utero (E9-16) exposure to 10 μg/kg DES, there is hyper-methylation in the promoter and intron 1 regions of Hoxa-10 gene, in the caudal part of the uterus with a concomitant increase in the Hoxa-10 expression in the same region [264]. While these data are interesting from the point of epigenetic regulation of the regionalization of the uterus, the authors suggest that the apparently conflicting data i.e., increased methylation vs increased gene expression might be due to differential binding to transcriptional repressors. Since previous studies have shown that no epigenetic changes were detected in the promoters of Hoxa-10 and Hoxa-11 genes [265], continuation of these studies is warranted.
Alworth and colleagues showed that in utero exposure (E12-18) of CD-1 mice to DES doses of 0.1–100 μg/kg followed by estradiol administration at 7–8 months of age caused opposite responses between low and high doses. The lower dose enhanced the response to exogenous estrogens, resulting in an increase in uterine weight, while the high dose dampened the response resulting in lighter uteri. A global methylation assay was conducted employing DMH (see Supplemental Material, Section 2.1, Table 1 and Figure 1), which was suitable to detect hypermethylation events. Over 300 CpG island loci were examined and five candidates were identified in 18S rDNA and 45S pre-rDNA methylation, suggesting a role for ribosomal assembly and protein synthesis in the mediation of DES effects [266].
Couse and colleagues have shown that ERα is essential for the mediation of DES effects in the uterus: αERKO female mice exhibited a complete resistance to the effects of DES while βERKO mice did not [267]. Additionally, as mentioned earlier, ERα induction is necessary for activation of estrogen responsive gene expression including that of the lactoferrin and c-fos genes [249; 252; 253; 268]. Since these genes are all downstream of ERα signaling, it is imperative to thoroughly examine the potential role of epigenetic mechanisms in the regulation of ERα expression after EDC exposure. Interesting new studies by Bredfeldt and colleagues have now provided a link between ER signaling and regulation of histone modifications. It was found that rapid PI3K/Akt signaling downstream of membrane-associated ER, in response to estradiol as well as DES, caused reduction in trimethylation of H3K27 (see Section 2.1.2). More interestingly, activation of this non-genomic signaling caused reprogramming of the uterine gene expression profile [56]. Whether such altered epigenetic mechanisms are transgenerational would be of great interest to the field [269].
4.3. Genistein
Genistein is a well-studied isoflavonoid phytoestrogen that is derived from soy products. Phytoestrogens were originally shown to have endocrine disrupting-potential in domestic species: newborn lambs born to ewes fed clover had reproductive abnormalities (in the late 1940s [270]). Soy products are popular since they are a lactose-free substitute for dairy products. Recent reports of low incidence of breast cancer in Asian women and other positive effects on breast cancer risks [271] have further spurred the use and study of soy products. United States FDA has approved 25 g/day soy consumption, approximately equivalent to 75 mg of isoflavones/day (1 mg/kg/day), as being beneficial against coronary artery disease (FDA, 1999). However, a cause for concern is that babies who are fed soy formula consume on average of 6–9 mg/kg body weight, which would result in babies being exposed to 4–7 times higher amounts of soy as compared to adults that are on a soy-rich diet or as per FDA guidelines [272; 273]. In utero or neonatal genistein exposure has been shown to cause specific reproductive effects in mice. It has also been demonstrated that the estrogenic action of genistein is mediated via ER mediated pathways [154; 274].
Neonatal administration of 0.5–50 mg/kg genistein (PND1-5) caused an increase in ano-genital distance (masculinization), accelerated puberty, and irregular estrous cycles in adult CD-1 mice [23]. In this context, genistein-treated (50 mg/kg/d) mice exhibited defects in the ovary such as the MOF phenotype, which correlated with a reduction in the number of apoptotic oocytes, previously shown to involve ERβ mediated actions [103; 156; 157]. This was also associated with fewer pups born to these females over their shortened reproductive lifespan [275; 276]. Genistein and other phytoestrogens have been shown to readily cross the placenta [277] and exposure in utero between E15 and E19 has shown similar effects as mentioned above [278]. A most recent report on the oral administration of genistin (the glycosylated form of genistein) revealed that exposure between PND 1 and PND 5 also resulted in ovaries with MOFs, delayed puberty, irregular estrous cycles and reduced litter sizes [279].
Phytoestrogen administration during development has been shown to diminish the size differences of the SDN-POA between males and females [280]. Bateman and Patisaul have reported that a short 2-day neonatal exposure to a 10 mg/kg genistein dose caused an advancement in pubertal age and abnormal estrous cyclicity. Additionally, they showed that there is an impaired ability to stimulate GnRH neuronal activity, suggesting that genistein could cause defeminization of the female HPG axis. It is well documented that neuronal inputs from the Kisspeptin system are able to regulate the GnRH neuronal activity and the coordination of puberty and steroid feedback. AVPV Kisspeptin neurons are more numerous in females than males, and genistein exposure significantly reduced their density in females, thus defeminizing the AVPV [281].
Protection against apoptosis is necessary, since the numbers of neurons are critical for the normal regionalization and function of the nuclei and ERs have been shown to be involved. ERβ expression is usually down-regulated by estradiol in the PVN; however, it is increased by phytoestrogens [280]. Furthermore, it has been reported that a short neonatal exposure to genistein resulted in ERα and tyrosine hydroxylase (TH) expression changes in the AVPV at PND19. Females had larger AVPV and higher number of TH expressing cells and three times more ERα expressing cells than in males [282]. Overall, studies on the effects of genistein on the expression of various genes involved in the HPG axis have varied depending on the treatment dose and animal species examined, but females generally appear to be more susceptible to its effects [283].
Numerous uterine defects have been documented in CD-1 mice that were neonatally exposed (PND1-5) to genistein (50 mg/kg/day) [23; 284; 285] supporting epidemiological data from women who were soy-fed as babies that had irregular menstrual cycle lengths and pain during cycles or uterine fibroids [18; 286]. A recent paper showed that the oocytes are themselves competent for fertilization and early embryonic development, but the uteri are unable to produce viable implantations: the sites were smaller and fewer in number [287].
Tang and colleagues recently investigated whether neonatal DES/genistein exposure could cause epigenetic changes and alter gene expression in adult uteri and whether there are interactions between adult ovarian hormones and such epigenetic reprogramming. CD-1 mice were exposed to DES (1 μg and 1000 μg/kg) or genistein (50 mg/kg) from PND1-5. Subsequently, some animals were sacrificed at PND19 while others were aged to 6 and 18 months with or without ovariectomies. Genome-wide methylation analysis was conducted with MSRF and candidate genes were identified. Of interest was the nucleosomal binding protein 1 (Nsbp1), which was shown to be hypomethylated at PND19 and hyper-methylated by puberty, in the control. Low-dose DES- and genistein- treated vs high-dose DES-treated animals had opposing methylation patterns. Furthermore, it was shown that in the aged animals, both DES and genistein caused hyper-methylation in the ovariectomized animals but remained hypomethylated in non-ovariectomized animals. These data suggest that Nsbp1 is hyper-methylated in intact mice with age and that DES and genistein have opposing effects on the methylation patterns in intact vs ovariectomized aging animals (hypomethylation vs hyper-methylation), respectively. These studies highlighted the age-dependent aspect of epigenetic reprogramming and also its interaction with steroid hormones [204].
4.4. Bisphenol A (BPA)
BPA is a plasticizer used in the manufacture of polycarbonate plastics, to make them clear and hardened and also in the manufacture of epoxy resins (reviewed in [14]). Total worldwide production of BPA exceeds 6 million tons per year. Nearly 95 % of adults that were tested had detectable levels of BPA in their urine (CDC report, [288]). Daily exposure occurs via plastic food containers (especially when heated or microwaved), lining inside cans, baby formula cans, carbonless print paper, and numerous other sources. Infants in neonatal intensive care units have particularly high exposure to BPA, presumably from its use in medical devices and from the migration of BPA into infant formula from the container. It has also been found in detectable amounts in dust [289; 290; 291; 292]. Early studies with BPA showed that it had estrogenic properties and that it is transferred both lactationally as well as transplacentally [13; 14]. BPA has a lower binding affinity to ERs than estradiol or DES [155; 293]. The “safe” exposure limit for BPA is 50 μg/kg/day but studies with lower doses than the “safe” dose demonstrated numerous detrimental defects in the female reproductive system [14].
Perinatal exposure to low environmentally relevant BPA doses (25–250 ng/kg) caused accelerated puberty, altered estrous cyclicity and disrupted ovarian morphology associated with changes in body weight and LH levels [294; 295; 296]. An increased occurrence of ovarian cysts with blood filled bursae, abnormal numbers of antral follicles, and decreased CL was found in aged mice that were neonatally exposed to a 100 μg/kg dose of BPA [297]. Another study by Adewale and Patisaul [298], demonstrated that exposure of rats to 50 μg/kg and 50 mg/kg doses during the period of hypothalamic neuronal establishment (PND0-3), resulted in a reduction in CL and increase in numbers of MOF and hemorrhagic follicles confirming that BPA has direct effects on the ovary that are independent of GnRH neuronal activity. MOFs were also observed in studies with neonatal BPA exposure (150 μg/kg dose), in mice [299].
Another effect of BPA is exerted at the level of oogenesis and is of very high concern [16]. Studies from Hunt and colleagues demonstrated that BPA released from damaged animal cages and water bottles, which were inadvertently treated with harsh alkaline detergent, induced defects in the meiotic prophase stage of oocyte development in mice: oocytes had increased levels of meiotic aneuploidy due to congression failure. This effect was mimicked when cages were intentionally damaged, or when 20 to 22 day old mice were exposed to a similar dose of BPA (20 ng/g body weight) for as few as 7 days [300]. Further studies demonstrated that BPA caused defects in synapsis and recombination in the homologous chromosomes in the fetal ovary. Interestingly, βERKO animals exhibited very similar meiotic defects in the pachytene oocytes of their fetal gonads. In utero treatment of ERKO females with low doses of BPA did not enhance the oocyte defects, suggesting that BPA could act via the ERβ signaling pathway alongside other non-genomic mechanisms [301].
In ArKO mice that were given BPA (0.1 or 1.0% w/w in chow), the ovarian expression of IGF-I, IGF-I receptor, GDF9, and BMP-15 were increased to normal levels, an effect resembling that of ArKO mice given estradiol replacement [302]. These authors further reported that BPA exerted “little effect” within ovarian and other estradiol-dependent tissues of wild-type mice.
BPA (50 μg/kg or lower) had similar effects on the HPG axis as genistein, with AVPV sexual dimorphism being disrupted after a 2-day neonatal exposure to 500 μg BPA [303]. Rubin and colleagues also showed that environmentally relevant doses do indeed have an effect on AVPV neuronal numbers [304]. Other studies showed that BPA exposure altered the HPG axis by precocious hypothalamic–pituitary maturation leading to precocious puberty, altered GnRH pulsatility in neonates and adults, and severe effects on GnRH signaling in the adult pituitary [305]. Interestingly, as mentioned above, Adewale and colleagues [298] have shown that the ovary can be impacted independent of GnRH activity.
In the uterus, neonatal BPA exposure has been shown to cause long-term adverse effects, including cystic endometrial hyperplasia, as well as the occurrence of more serious uterine pathologies such as adenomyosis, leiomyomas (fibroids), atypical hyperplasia, and stromal polyps [297]. Furthermore, paraovarian cysts, progressive proliferative lesions of the oviduct, and cystic mesonephric (Wolffian) duct remnants in the uterus were found in the BPA-treated mice after in utero exposure [306].
Similar defects were shown in in utero BPA-exposed mice (25 to 250 ng/kg), using Alzet osmotic pumps [296]. Vaginal wet weight was decreased and lamina propria of the endometrium was decreased as well, with concomitant increase in glandular epithelial proliferation at 3 months of age. BPA caused an increase in ERα and PR expression in the lumina typifying a hyper estrogenic response of the uterus. It would be of interest to examine if hypomethylation is associated with such an increase in gene expression. A recent study by Varayoud and colleagues showed that in an ovarectomized, neonatally BPA or DES exposed mouse model, progesterone priming followed by estradiol treatment caused an impaired proliferative response and altered PR and ERα expression in the sub-epithelial stroma of the uterus suggesting that the uteri were unable to respond to ovarian steroids [307]. In addition, Hoxa-10 expression was decreased even though methylation of its promoter was unaffected. Furthermore, an abnormal overexpression of the corepressor, silencing mediator for retinoic acid and thyroid hormone receptor (SMRT), was found in the same stromal cells in which Hoxa-10 expression was reduced. Further epigenetic analyses on BPA-treated uteri are warranted to obtain further clarifications of these interesting studies.
5. Transgenerational epigenetic effects of EDCs
Transgenerational epigenetic effects, including those that are induced by EDCs have been discussed in recent reviews [308; 309]. Epidemiological studies have suggested that transgenerational epigenetic effects occur in humans. In the Dutch famine of the 1944, not only did the fetuses that were exposed to under-nutrition in utero suffer from cardiovascular or metabolic disorders during their adulthood, but the grand-children also showed a lower birth weight [310]). In an independent study, an association between paternal grandfathers access to excessive food during prepuberty and the occurrence of cardiac disease or diabetes in their grandsons was shown [311]. Although molecular mechanisms remain to be detailed, these observations suggest that effects of environment are transmitted between generations via epigenetic (non-Mendelian) inheritance [312].
For over two decades, scientists have considered the possibility that epigenetic changes in the germline induced by exogenous factors can be inherited [313; 314]. Mitotic inheritance of the epigenetic marks was discussed in Section 2.1.1, and meiotic inheritance is described as follows. The epigenetic marks that are transmitted between generations and are described loosely as transgenerational epigenetic effects do not necessarily qualify as ‘transgenerational epigenetic inheritance’ [308]. The latter involves epigenetic modifications that are transmitted via germline. This distinction is important because the epigenetic marks are cleared between generations with some exception, such as imprinted genes (see below). In order for an transgenerational epigenetic effect to be considered inherited, the germline that gave rise to the affected offspring should not have been exposed to the environmental factors directly [315; 316]. When deciding whether a transgenerational epigenetic effect is inherited, the following criteria have to be fulfilled: the exposure specifically targets the germline, and if it does not, the exposure should occur before or during germ cell lineage specification [317] It was proposed that epigenetic inheritance is less commonly observed in mammals as compared to other organisms (e.g., plants) because the germ cells are specified much earlier in mammalian development. Thus, an event that may affect the embryo may not necessarily affect the germline [317].
5.1. General observations of transgenerational epigenetic effects
There are well-established examples of transgenerational inheritance of epigenetic patterns in some animal models [318] and plants [319]. In mice, results from studies at loci with metastable epialleles, such as viable yellow agouti (Avy) and axin fused (Axinfu) [318; 320; 321], show epigenetic transgenerational inheritance. The Avy, which is one of the best-studied examples of metastable epialleles and is at agouti (A) locus. Agouti gene is normally expressed in skin and gives the normal coat color of agouti mice. In the Avy allele, a transposon, intracisternal A particle (IAP) is inserted into the regulatory region of the gene, whose methylation status determines the expression level of the gene and gives variation of coat color ranging from complete agouti (pseudo-agouti) to yellow coat color [318]. The methylation of IAP silences the gene leading to pseudo-agouti and the lack of methylation leads to expression, and in turn yellow coat color. These alterations in the coat color are also associated with the overall health of the animals with pseudo-agouti being healthy and yellow being obese, diabetic, and susceptible to tumor development. Recent experiments have shown that both nutrients and EDCs (i.e., genistein or BPA) that these animals are exposed to during their development in utero affect the methylation status of IAP and hence the health of the mice [322; 323]. The relevant feature of these isogenic mouse strains to transgenerational epigenetic effect is that the epigenetic phenotype of the mother is a determinant of the epigenetic phenotype of the offspring. Thus, the epigenetic marks on the IAP are not erased during meiosis in the female germ cells but are inherited [318]. In plants, a similar type of inheritance has been observed for numerous loci for multiple generations [308]).
In humans, increased familial risk of colorectal cancer was linked to epimutations of tumor suppressor genes (see [324] for details). In addition, the abundance of transposons in the genome, lead one to speculate similar transgenerational epigenetic inheritance in humans [325]. However, given the outbred nature of humans, it would be nearly impossible to rule out the role of genetics in such transgenerational effects [326].
5.2. Transgenerational epigenetic effects associated with EDC exposures
Documentation of the transgenerational epigenetic effects of EDCs has been accumulating such as the case of transgenerational effects of DES exposure reported in humans [228]. Exposure to DES while in utero was shown to affect the female F2 generation but not males in mice [231]. Later studies with DES exposure showed that it leads to reproductive tract tumors in both F2 generation males and female mice [232; 233]. Exposure to vinclozolin and MXC during in utero testis development (E8-15) was shown to affect sperm parameters and testis function; these effects were transmitted through the male germline up to the four generations and were associated with DNA methylation alterations in the F2 and F3 generations [87]. Additional pathologies in other organ systems (e.g., kidney and prostate) were observed in 17–50% of the males [327]. Furthermore, F3 males displayed reproductive behavior and anxiety problems [328; 329]. However, milder pathologies (e.g., uterine bleeding and anemia) with a much weaker occurrence rate (6.5–8.6%) were observed in F1-F3 females when both parents were exposed to the EDC, but not when only one parent was exposed [330]. The reasons for lower penetrance and a requirement for both parents exposure in females can be because the timing of the exposure and establishment of genomic imprinting varies between males and females (see Section 3.1.1). To be most effective, the treatment for females should include in utero as well as early postnatal life, when the epigenetic reprogramming of female germ cells takes place (Figure 2). Alternatively, if the demethylation process could be influenced by EDCs, an exposure during this period should affect both males and females.
More recently, two research groups reported a failure to observe transgenerational effects of vinclozolin [331; 332]. However, another study demonstrated the transgenerational (F2 and F3 generation) effects of vinclozolin on imprinted genes in sperm (see below, [333]). The discrepancy between these studies can be due to differences in the route of exposure or in sub-strains of animals used in the studies [309]. Other EDCs were also reported to cause transgenerational epigenetic effects. For example, perinatal exposure to BPA can induce gene expression alterations in testicular steroid receptor co-regulators [334]. More recently, transgenerational epigenetic effects of phthalates in the gonad and dioxins in the uterus were also reported [335; 336].
5.3. Potential mechanisms of transgenerational epigenetic effects
In mammals, transgenerational epigenetic effects of EDCs can be potentially transmitted via multiple mechanisms, including histone proteins [269], and RNA [348] as well as DNA methylation. Here, we focus on DNA methylation.
DNA methylation alterations in different DNA sequences can be transmitted via (1) imprinted genes, (2) parasitic DNA sequences, and (3) imprinted-like sequences.
Imprinted genes. In the mammalian genome, approximately 1% of the autosomal genes are imprinted (i.e., expressed from only one parental allele). Genomic imprinting refers to the epigenetic form of gene regulation that involves DNA methylation in an allele-specific manner established in the gametes [28]. Genomic imprinting plays an important role in normal development and growth, and a disturbance of the genomic imprinting has been shown in developmental disorders such as Beckwith-Wiedemann and Prader-Willi/Angelman syndromes [337], and cancer [338]. One of the well-studied imprinted gene sets is Igf2 and H19, located on mouse chromosome 7 [339]. Igf2 is expressed from the paternal allele, and H19 is expressed from the maternal allele. This expression pattern is regulated by differential methylation that is established in a gender-specific development period (see Section 3.1.1); the methylation status of the differentially methylated region is shared between these two genes [340]. A disturbance during the imprinting process can affect the germ cells of F1 individual in a sex-specific manner, and the effect can be transmitted to the F2. Due to the epigenetic reprogramming of the germline, DNA methylation changes are less likely to be transmitted beyond F2. However, in the recent vinclozolin study, the effects of vinclozolin are reported on several imprinted genes, including H19, through the F3 generation [333]. Although effects on imprinted genes are reduced in F3, they did not completely disappear [333], suggesting a mechanism that makes EDC-induced changes are resistant to the germline epigenetic reprogramming and requires further investigation.
Parasitic DNA sequences. During the erasure and establishment of methylation patterns in imprinted genes [341], parasitic DNA sequences such as retrotransposons (e.g., LINE-1 and IAP) remain mostly methylated [89; 342]. Even a minor disruption of methylation patterns of these sequences can lead to a broad spectrum of abnormalities ranging from metabolic disorders to cancer [325; 343; 344]. As in the case of viable yellow agouti mice, these sites can be altered by estrogenic EDCs such as BPA [323] or genistein [345]. Since these sites remain altered during epigenetic reprogramming, the changes can be transmitted to the next generation.
Imprinted-like DNA sequences. It was proposed that EDC exposure could generate imprinted-like DNA sequences [346]. Since these sequences are not normally set for reprogramming, the changes on them may not be corrected between generations and thus may be transmitted to the F3 generation and beyond, but further studies are needed to test this hypothesis.
6. Conclusions and future directions
Data from the reviewed studies collectively suggest that perinatal EDC exposure affects adult ovaries and female reproductive tissues, leading to reproductive dysfunction, supporting the DoHAD concept. In addition, these effects are mediated primarily by steroid hormone receptors, especially the ERs: via ERβ in the ovary and ERα in the uterus and perhaps both in the hypothalamus and pituitary [143; 156; 157; 202; 267]. Furthermore, most of the EDCs discussed in this article lead to MOF (a potential precursor to premature ovarian failure), with the process being mediated by ERβ agonistic actions [158]. On the other hand, EDCs with ERβ antagonistic actions showed inhibitory effects on follicular maturation, and also lead to reduced fertility in females.
An emerging theme from these animal studies is that perinatal EDC exposure can cause epigenetic alterations, and these can be modified by later hormone stimulation. Studies have confirmed that the proliferative response to can be modulated by endogenous hormones. Furthermore, hypomethylation of ER-responsive genes are closely tied to the process of overexpression of proto-oncogenes and thereby carcionogenesis [349]. Some studies suggest that ERs are themselves epigenetically modified after EDC exposure [143]. Cancer epigenomic studies have already demonstrated a role for ERs in the proliferation and progression of tumors in human cancers. For example, increased ERβ gene expression and decreased DNA methylation were observed in endometriotic tissues as compared to normal endometrial tissues [350]. Hyper-methylation and reduced gene expression of ERβ were also observed in breast and prostate cancers [351; 352; 353; 354].
It is also clear that low doses of EDCs have effects on the epigenetic signature of a tissue, which may not necessarily translate into physiological changes in the exposed animals [143]. Studies using mixtures of EDCs show that while the dose of a given EDC may not have any obvious effects when used alone, it becomes effective when in mixtures [24]. Humans are exposed to mixtures of EDCs over their lifetime, and it is quite possible that they may accumulate numerous patterns of epigenetic alterations and yet be asymptomatic until an additional stressor is applied, at which time the symptoms become apparent. Validating such a hypothesis in a biologically relevant model system is critical.
Current epigenetic analysis methods used for discovering new genes in this area include MSRF and AP-PCR which have relatively low coverage (Table 1). Although results from these methods provided valuable “proof-of-principle” type of information, more comprehensive methods of analysis are needed for a better understanding of the broader effects of EDCs. For example, Tang and colleagues showed that neonatal DES and genistein exposures with or without ovarian steroid exposure caused vastly different epigenotypes of the Nsbp1 gene, which in turn resulted in alterations in its gene expression in aged animals [204]. These authors used MSRF to identify the targets. Earlier studies by Li and coworkers used the bisulfite-sequencing technique for examining individual uterine genes after DES exposure [349]. The genes examined in the latter studies, c-fos and lactoferrin, were not identified as candidates using the MSRF technique. Similarly, bisulfite-sequencing of the ERβ promoter in MXC-treated ovaries showed that it was hypermethylated. However, ERβ was not among the candidates discovered by AP-PCR analysis [143]. Distribution of RE-digestion sites may be a limiting factor since they may not be ubiquitous. Therefore, use of targeted array-based technologies to identify entire pathways is warranted. Now, with a combination of deep sequencing after ChIP (ChIP-seq) or bisulfite treated DNA on targeted arrays or immunoprecipitated DNA on targeted arrays, we can identify the exact methylation changes within a resolution of 2–3 bases. These new approaches can be used to determine epigenetic alterations across time (neonatal to pubertal to aged animals) and across dose (ranging from environmentally relevant low doses to high doses). Once pathways are identified, comparisons can be drawn across estrogenic EDCs as to whether they are relative in terms of estrogenic effects or specific to the particular EDC. A case in point are data from microarray analyses that show that different doses of estrogens result in completely different sets of genes that were up- or down-regulated, suggesting a non-monotonic effect [266; 355; 356]. To further test such a hypothesis, for example, that all ER responsive gene pathways are epigenetically modified by a particular EDC, collaborative multi- lab, multi-platform, and multi-tissue studies would have to be conducted. Biobanking of samples that are appropriate for purposes of various analyses from multiple tissues would be necessary.
Biomarker panels can be developed for diagnosis of past exposures at perinatal and pubertal periods so that potential risks can be assessed for preventative and therapeutic purposes. The demonstration that epigenetic changes can be reversed with nutrient supplementation, such as with the Avy mice, has been an important step in the field. However, can this be applicable to reversing or preventing epigenetic alterations induced by developmental EDC exposure?
There are a few reports as to the effects of EDCs transmitted to subsequent generations by the male germline, however very little is known about the female germline in such instances. In addition, the role of epigenetic mechanisms that are affected by the EDC exposures and importantly, the quality of the oocytes from such exposures need to be closely examined. If such effects do exist, the public health risk would be enormous. Future studies are likely to bring fruitful discoveries with an ultimate goal of using the obtained information for improving public health.
Supplementary Material
Acknowledgments
The authors wish to thank Dr. Kathy Manger for her assistance in the preparation of this manuscript. The research results from our laboratory described in this review supported in part by the NIEHS grant ES013854 and the NIEHS sponsored UMDNJ Center for Environmental Exposures and Disease grant NIEHS P30ES005022.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Crain DA, Janssen SJ, Edwards TM, Heindel J, Ho SM, Hunt P, Iguchi T, Juul A, McLachlan JA, Schwartz J, Skakkebaek N, Soto AM, Swan S, Walker C, Woodruff TK, Woodruff TJ, Giudice LC, Guillette LJ., Jr Female reproductive disorders: the roles of endocrine-disrupting compounds and developmental timing. Fertil Steril. 2008;90:911–40. doi: 10.1016/j.fertnstert.2008.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barrett J, Gonzalez S, Sarantis H, Varshavsky J. Girl, Disrupted: Hormone Disruptors and Women’s Reproductive Health Collaborative on Health and the Environment. Bolinas, CA: 2009. p. 35. [Google Scholar]
- 3.Diamanti-Kandarakis E, Bourguignon JP, Giudice LC, Hauser R, Prins GS, Soto AM, Zoeller RT, Gore AC. Endocrine-disrupting chemicals: an Endocrine Society scientific statement. Endocr Rev. 2009;30:293–342. doi: 10.1210/er.2009-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker DJ. Fetal origins of coronary heart disease. BMJ. 1995;311:171–4. doi: 10.1136/bmj.311.6998.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barker DJ. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58:114–5. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA, Bateson P, Beedle AS, Law CM, Bhutta ZA, Anokhin KV, Bougneres P, Chandak GR, Dasgupta P, Smith GD, Ellison PT, Forrester TE, Gilbert SF, Jablonka E, Kaplan H, Prentice AM, Simpson SJ, Uauy R, West-Eberhard MJ. Towards a new developmental synthesis: adaptive developmental plasticity and human disease. Lancet. 2009;373:1654–7. doi: 10.1016/S0140-6736(09)60234-8. [DOI] [PubMed] [Google Scholar]
- 7.Crisp TM, Clegg ED, Cooper RL, Wood WP, Anderson DG, Baetcke KP, Hoffmann JL, Morrow MS, Rodier DJ, Schaeffer JE, Touart LW, Zeeman MG, Patel YM. Environmental endocrine disruption: an effects assessment and analysis. Environ Health Perspect 106 Suppl. 1998;1:11–56. doi: 10.1289/ehp.98106s111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Toppari J, Larsen JC, Christiansen P, Giwercman A, Grandjean P, Guillette LJ, Jr, Jegou B, Jensen TK, Jouannet P, Keiding N, Leffers H, McLachlan JA, Meyer O, Muller J, Rajpert-De Meyts E, Scheike T, Sharpe R, Sumpter J, Skakkebaek NE. Male reproductive health and environmental xenoestrogens. Environ Health Perspect 104 Suppl. 1996;4:741–803. doi: 10.1289/ehp.96104s4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carsen R. Silent Spring. 1962. [Google Scholar]
- 10.Hamilton BE, Ventura SJ. Fertility and abortion rates in the United States, 1960–2002. Int J Androl. 2006;29:34–45. doi: 10.1111/j.1365-2605.2005.00638.x. [DOI] [PubMed] [Google Scholar]
- 11.Guzick DS, Swan S. The decline of infertility: apparent or real? Fertil Steril. 2006;86:524–6. doi: 10.1016/j.fertnstert.2006.05.027. discussion 534. [DOI] [PubMed] [Google Scholar]
- 12.Cohn BA, Cirillo PM, Wolff MS, Schwingl PJ, Cohen RD, Sholtz RI, Ferrara A, Christianson RE, van den Berg BJ, Siiteri PK. DDT and DDE exposure in mothers and time to pregnancy in daughters. Lancet. 2003;361:2205–6. doi: 10.1016/S0140-6736(03)13776-2. [DOI] [PubMed] [Google Scholar]
- 13.vom Saal FS, Akingbemi BT, Belcher SM, Birnbaum LS, Crain DA, Eriksen M, Farabollini F, Guillette LJ, Jr, Hauser R, Heindel JJ, Ho SM, Hunt PA, Iguchi T, Jobling S, Kanno J, Keri RA, Knudsen KE, Laufer H, LeBlanc GA, Marcus M, McLachlan JA, Myers JP, Nadal A, Newbold RR, Olea N, Prins GS, Richter CA, Rubin BS, Sonnenschein C, Soto AM, Talsness CE, Vandenbergh JG, Vandenberg LN, Walser-Kuntz DR, Watson CS, Welshons WV, Wetherill Y, Zoeller RT. Chapel Hill bisphenol A expert panel consensus statement: integration of mechanisms, effects in animals and potential to impact human health at current levels of exposure. Reprod Toxicol. 2007;24:131–8. doi: 10.1016/j.reprotox.2007.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vandenberg LN, Maffini MV, Sonnenschein C, Rubin BS, Soto AM. Bisphenol-A and the great divide: a review of controversies in the field of endocrine disruption. Endocr Rev. 2009;30:75–95. doi: 10.1210/er.2008-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.NTP-CERHR. Expert panel report on the reproductive and developmental toxicity of bisphenol A. 2007 doi: 10.1002/bdrb.20147. http://cerhr.niehs.nih.gov/chemicals/bisphenol/BPAFinalEPVF112607.pdf. [DOI] [PubMed]
- 16.Mok-Lin E, Ehrlich S, Williams PL, Petrozza J, Wright DL, Calafat AM, Ye X, Hauser R. Urinary bisphenol A concentrations and ovarian response among women undergoing IVF. Int J Androl. 2009 doi: 10.1111/j.1365-2605.2009.01014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada H, Furuta I, Kato EH, Kataoka S, Usuki Y, Kobashi G, Sata F, Kishi R, Fujimoto S. Maternal serum and amniotic fluid bisphenol A concentrations in the early second trimester. Reprod Toxicol. 2002;16:735–9. doi: 10.1016/s0890-6238(02)00051-5. [DOI] [PubMed] [Google Scholar]
- 18.D’Aloisio AA, Baird DD, DeRoo LA, Sandler DP. Association of intrauterine and early-life exposures with diagnosis of uterine leiomyomata by 35 years of age in the sister study. Environ Health Perspect. 2010;118:375–81. doi: 10.1289/ehp.0901423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newbold RR. Lessons learned from perinatal exposure to diethylstilbestrol. Toxicol Appl Pharmacol. 2004;199:142–50. doi: 10.1016/j.taap.2003.11.033. [DOI] [PubMed] [Google Scholar]
- 20.Guillette LJ, Jr, Gross TS, Masson GR, Matter JM, Percival HF, Woodward AR. Developmental abnormalities of the gonad and abnormal sex hormone concentrations in juvenile alligators from contaminated and control lakes in Florida. Environ Health Perspect. 1994;102:680–8. doi: 10.1289/ehp.94102680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guillette LJ, Jr, Gunderson MP. Alterations in development of reproductive and endocrine systems of wildlife populations exposed to endocrine-disrupting contaminants. Reproduction. 2001;122:857–64. doi: 10.1530/rep.0.1220857. [DOI] [PubMed] [Google Scholar]
- 22.Iguchi T, Takasugi N, Bern HA, Mills KT. Frequent occurrence of polyovular follicles in ovaries of mice exposed neonatally to diethylstilbestrol. Teratology. 1986;34:29–35. doi: 10.1002/tera.1420340105. [DOI] [PubMed] [Google Scholar]
- 23.Jefferson WN, Padilla-Banks E, Newbold RR. Adverse effects on female development and reproduction in CD-1 mice following neonatal exposure to the phytoestrogen genistein at environmentally relevant doses. Biol Reprod. 2005;73:798–806. doi: 10.1095/biolreprod.105.041277. [DOI] [PubMed] [Google Scholar]
- 24.Kortenkamp A. Low dose mixture effects of endocrine disrupters: implications for risk assessment and epidemiology. Int J Androl. 2008;31:233–40. doi: 10.1111/j.1365-2605.2007.00862.x. [DOI] [PubMed] [Google Scholar]
- 25.Kringlen E. Twin studies in schizophrenia with special emphasis on concordance figures. Am J Med Genet. 2000;97:4–11. doi: 10.1002/(sici)1096-8628(200021)97:1<4::aid-ajmg2>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 26.Wong AH, Gottesman II, Petronis A. Phenotypic differences in genetically identical organisms: the epigenetic perspective. Hum Mol Genet. 2005;14(Spec No 1):R11–8. doi: 10.1093/hmg/ddi116. [DOI] [PubMed] [Google Scholar]
- 27.Fraga MF, Ballestar E, Paz MF, Ropero S, Setien F, Ballestar ML, Heine-Suner D, Cigudosa JC, Urioste M, Benitez J, Boix-Chornet M, Sanchez-Aguilera A, Ling C, Carlsson E, Poulsen P, Vaag A, Stephan Z, Spector TD, Wu YZ, Plass C, Esteller M. Epigenetic differences arise during the lifetime of monozygotic twins. Proc Natl Acad Sci U S A. 2005;102:10604–9. doi: 10.1073/pnas.0500398102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–62. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jablonka E, Lamb MJ. The changing concept of epigenetics. Ann N Y Acad Sci. 2002;981:82–96. doi: 10.1111/j.1749-6632.2002.tb04913.x. [DOI] [PubMed] [Google Scholar]
- 30.Holliday R. Epigenetics: a historical overview. Epigenetics. 2006;1:76–80. doi: 10.4161/epi.1.2.2762. [DOI] [PubMed] [Google Scholar]
- 31.Van Speybroeck L. From epigenesis to epigenetics: the case of C. H. Waddington. Ann N Y Acad Sci. 2002;981:61–81. [PubMed] [Google Scholar]
- 32.Wu C, Morris JR. Genes, genetics, and epigenetics: a correspondence. Science. 2001;293:1103–5. doi: 10.1126/science.293.5532.1103. [DOI] [PubMed] [Google Scholar]
- 33.Rubin H. Etymology of epigenetics. Science. 2001;294:2477–8. doi: 10.1126/science.294.5551.2477c. [DOI] [PubMed] [Google Scholar]
- 34.Riggs AD. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14:9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- 35.Holliday R, Pugh JE. DNA modification mechanisms and gene activity during development. Science. 1975;187:226–32. [PubMed] [Google Scholar]
- 36.Ooi SK, Bestor TH. The colorful history of active DNA demethylation. Cell. 2008;133:1145–8. doi: 10.1016/j.cell.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 37.Turner BM. Epigenetic responses to environmental change and their evolutionary implications. Philos Trans R Soc Lond B Biol Sci. 2009;364:3403–18. doi: 10.1098/rstb.2009.0125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Illingworth RS, Bird AP. CpG islands--‘a rough guide’. FEBS Lett. 2009;583:1713–20. doi: 10.1016/j.febslet.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Sharma S, Kelly TK, Jones PA. Epigenetics in cancer. Carcinogenesis. 2010;31:27–36. doi: 10.1093/carcin/bgp220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bird A. DNA methylation patterns and epigenetic memory. Genes Dev. 2002;16:6–21. doi: 10.1101/gad.947102. [DOI] [PubMed] [Google Scholar]
- 41.Tang WY, Ho SM. Epigenetic reprogramming and imprinting in origins of disease. Rev Endocr Metab Disord. 2007;8:173–82. doi: 10.1007/s11154-007-9042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yagi M, Koshland ME. Expression of the J chain gene during B cell differentiation is inversely correlated with DNA methylation. Proc Natl Acad Sci U S A. 1981;78:4907–11. doi: 10.1073/pnas.78.8.4907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarrey J. The Role of DNA Methylation in Regulating Tissue-Specific Gene Expression During Gametogenesis. The 42nd Annual Meeting of the Society for Study of Reproduction; Pittsburgh, PA. 2009. [Google Scholar]
- 44.Mohn F, Schubeler D. Genetics and epigenetics: stability and plasticity during cellular differentiation. Trends Genet. 2009;25:129–36. doi: 10.1016/j.tig.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 45.Kelly TL, Trasler JM. Reproductive epigenetics. Clin Genet. 2004;65:247–60. doi: 10.1111/j.0009-9163.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 46.Li E. Chromatin modification and epigenetic reprogramming in mammalian development. Nat Rev Genet. 2002;3:662–73. doi: 10.1038/nrg887. [DOI] [PubMed] [Google Scholar]
- 47.La Salle S, Mertineit C, Taketo T, Moens PB, Bestor TH, Trasler JM. Windows for sex-specific methylation marked by DNA methyltransferase expression profiles in mouse germ cells. Dev Biol. 2004;268:403–15. doi: 10.1016/j.ydbio.2003.12.031. [DOI] [PubMed] [Google Scholar]
- 48.Lucifero D, Chaillet JR, Trasler JM. Potential significance of genomic imprinting defects for reproduction and assisted reproductive technology. Hum Reprod Update. 2004;10:3–18. doi: 10.1093/humupd/dmh002. [DOI] [PubMed] [Google Scholar]
- 49.Hiura H, Obata Y, Komiyama J, Shirai M, Kono T. Oocyte growth-dependent progression of maternal imprinting in mice. Genes Cells. 2006;11:353–61. doi: 10.1111/j.1365-2443.2006.00943.x. [DOI] [PubMed] [Google Scholar]
- 50.Schaefer CB, Ooi SK, Bestor TH, Bourc’his D. Epigenetic decisions in mammalian germ cells. Science. 2007;316:398–9. doi: 10.1126/science.1137544. [DOI] [PubMed] [Google Scholar]
- 51.Ueda T, Abe K, Miura A, Yuzuriha M, Zubair M, Noguchi M, Niwa K, Kawase Y, Kono T, Matsuda Y, Fujimoto H, Shibata H, Hayashizaki Y, Sasaki H. The paternal methylation imprint of the mouse H19 locus is acquired in the gonocyte stage during foetal testis development. Genes Cells. 2000;5:649–59. doi: 10.1046/j.1365-2443.2000.00351.x. [DOI] [PubMed] [Google Scholar]
- 52.Turner BM. Reading signals on the nucleosome with a new nomenclature for modified histones. Nat Struct Mol Biol. 2005;12:110–2. doi: 10.1038/nsmb0205-110. [DOI] [PubMed] [Google Scholar]
- 53.Hirose M, Sarui K, Sunagawa A. Correlation between nuclear histone acetylation and casein messenger RNA induction in the mammary gland. J Biochem. 1985;97:781–9. doi: 10.1093/oxfordjournals.jbchem.a135118. [DOI] [PubMed] [Google Scholar]
- 54.Sharma D, Blum J, Yang X, Beaulieu N, Macleod AR, Davidson NE. Release of methyl CpG binding proteins and histone deacetylase 1 from the Estrogen receptor alpha (ER) promoter upon reactivation in ER-negative human breast cancer cells. Mol Endocrinol. 2005;19:1740–51. doi: 10.1210/me.2004-0011. [DOI] [PubMed] [Google Scholar]
- 55.Yoo CB, Jones PA. Epigenetic therapy of cancer: past, present and future. Nat Rev Drug Discov. 2006;5:37–50. doi: 10.1038/nrd1930. [DOI] [PubMed] [Google Scholar]
- 56.Bredfeldt TG, Greathouse KL, Safe SH, Hung MC, Bedford MT, Walker CL. Xenoestrogen-induced regulation of EZH2 and histone methylation via estrogen receptor signaling to PI3K/AKT. Mol Endocrinol. 2010;24:993–1006. doi: 10.1210/me.2009-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kouzarides T. Chromatin modifications and their function. Cell. 2007;128:693–705. doi: 10.1016/j.cell.2007.02.005. [DOI] [PubMed] [Google Scholar]
- 58.Chang SC, Tucker T, Thorogood NP, Brown CJ. Mechanisms of X-chromosome inactivation. Front Biosci. 2006;11:852–66. doi: 10.2741/1842. [DOI] [PubMed] [Google Scholar]
- 59.Brosnan CA, Voinnet O. The long and the short of noncoding RNAs. Curr Opin Cell Biol. 2009;21:416–25. doi: 10.1016/j.ceb.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 60.Costa FF. Non-coding RNAs: new players in eukaryotic biology. Gene. 2005;357:83–94. doi: 10.1016/j.gene.2005.06.019. [DOI] [PubMed] [Google Scholar]
- 61.Costa FF. Non-coding RNAs, epigenetics and complexity. Gene. 2008;410:9–17. doi: 10.1016/j.gene.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 62.Namihira M, Kohyama J, Abematsu M, Nakashima K. Epigenetic mechanisms regulating fate specification of neural stem cells. Philos Trans R Soc Lond B Biol Sci. 2008;363:2099–109. doi: 10.1098/rstb.2008.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stearns V, Zhou Q, Davidson NE. Epigenetic regulation as a new target for breast cancer therapy. Cancer Invest. 2007;25:659–65. doi: 10.1080/07357900701719234. [DOI] [PubMed] [Google Scholar]
- 64.Cedar H, Bergman Y. Linking DNA methylation and histone modification: patterns and paradigms. Nat Rev Genet. 2009;10:295–304. doi: 10.1038/nrg2540. [DOI] [PubMed] [Google Scholar]
- 65.Dhasarathy A, Wade PA. The MBD protein family-reading an epigenetic mark? Mutat Res. 2008;647:39–43. doi: 10.1016/j.mrfmmm.2008.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Slotkin RK, Martienssen R. Transposable elements and the epigenetic regulation of the genome. Nat Rev Genet. 2007;8:272–85. doi: 10.1038/nrg2072. [DOI] [PubMed] [Google Scholar]
- 67.Chan SW. Inputs and outputs for chromatin-targeted RNAi. Trends Plant Sci. 2008;13:383–9. doi: 10.1016/j.tplants.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 68.Avner P, Heard E. X-chromosome inactivation: counting, choice and initiation. Nat Rev Genet. 2001;2:59–67. doi: 10.1038/35047580. [DOI] [PubMed] [Google Scholar]
- 69.Bartolomei MS. Genomic imprinting: employing and avoiding epigenetic processes. Genes Dev. 2009;23:2124–33. doi: 10.1101/gad.1841409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lister R, Pelizzola M, Dowen RH, Hawkins RD, Hon G, Tonti-Filippini J, Nery JR, Lee L, Ye Z, Ngo QM, Edsall L, Antosiewicz-Bourget J, Stewart R, Ruotti V, Millar AH, Thomson JA, Ren B, Ecker JR. Human DNA methylomes at base resolution show widespread epigenomic differences. Nature. 2009;462:315–22. doi: 10.1038/nature08514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Edson MA, Nagaraja AK, Matzuk MM. The mammalian ovary from genesis to revelation. Endocr Rev. 2009;30:624–712. doi: 10.1210/er.2009-0012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Saitou M, Barton SC, Surani MA. A molecular programme for the specification of germ cell fate in mice. Nature. 2002;418:293–300. doi: 10.1038/nature00927. [DOI] [PubMed] [Google Scholar]
- 73.Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–8. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- 74.McCarrey JR. Development of the germ cell. In: Desjardins C, Ewing LL, editors. Cell and Molecular Biology of the Testis. Oxford Univesity Press; New York: 1993. pp. 58–89. [Google Scholar]
- 75.McLaren A. Mammalian germ cells: birth, sex, and immortality. Cell Struct Funct. 2001;26:119–22. doi: 10.1247/csf.26.119. [DOI] [PubMed] [Google Scholar]
- 76.Berta P, Hawkins JR, Sinclair AH, Taylor A, Griffiths BL, Goodfellow PN, Fellous M. Genetic evidence equating SRY and the testis-determining factor. Nature. 1990;348:448–50. doi: 10.1038/348448A0. [DOI] [PubMed] [Google Scholar]
- 77.Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–21. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- 78.DiNapoli L, Capel B. SRY and the standoff in sex determination. Mol Endocrinol. 2008;22:1–9. doi: 10.1210/me.2007-0250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brennan J, Capel B. One tissue, two fates: molecular genetic events that underlie testis versus ovary development. Nat Rev Genet. 2004;5:509–21. doi: 10.1038/nrg1381. [DOI] [PubMed] [Google Scholar]
- 80.Sekido R, Lovell-Badge R. Sex determination and SRY: down to a wink and a nudge? Trends Genet. 2009;25:19–29. doi: 10.1016/j.tig.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 81.Small CL, Shima JE, Uzumcu M, Skinner MK, Griswold MD. Profiling gene expression during the differentiation and development of the murine embryonic gonad. Biol Reprod. 2005;72:492–501. doi: 10.1095/biolreprod.104.033696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nef S, Schaad O, Stallings NR, Cederroth CR, Pitetti JL, Schaer G, Malki S, Dubois-Dauphin M, Boizet-Bonhoure B, Descombes P, Parker KL, Vassalli JD. Gene expression during sex determination reveals a robust female genetic program at the onset of ovarian development. Dev Biol. 2005;287:361–77. doi: 10.1016/j.ydbio.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Cederroth CR, Pitetti JL, Papaioannou MD, Nef S. Genetic programs that regulate testicular and ovarian development. Mol Cell Endocrinol. 2007;265–266:3–9. doi: 10.1016/j.mce.2006.12.029. [DOI] [PubMed] [Google Scholar]
- 84.Clement TM, Anway MD, Uzumcu M, Skinner MK. Regulation of the gonadal transcriptome during sex determination and testis morphogenesis: comparative candidate genes. Reproduction. 2007;134:455–72. doi: 10.1530/REP-06-0341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yao HH. The pathway to femaleness: current knowledge on embryonic development of the ovary. Mol Cell Endocrinol. 2005;230:87–93. doi: 10.1016/j.mce.2004.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Reik W, Dean W, Walter J. Epigenetic reprogramming in mammalian development. Science. 2001;293:1089–93. doi: 10.1126/science.1063443. [DOI] [PubMed] [Google Scholar]
- 87.Anway MD, Cupp AS, Uzumcu M, Skinner MK. Epigenetic transgenerational actions of endocrine disruptors and male fertility. Science. 2005;308:1466–9. doi: 10.1126/science.1108190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allegrucci C, Thurston A, Lucas E, Young L. Epigenetics and the germline. Reproduction. 2005;129:137–49. doi: 10.1530/rep.1.00360. [DOI] [PubMed] [Google Scholar]
- 89.Hajkova P, Erhardt S, Lane N, Haaf T, El-Maarri O, Reik W, Walter J, Surani MA. Epigenetic reprogramming in mouse primordial germ cells. Mech Dev. 2002;117:15–23. doi: 10.1016/s0925-4773(02)00181-8. [DOI] [PubMed] [Google Scholar]
- 90.Coffigny H, Bourgeois C, Ricoul M, Bernardino J, Vilain A, Niveleau A, Malfoy B, Dutrillaux B. Alterations of DNA methylation patterns in germ cells and Sertoli cells from developing mouse testis. Cytogenet Cell Genet. 1999;87:175–81. doi: 10.1159/000015460. [DOI] [PubMed] [Google Scholar]
- 91.McGee EA, Perlas E, LaPolt PS, Tsafriri A, Hsueh AJ. Follicle-stimulating hormone enhances the development of preantral follicles in juvenile rats. Biol Reprod. 1997;57:990–8. doi: 10.1095/biolreprod57.5.990. [DOI] [PubMed] [Google Scholar]
- 92.Obata Y, Kono T. Maternal primary imprinting is established at a specific time for each gene throughout oocyte growth. J Biol Chem. 2002;277:5285–9. doi: 10.1074/jbc.M108586200. [DOI] [PubMed] [Google Scholar]
- 93.Pepling ME, Spradling AC. Mouse ovarian germ cell cysts undergo programmed breakdown to form primordial follicles. Dev Biol. 2001;234:339–51. doi: 10.1006/dbio.2001.0269. [DOI] [PubMed] [Google Scholar]
- 94.Perez GI, Robles R, Knudson CM, Flaws JA, Korsmeyer SJ, Tilly JL. Prolongation of ovarian lifespan into advanced chronological age by Bax-deficiency. Nat Genet. 1999;21:200–3. doi: 10.1038/5985. [DOI] [PubMed] [Google Scholar]
- 95.Skinner MK. Regulation of primordial follicle assembly and development. Hum Reprod Update. 2005;11:461–71. doi: 10.1093/humupd/dmi020. [DOI] [PubMed] [Google Scholar]
- 96.Merchant H. Rat gonadal and ovarioan organogenesis with and without germ cells. An ultrastructural study. Dev Biol. 1975;44:1–21. doi: 10.1016/0012-1606(75)90372-3. [DOI] [PubMed] [Google Scholar]
- 97.Soyal SM, Amleh A, Dean J, Galpha FI. a germ cell-specific transcription factor required for ovarian follicle formation. Development. 2000;127:4645–54. doi: 10.1242/dev.127.21.4645. [DOI] [PubMed] [Google Scholar]
- 98.Morrison LJ, Marcinkiewicz JL. Tumor necrosis factor alpha enhances oocyte/follicle apoptosis in the neonatal rat ovary. Biol Reprod. 2002;66:450–7. doi: 10.1095/biolreprod66.2.450. [DOI] [PubMed] [Google Scholar]
- 99.Dissen GA, Hirshfield AN, Malamed S, Ojeda SR. Expression of neurotrophins and their receptors in the mammalian ovary is developmentally regulated: changes at the time of folliculogenesis. Endocrinology. 1995;136:4681–92. doi: 10.1210/endo.136.10.7664689. [DOI] [PubMed] [Google Scholar]
- 100.Billiar RB, Zachos NC, Burch MG, Albrecht ED, Pepe GJ. Up-regulation of alpha-inhibin expression in the fetal ovary of estrogen-suppressed baboons is associated with impaired fetal ovarian folliculogenesis. Biol Reprod. 2003;68:1989–96. doi: 10.1095/biolreprod.102.011908. [DOI] [PubMed] [Google Scholar]
- 101.Bristol-Gould SK, Kreeger PK, Selkirk CG, Kilen SM, Cook RW, Kipp JL, Shea LD, Mayo KE, Woodruff TK. Postnatal regulation of germ cells by activin: the establishment of the initial follicle pool. Dev Biol. 2006;298:132–48. doi: 10.1016/j.ydbio.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 102.Kezele P, Skinner MK. Regulation of ovarian primordial follicle assembly and development by estrogen and progesterone: endocrine model of follicle assembly. Endocrinology. 2003;144:3329–37. doi: 10.1210/en.2002-0131. [DOI] [PubMed] [Google Scholar]
- 103.Chen Y, Jefferson WN, Newbold RR, Padilla-Banks E, Pepling ME. Estradiol, progesterone, and genistein inhibit oocyte nest breakdown and primordial follicle assembly in the neonatal mouse ovary in vitro and in vivo. Endocrinology. 2007;148:3580–90. doi: 10.1210/en.2007-0088. [DOI] [PubMed] [Google Scholar]
- 104.Nilsson EE, Stanfield J, Skinner MK. Interactions between progesterone and tumor necrosis factor-alpha in the regulation of primordial follicle assembly. Reproduction. 2006;132:877–86. doi: 10.1530/REP-06-0045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim H, Nakajima T, Hayashi S, Chambon P, Watanabe H, Iguchi T, Sato T. Effects of diethylstilbestrol on programmed oocyte death and induction of polyovular follicles in neonatal mouse ovaries. Biol Reprod. 2009;81:1002–9. doi: 10.1095/biolreprod.108.070599. [DOI] [PubMed] [Google Scholar]
- 106.Kipp JL, Kilen SM, Bristol-Gould S, Woodruff TK, Mayo KE. Neonatal exposure to estrogens suppresses activin expression and signaling in the mouse ovary. Endocrinology. 2007;148:1968–76. doi: 10.1210/en.2006-1083. [DOI] [PubMed] [Google Scholar]
- 107.McGee EA, Hsueh AJ. Initial and cyclic recruitment of ovarian follicles. Endocr Rev. 2000;21:200–14. doi: 10.1210/edrv.21.2.0394. [DOI] [PubMed] [Google Scholar]
- 108.Hutt KJ, McLaughlin EA, Holland MK. Kit ligand and c-Kit have diverse roles during mammalian oogenesis and folliculogenesis. Mol Hum Reprod. 2006;12:61–9. doi: 10.1093/molehr/gal010. [DOI] [PubMed] [Google Scholar]
- 109.Motro B, Bernstein A. Dynamic changes in ovarian c-kit and Steel expression during the estrous reproductive cycle. Dev Dyn. 1993;197:69–79. doi: 10.1002/aja.1001970107. [DOI] [PubMed] [Google Scholar]
- 110.Huang EJ, Manova K, Packer AI, Sanchez S, Bachvarova RF, Besmer P. The murine steel panda mutation affects kit ligand expression and growth of early ovarian follicles. Dev Biol. 1993;157:100–9. doi: 10.1006/dbio.1993.1115. [DOI] [PubMed] [Google Scholar]
- 111.Bedell MA, Brannan CI, Evans EP, Copeland NG, Jenkins NA, Donovan PJ. DNA rearrangements located over 100 kb 5′ of the Steel (Sl)-coding region in Steel-panda and Steel-contrasted mice deregulate Sl expression and cause female sterility by disrupting ovarian follicle development. Genes Dev. 1995;9:455–70. doi: 10.1101/gad.9.4.455. [DOI] [PubMed] [Google Scholar]
- 112.Packer AI, Hsu YC, Besmer P, Bachvarova RF. The ligand of the c-kit receptor promotes oocyte growth. Dev Biol. 1994;161:194–205. doi: 10.1006/dbio.1994.1020. [DOI] [PubMed] [Google Scholar]
- 113.Lees-Murdock DJ, Lau HT, Castrillon DH, De Felici M, Walsh CP. DNA methyltransferase loading, but not de novo methylation, is an oocyte-autonomous process stimulated by SCF signalling. Dev Biol. 2008;321:238–50. doi: 10.1016/j.ydbio.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 114.Durlinger AL, Kramer P, Karels B, de Jong FH, Uilenbroek JT, Grootegoed JA, Themmen AP. Control of primordial follicle recruitment by anti-Mullerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–96. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 115.Durlinger AL, Gruijters MJ, Kramer P, Karels B, Ingraham HA, Nachtigal MW, Uilenbroek JT, Grootegoed JA, Themmen AP. Anti-Mullerian hormone inhibits initiation of primordial follicle growth in the mouse ovary. Endocrinology. 2002;143:1076–84. doi: 10.1210/endo.143.3.8691. [DOI] [PubMed] [Google Scholar]
- 116.Durlinger AL, Visser JA, Themmen AP. Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124:601–9. doi: 10.1530/rep.0.1240601. [DOI] [PubMed] [Google Scholar]
- 117.Uzumcu M, Kuhn PE, Marano JE, Armenti AE, Passantino L. Early postnatal methoxychlor exposure inhibits folliculogenesis and stimulates anti-Mullerian hormone production in the rat ovary. J Endocrinol. 2006;191:549–58. doi: 10.1677/joe.1.06592. [DOI] [PubMed] [Google Scholar]
- 118.Guerrier D, Boussin L, Mader S, Josso N, Kahn A, Picard JY. Expression of the gene for anti-Mullerian hormone. J Reprod Fertil. 1990;88:695–706. doi: 10.1530/jrf.0.0880695. [DOI] [PubMed] [Google Scholar]
- 119.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Neonatal estrogen exposure inhibits steroidogenesis in the developing rat ovary. Dev Dyn. 2001;221:443–53. doi: 10.1002/dvdy.1162. [DOI] [PubMed] [Google Scholar]
- 120.Ikeda Y, Nagai A, Ikeda MA, Hayashi S. Increased expression of Mullerian-inhibiting substance correlates with inhibition of follicular growth in the developing ovary of rats treated with E2 benzoate. Endocrinology. 2002;143:304–12. doi: 10.1210/endo.143.1.8603. [DOI] [PubMed] [Google Scholar]
- 121.Britt KL, Saunders PK, McPherson SJ, Misso ML, Simpson ER, Findlay JK. Estrogen actions on follicle formation and early follicle development. Biol Reprod. 2004;71:1712–23. doi: 10.1095/biolreprod.104.028175. [DOI] [PubMed] [Google Scholar]
- 122.Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor Foxo3a. Science. 2003;301:215–8. doi: 10.1126/science.1086336. [DOI] [PubMed] [Google Scholar]
- 123.Yang JL, Zhang CP, Li L, Huang L, Ji SY, Lu CL, Fan CH, Cai H, Ren Y, Hu ZY, Gao F, Liu YX. Testosterone induces redistribution of forkhead box-3a and down-regulation of growth and differentiation factor 9 messenger ribonucleic acid expression at early stage of mouse folliculogenesis. Endocrinology. 151:774–82. doi: 10.1210/en.2009-0751. [DOI] [PubMed] [Google Scholar]
- 124.Hirshfield AN. Theca cells may be present at the outset of follicular growth. Biol Reprod. 1991;44:1157–62. doi: 10.1095/biolreprod44.6.1157. [DOI] [PubMed] [Google Scholar]
- 125.Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–5. doi: 10.1038/383531a0. [DOI] [PubMed] [Google Scholar]
- 126.Nilsson EE, Skinner MK. Growth and differentiation factor-9 stimulates progression of early primary but not primordial rat ovarian follicle development. Biol Reprod. 2002;67:1018–24. doi: 10.1095/biolreprod.101.002527. [DOI] [PubMed] [Google Scholar]
- 127.Parrott JA, Skinner MK. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology. 1999;140:4262–71. doi: 10.1210/endo.140.9.6994. [DOI] [PubMed] [Google Scholar]
- 128.Parrott JA, Skinner MK. Kit ligand actions on ovarian stromal cells: effects on theca cell recruitment and steroid production. Mol Reprod Dev. 2000;55:55–64. doi: 10.1002/(SICI)1098-2795(200001)55:1<55::AID-MRD8>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 129.Ojeda SR, Ramirez VD. Plasma level of LH and FSH in maturing rats: response to hemigonadectomy. Endocrinology. 1972;90:466–72. doi: 10.1210/endo-90-2-466. [DOI] [PubMed] [Google Scholar]
- 130.Sokka T, Huhtaniemi I. Ontogeny of gonadotrophin receptors and gonadotrophin-stimulated cyclic AMP production in the neonatal rat ovary. J Endocrinol. 1990;127:297–303. doi: 10.1677/joe.0.1270297. [DOI] [PubMed] [Google Scholar]
- 131.Kumar TR. What have we learned about gonadotropin function from gonadotropin subunit and receptor knockout mice? Reproduction. 2005;130:293–302. doi: 10.1530/rep.1.00660. [DOI] [PubMed] [Google Scholar]
- 132.Uzumcu M, Zachow R. Developmental exposure to environmental endocrine disruptors: consequences within the ovary and on female reproductive function. Reprod Toxicol. 2007;23:337–52. doi: 10.1016/j.reprotox.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Magoffin DA. Ovarian theca cell. Int J Biochem Cell Biol. 2005;37:1344–9. doi: 10.1016/j.biocel.2005.01.016. [DOI] [PubMed] [Google Scholar]
- 134.Palermo R. Differential actions of FSH and LH during folliculogenesis. Reprod Biomed Online. 2007;15:326–37. doi: 10.1016/s1472-6483(10)60347-1. [DOI] [PubMed] [Google Scholar]
- 135.Zachow R, Uzumcu M. The hepatocyte growth factor system as a regulator of female and male gonadal function. J Endocrinol. 2007;195:359–71. doi: 10.1677/JOE-07-0466. [DOI] [PubMed] [Google Scholar]
- 136.Kwintkiewicz J, Giudice LC. The interplay of insulin-like growth factors, gonadotropins, and endocrine disruptors in ovarian follicular development and function. Semin Reprod Med. 2009;27:43–51. doi: 10.1055/s-0028-1108009. [DOI] [PubMed] [Google Scholar]
- 137.Mazerbourg S, Bondy CA, Zhou J, Monget P. The insulin-like growth factor system: a key determinant role in the growth and selection of ovarian follicles? a comparative species study. Reprod Domest Anim. 2003;38:247–58. doi: 10.1046/j.1439-0531.2003.00440.x. [DOI] [PubMed] [Google Scholar]
- 138.DdeMoura M, Choi D, Adashi EY, Payne DW. Insulin-like growth factor-I-mediated amplification of follicle-stimulating hormone-supported progesterone accumulation by cultured rat granulosa cells: enhancement of steroidogenic enzyme activity and expression. Biol Reprod. 1997;56:946–53. doi: 10.1095/biolreprod56.4.946. [DOI] [PubMed] [Google Scholar]
- 139.Ui M, Shimonaka M, Shimasaki S, Ling N. An insulin-like growth factor-binding protein in ovarian follicular fluid blocks follicle-stimulating hormone-stimulated steroid production by ovarian granulosa cells. Endocrinology. 1989;125:912–6. doi: 10.1210/endo-125-2-912. [DOI] [PubMed] [Google Scholar]
- 140.Cataldo NA, Woodruff TK, Giudice LC. Regulation of insulin-like growth factor binding protein production by human luteinizing granulosa cells cultured in defined medium. J Clin Endocrinol Metab. 1993;76:207–15. doi: 10.1210/jcem.76.1.7678423. [DOI] [PubMed] [Google Scholar]
- 141.Conover CA, Faessen GF, Ilg KE, Chandrasekher YA, Christiansen M, Overgaard MT, Oxvig C, Giudice LC. Pregnancy-associated plasma protein-a is the insulin-like growth factor binding protein-4 protease secreted by human ovarian granulosa cells and is a marker of dominant follicle selection and the corpus luteum. Endocrinology. 2001;142:2155. doi: 10.1210/endo.142.5.8286. [DOI] [PubMed] [Google Scholar]
- 142.Nyegaard M, Overgaard MT, Su YQ, Hamilton AE, Kwintkiewicz J, Hsieh M, Nayak NR, Conti M, Conover CA, Giudice LC. Lack of Functional Pregnancy-Associated Plasma Protein-A (PAPPA) Compromises Mouse Ovarian Steroidogenesis and Female Fertility. Biol Reprod. doi: 10.1095/biolreprod.109.079517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zama AM, Uzumcu M. Fetal and neonatal exposure to the endocrine disruptor methoxychlor causes epigenetic alterations in adult ovarian genes. Endocrinology. 2009;150:4681–91. doi: 10.1210/en.2009-0499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Richards JS. Genetics of ovulation. Semin Reprod Med. 2007;25:235–42. doi: 10.1055/s-2007-980217. [DOI] [PubMed] [Google Scholar]
- 145.Henkes LE, Davis JS, Rueda BR. Mutant mouse models and their contribution to our knowledge of corpus luteum development, function and regression. Reprod Biol Endocrinol. 2003;1:87. doi: 10.1186/1477-7827-1-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Stocco C, Telleria C, Gibori G. The molecular control of corpus luteum formation, function, and regression. Endocr Rev. 2007;28:117–49. doi: 10.1210/er.2006-0022. [DOI] [PubMed] [Google Scholar]
- 147.Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 54:397–408. doi: 10.1387/ijdb.082758ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Nilsson EE, Skinner MK. Progesterone regulation of primordial follicle assembly in bovine fetal ovaries. Mol Cell Endocrinol. 2009;313:9–16. doi: 10.1016/j.mce.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Sar M, Welsch F. Differential expression of estrogen receptor-beta and estrogen receptor-alpha in the rat ovary. Endocrinology. 1999;140:963–71. doi: 10.1210/endo.140.2.6533. [DOI] [PubMed] [Google Scholar]
- 150.Chen Y, Breen K, Pepling ME. Estrogen can signal through multiple pathways to regulate oocyte cyst breakdown and primordial follicle assembly in the neonatal mouse ovary. J Endocrinol. 2009;202:407–17. doi: 10.1677/JOE-09-0109. [DOI] [PubMed] [Google Scholar]
- 151.Couse JF, Korach KS. Estrogen receptor null mice: what have we learned and where will they lead us? Endocr Rev. 1999;20:358–417. doi: 10.1210/edrv.20.3.0370. [DOI] [PubMed] [Google Scholar]
- 152.Couse JF, Yates MM, Deroo BJ, Korach KS. Estrogen receptor-beta is critical to granulosa cell differentiation and the ovulatory response to gonadotropins. Endocrinology. 2005;146:3247–62. doi: 10.1210/en.2005-0213. [DOI] [PubMed] [Google Scholar]
- 153.Emmen JM, Couse JF, Elmore SA, Yates MM, Kissling GE, Korach KS. In vitro growth and ovulation of follicles from ovaries of estrogen receptor (ER){alpha} and ER{beta} null mice indicate a role for ER{beta} in follicular maturation. Endocrinology. 2005;146:2817–26. doi: 10.1210/en.2004-1108. [DOI] [PubMed] [Google Scholar]
- 154.Drummond A, Fuller P. The importance of ER{beta} signalling in ovarian function. J Endocrinol. 2009 [Google Scholar]
- 155.Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology. 1998;139:4252–63. doi: 10.1210/endo.139.10.6216. [DOI] [PubMed] [Google Scholar]
- 156.Jefferson W, Newbold R, Padilla-Banks E, Pepling M. Neonatal genistein treatment alters ovarian differentiation in the mouse: inhibition of oocyte nest breakdown and increased oocyte survival. Biol Reprod. 2006;74:161–8. doi: 10.1095/biolreprod.105.045724. [DOI] [PubMed] [Google Scholar]
- 157.Jefferson WN, Couse JF, Padilla-Banks E, Korach KS, Newbold RR. Neonatal exposure to genistein induces estrogen receptor (ER)alpha expression and multioocyte follicles in the maturing mouse ovary: evidence for ERbeta-mediated and nonestrogenic actions. Biol Reprod. 2002;67:1285–96. doi: 10.1095/biolreprod67.4.1285. [DOI] [PubMed] [Google Scholar]
- 158.Kirigaya A, Kim H, Hayashi S, Chambon P, Watanabe H, Lguchi T, Sato T. Involvement of estrogen receptor beta in the induction of polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. Zoolog Sci. 2009;26:704–12. doi: 10.2108/zsj.26.704. [DOI] [PubMed] [Google Scholar]
- 159.Woodruff TK, Mayo KE. To beta or not to beta: estrogen receptors and ovarian function. Endocrinology. 2005;146:3244–6. doi: 10.1210/en.2005-0630. [DOI] [PubMed] [Google Scholar]
- 160.Couse JF, Bunch DO, Lindzey J, Schomberg DW, Korach KS. Prevention of the polycystic ovarian phenotype and characterization of ovulatory capacity in the estrogen receptor-alpha knockout mouse. Endocrinology. 1999;140:5855–65. doi: 10.1210/endo.140.12.7222. [DOI] [PubMed] [Google Scholar]
- 161.Szoltys M, Slomczynska M. Changes in distribution of androgen receptor during maturation of rat ovarian follicles. Exp Clin Endocrinol Diabetes. 2000;108:228–34. doi: 10.1055/s-2000-7747. [DOI] [PubMed] [Google Scholar]
- 162.Hillier SG, Tetsuka M, Fraser HM. Location and developmental regulation of androgen receptor in primate ovary. Hum Reprod. 1997;12:107–11. doi: 10.1093/humrep/12.1.107. [DOI] [PubMed] [Google Scholar]
- 163.Sajjad Y, Quenby SM, Nickson P, Lewis-Jones DI, Vince G. Expression of androgen receptors in upper human fetal reproductive tract. Hum Reprod. 2004;19:1659–65. doi: 10.1093/humrep/deh295. [DOI] [PubMed] [Google Scholar]
- 164.Juengel JL, Heath DA, Quirke LD, McNatty KP. Oestrogen receptor {alpha} and {beta}, androgen receptor and progesterone receptor mRNA and protein localisation within the developing ovary and in small growing follicles of sheep. Reproduction. 2006;131:81–92. doi: 10.1530/rep.1.00704. [DOI] [PubMed] [Google Scholar]
- 165.Pelletier G. Localization of androgen and estrogen receptors in rat and primate tissues. Histol Histopathol. 2000;15:1261–70. doi: 10.14670/HH-15.1261. [DOI] [PubMed] [Google Scholar]
- 166.Slomczynska M, Szoltys M. Immunohistochemical localization of androgen receptor (AR) in rat ovary. Folia Histochem Cytobiol. 1997;35:101–2. [PubMed] [Google Scholar]
- 167.Tetsuka M, Hillier SG. Androgen receptor gene expression in rat granulosa cells: the role of follicle-stimulating hormone and steroid hormones. Endocrinology. 1996;137:4392–7. doi: 10.1210/endo.137.10.8828500. [DOI] [PubMed] [Google Scholar]
- 168.Lyon MF, Glenister PH. Reduced reproductive performance in androgen-resistant Tfm/Tfm female mice. Proc R Soc Lond B Biol Sci. 1980;208:1–12. doi: 10.1098/rspb.1980.0040. [DOI] [PubMed] [Google Scholar]
- 169.Shiina H, Matsumoto T, Sato T, Igarashi K, Miyamoto J, Takemasa S, Sakari M, Takada I, Nakamura T, Metzger D, Chambon P, Kanno J, Yoshikawa H, Kato S. Premature ovarian failure in androgen receptor-deficient mice. Proc Natl Acad Sci U S A. 2006;103:224–9. doi: 10.1073/pnas.0506736102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Taniguchi F, Couse JF, Rodriguez KF, Emmen JM, Poirier D, Korach KS. Estrogen receptor-alpha mediates an intraovarian negative feedback loop on thecal cell steroidogenesis via modulation of Cyp17a1 (cytochrome P450, steroid 17alpha-hydroxylase/17,20 lyase) expression. FASEB J. 2007;21:586–95. doi: 10.1096/fj.06-6681com. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Gorski RA. Sexual differentiation of the brain. Hosp Pract. 1978;13:55–62. doi: 10.1080/21548331.1978.11707415. [DOI] [PubMed] [Google Scholar]
- 172.Bakker J, De Mees C, Douhard Q, Balthazart J, Gabant P, Szpirer J, Szpirer C. Alpha-fetoprotein protects the developing female mouse brain from masculinization and defeminization by estrogens. Nat Neurosci. 2006;9:220–6. doi: 10.1038/nn1624. [DOI] [PubMed] [Google Scholar]
- 173.Ikezuki Y, Tsutsumi O, Takai Y, Kamei Y, Taketani Y. Determination of bisphenol A concentrations in human biological fluids reveals significant early prenatal exposure. Hum Reprod. 2002;17:2839–41. doi: 10.1093/humrep/17.11.2839. [DOI] [PubMed] [Google Scholar]
- 174.Gore AC. Gonadotropin-releasing hormone neurons: multiple inputs, multiple outputs. Endocrinology. 2004;145:4016–7. doi: 10.1210/en.2004-0855. [DOI] [PubMed] [Google Scholar]
- 175.Quadros PS, Wagner CK. Regulation of progesterone receptor expression by estradiol is dependent on age, sex and region in the rat brain. Endocrinology. 2008;149:3054–61. doi: 10.1210/en.2007-1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Orikasa C, Sakuma Y. Possible involvement of preoptic estrogen receptor beta positive cells in luteinizing hormone surge in the rat. Domest Anim Endocrinol. 2003;25:83–92. doi: 10.1016/s0739-7240(03)00047-x. [DOI] [PubMed] [Google Scholar]
- 177.Chakraborty TR, Hof PR, Ng L, Gore AC. Stereologic analysis of estrogen receptor alpha (ER alpha) expression in rat hypothalamus and its regulation by aging and estrogen. J Comp Neurol. 2003;466:409–21. doi: 10.1002/cne.10906. [DOI] [PubMed] [Google Scholar]
- 178.Chakraborty TR, Ng L, Gore AC. Age-related changes in estrogen receptor beta in rat hypothalamus: a quantitative analysis. Endocrinology. 2003;144:4164–71. doi: 10.1210/en.2003-0052. [DOI] [PubMed] [Google Scholar]
- 179.Orvis GD, Behringer RR. Cellular mechanisms of Mullerian duct formation in the mouse. Dev Biol. 2007;306:493–504. doi: 10.1016/j.ydbio.2007.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kurita T, Cooke PS, Cunha GR. Epithelial-stromal tissue interaction in paramesonephric (Mullerian) epithelial differentiation. Dev Biol. 2001;240:194–211. doi: 10.1006/dbio.2001.0458. [DOI] [PubMed] [Google Scholar]
- 181.Robboy SJ, Taguchi O, Cunha GR. Normal development of the human female reproductive tract and alterations resulting from experimental exposure to diethylstilbestrol. Hum Pathol. 1982;13:190–8. doi: 10.1016/s0046-8177(82)80177-9. [DOI] [PubMed] [Google Scholar]
- 182.Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: I. Normal development. Am J Anat. 1989;186:1–20. doi: 10.1002/aja.1001860102. [DOI] [PubMed] [Google Scholar]
- 183.Gray CA, Bartol FF, Tarleton BJ, Wiley AA, Johnson GA, Bazer FW, Spencer TE. Developmental biology of uterine glands. Biol Reprod. 2001;65:1311–23. doi: 10.1095/biolreprod65.5.1311. [DOI] [PubMed] [Google Scholar]
- 184.Korach KS, Horigome T, Tomooka Y, Yamashita S, Newbold RR, McLachlan JA. Immunodetection of estrogen receptor in epithelial and stromal tissues of neonatal mouse uterus. Proc Natl Acad Sci U S A. 1988;85:3334–7. doi: 10.1073/pnas.85.10.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Okada A, Ohta Y, Buchanan DL, Sato T, Inoue S, Hiroi H, Muramatsu M, Iguchi T. Changes in ontogenetic expression of estrogen receptor alpha and not of estrogen receptor beta in the female rat reproductive tract. J Mol Endocrinol. 2002;28:87–97. doi: 10.1677/jme.0.0280087. [DOI] [PubMed] [Google Scholar]
- 186.Cummings AM. Methoxychlor as a model for environmental estrogens. Crit Rev Toxicol. 1997;27:367–79. doi: 10.3109/10408449709089899. [DOI] [PubMed] [Google Scholar]
- 187.Gaido KW, Maness SC, McDonnell DP, Dehal SS, Kupfer D, Safe S. Interaction of methoxychlor and related compounds with estrogen receptor alpha and beta, and androgen receptor: structure-activity studies. Mol Pharmacol. 2000;58:852–8. [PubMed] [Google Scholar]
- 188.Gaido KW, Leonard LS, Maness SC, Hall JM, McDonnell DP, Saville B, Safe S. Differential interaction of the methoxychlor metabolite 2,2-bis-(p-hydroxyphenyl)-1,1,1-trichloroethane with estrogen receptors alpha and beta. Endocrinology. 1999;140:5746–53. doi: 10.1210/endo.140.12.7191. [DOI] [PubMed] [Google Scholar]
- 189.Bretveld RW, Hooiveld M, Zielhuis GA, Pellegrino A, van Rooij IA, Roeleveld N. Reproductive disorders among male and female greenhouse workers. Reprod Toxicol. 2008;25:107–14. doi: 10.1016/j.reprotox.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 190.Bretveld R, Zielhuis GA, Roeleveld N. Time to pregnancy among female greenhouse workers. Scand J Work Environ Health. 2006;32:359–67. doi: 10.5271/sjweh.1031. [DOI] [PubMed] [Google Scholar]
- 191.Fuortes L, Clark MK, Kirchner HL, Smith EM. Association between female infertility and agricultural work history. Am J Ind Med. 1997;31:445–51. doi: 10.1002/(sici)1097-0274(199704)31:4<445::aid-ajim11>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 192.Harris SJ, Cecil HC, Bitman J. Effect of several dietary levels of technical methoxychlor on reproduction in rats. J Agric Food Chem. 1974;22:969–73. doi: 10.1021/jf60196a018. [DOI] [PubMed] [Google Scholar]
- 193.Martinez EM, Swartz WJ. Effects of methoxychlor on the reproductive system of the adult female mouse. 1. Gross and histologic observations. Reprod Toxicol. 1991;5:139–47. doi: 10.1016/0890-6238(91)90042-e. [DOI] [PubMed] [Google Scholar]
- 194.Hall DL, Payne LA, Putnam JM, Huet-Hudson YM. Effect of methoxychlor on implantation and embryo development in the mouse. Reprod Toxicol. 1997;11:703–8. doi: 10.1016/s0890-6238(97)00026-9. [DOI] [PubMed] [Google Scholar]
- 195.Cummings AM, Gray LE., Jr Antifertility effect of methoxychlor in female rats: dose- and time-dependent blockade of pregnancy. Toxicol Appl Pharmacol. 1989;97:454–62. doi: 10.1016/0041-008x(89)90250-0. [DOI] [PubMed] [Google Scholar]
- 196.Bal HS. Effect of methoxychlor on reproductive systems of the rat. Proc Soc Exp Biol Med. 1984;176:187–96. doi: 10.3181/00379727-176-41861. [DOI] [PubMed] [Google Scholar]
- 197.Okazaki K, Okazaki S, Nishimura S, Nakamura H, Kitamura Y, Hatayama K, Nakamura A, Tsuda T, Katsumata T, Nishikawa A, Hirose M. A repeated 28-day oral dose toxicity study of methoxychlor in rats, based on the ‘enhanced OECD test guideline 407’ for screening endocrine-disrupting chemicals. Arch Toxicol. 2001;75:513–21. doi: 10.1007/s002040100273. [DOI] [PubMed] [Google Scholar]
- 198.Borgeest C, Symonds D, Mayer LP, Hoyer PB, Flaws JA. Methoxychlor may cause ovarian follicular atresia and proliferation of the ovarian epithelium in the mouse. Toxicol Sci. 2002;68:473–8. doi: 10.1093/toxsci/68.2.473. [DOI] [PubMed] [Google Scholar]
- 199.Gray LE, Jr, Ostby J, Ferrell J, Rehnberg G, Linder R, Cooper R, Goldman J, Slott V, Laskey J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam Appl Toxicol. 1989;12:92–108. doi: 10.1016/0272-0590(89)90065-1. [DOI] [PubMed] [Google Scholar]
- 200.Chapin RE, Harris MW, Davis BJ, Ward SM, Wilson RE, Mauney MA, Lockhart AC, Smialowicz RJ, Moser VC, Burka LT, Collins BJ. The effects of perinatal/juvenile methoxychlor exposure on adult rat nervous, immune, and reproductive system function. Fundam Appl Toxicol. 1997;40:138–57. doi: 10.1006/faat.1997.2381. [DOI] [PubMed] [Google Scholar]
- 201.You L, Casanova M, Bartolucci EJ, Fryczynski MW, Dorman DC, Everitt JI, Gaido KW, Ross SM, Heck Hd H. Combined effects of dietary phytoestrogen and synthetic endocrine-active compound on reproductive development in Sprague-Dawley rats: genistein and methoxychlor. Toxicol Sci. 2002;66:91–104. doi: 10.1093/toxsci/66.1.91. [DOI] [PubMed] [Google Scholar]
- 202.Armenti AE, Zama AM, Passantino L, Uzumcu M. Developmental methoxychlor exposure affects multiple reproductive parameters and ovarian folliculogenesis and gene expression in adult rats. Toxicol Appl Pharmacol. 2008;233:286–96. doi: 10.1016/j.taap.2008.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Harvey CN, Esmail M, Wang Q, Brooks AI, Zachow R, Uzumcu M. Effect of the methoxychlor metabolite HPTE on the rat ovarian granulosa cell transcriptome in vitro. Toxicol Sci. 2009;110:95–106. doi: 10.1093/toxsci/kfp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Tang WY, Newbold R, Mardilovich K, Jefferson W, Cheng RY, Medvedovic M, Ho SM. Persistent hypomethylation in the promoter of nucleosomal binding protein 1 (Nsbp1) correlates with overexpression of Nsbp1 in mouse uteri neonatally exposed to diethylstilbestrol or genistein. Endocrinology. 2008;149:5922–31. doi: 10.1210/en.2008-0682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Gray LE, Jr, Ostby JS, Ferrell JM, Sigmon ER, Goldman JM. Methoxychlor induces estrogen-like alterations of behavior and the reproductive tract in the female rat and hamster: effects on sex behavior, running wheel activity, and uterine morphology. Toxicol Appl Pharmacol. 1988;96:525–40. doi: 10.1016/0041-008x(88)90012-9. [DOI] [PubMed] [Google Scholar]
- 206.Laviola G, Gioiosa L, Adriani W, Palanza P. D-amphetamine-related reinforcing effects are reduced in mice exposed prenatally to estrogenic endocrine disruptors. Brain Res Bull. 2005;65:235–40. doi: 10.1016/j.brainresbull.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 207.Gioiosa L, Fissore E, Ghirardelli G, Parmigiani S, Palanza P. Developmental exposure to low-dose estrogenic endocrine disruptors alters sex differences in exploration and emotional responses in mice. Horm Behav. 2007;52:307–16. doi: 10.1016/j.yhbeh.2007.05.006. [DOI] [PubMed] [Google Scholar]
- 208.Gore AC. Environmental toxicant effects on neuroendocrine function. Endocrine. 2001;14:235–46. doi: 10.1385/ENDO:14:2:235. [DOI] [PubMed] [Google Scholar]
- 209.Masutomi N, Shibutani M, Takagi H, Uneyama C, Takahashi N, Hirose M. Impact of dietary exposure to methoxychlor, genistein, or diisononyl phthalate during the perinatal period on the development of the rat endocrine/reproductive systems in later life. Toxicology. 2003;192:149–70. doi: 10.1016/s0300-483x(03)00269-5. [DOI] [PubMed] [Google Scholar]
- 210.Takagi H, Shibutani M, Lee KY, Masutomi N, Fujita H, Inoue K, Mitsumori K, Hirose M. Impact of maternal dietary exposure to endocrine-acting chemicals on progesterone receptor expression in microdissected hypothalamic medial preoptic areas of rat offspring. Toxicol Appl Pharmacol. 2005;208:127–36. doi: 10.1016/j.taap.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 211.Masutomi N, Shibutani M, Takagi H, Uneyama C, Lee KY, Hirose M. Alteration of pituitary hormone-immunoreactive cell populations in rat offspring after maternal dietary exposure to endocrine-active chemicals. Arch Toxicol. 2004;78:232–40. doi: 10.1007/s00204-003-0528-x. [DOI] [PubMed] [Google Scholar]
- 212.Walker DM, Gore AC, Armenti AE, Zama AM, Uzumcu M. Long-term effects of perinatal methoxychlor or estradiol on gene expression in the hypothalamus of the aging female rat. The 91th Annual Meeting of Endocrine Society; Washington D.C. 2009. [Google Scholar]
- 213.Tullner WW. Uterotrophic action of the insecticide methoxychlor. Science. 1961;133:647. doi: 10.1126/science.133.3453.647. [DOI] [PubMed] [Google Scholar]
- 214.Walters LM, Rourke AW, Eroschenko VP. Purified methoxychlor stimulates the reproductive tract in immature female mice. Reprod Toxicol. 1993;7:599–606. doi: 10.1016/0890-6238(93)90036-7. [DOI] [PubMed] [Google Scholar]
- 215.Rourke AW, Eroschenko VP, Washburn LJ. Protein secretions in mouse uterus after methoxychlor or estradiol exposure. Reprod Toxicol. 1991;5:437–42. doi: 10.1016/0890-6238(91)90007-3. [DOI] [PubMed] [Google Scholar]
- 216.Eroschenko VP, Rourke AW, Sims WF. Estradiol or methoxychlor stimulates estrogen receptor (ER) expression in uteri. Reprod Toxicol. 1996;10:265–71. doi: 10.1016/0890-6238(96)00055-x. [DOI] [PubMed] [Google Scholar]
- 217.Metcalf JL, Laws SC, Cummings AM. Methoxychlor mimics the action of 17 beta-estradiol on induction of uterine epidermal growth factor receptors in immature female rats. Reprod Toxicol. 1996;10:393–9. doi: 10.1016/0890-6238(96)00085-8. [DOI] [PubMed] [Google Scholar]
- 218.Cummings AM, Gray LE., Jr Methoxychlor affects the decidual cell response of the uterus but not other progestational parameters in female rats. Toxicol Appl Pharmacol. 1987;90:330–6. doi: 10.1016/0041-008x(87)90340-1. [DOI] [PubMed] [Google Scholar]
- 219.Ingermann RL, Bencic DC, Eroschenko VP. Methoxychlor effects on hatching and larval startle response in the salamander Ambystoma macrodactylum are independent of its estrogenic actions. Bull Environ Contam Toxicol. 1999;62:578–83. doi: 10.1007/s001289900914. [DOI] [PubMed] [Google Scholar]
- 220.Mwatibo JM, Green JD. Estradiol disrupts sea urchin embryogenesis differently from methoxychlor. Bull Environ Contam Toxicol. 1998;61:577–82. doi: 10.1007/pl00002974. [DOI] [PubMed] [Google Scholar]
- 221.Pickford DB, Morris ID. Effects of endocrine-disrupting contaminants on amphibian oogenesis: methoxychlor inhibits progesterone-induced maturation of Xenopus laevis oocytes in vitro. Environ Health Perspect. 1999;107:285–92. doi: 10.1289/ehp.99107285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Fei X, Chung H, Taylor HS. Methoxychlor disrupts uterine Hoxa10 gene expression. Endocrinology. 2005;146:3445–51. doi: 10.1210/en.2005-0341. [DOI] [PubMed] [Google Scholar]
- 223.Daftary GS, Taylor HS. Endocrine regulation of HOX genes. Endocr Rev. 2006;27:331–55. doi: 10.1210/er.2005-0018. [DOI] [PubMed] [Google Scholar]
- 224.Smith OW, Gabbe SG. Diethylstilbestrol in the prevention and treatment of complications of pregnancy. 1948. Am J Obstet Gynecol. 1999;181:1570–1. doi: 10.1016/s0002-9378(99)70409-6. [DOI] [PubMed] [Google Scholar]
- 225.Dieckmann WJ, Davis ME, Rynkiewicz LM, Pottinger RE. Does the administration of diethylstilbestrol during pregnancy have therapeutic value? Am J Obstet Gynecol. 1953;66:1062–81. doi: 10.1016/s0002-9378(16)38617-3. [DOI] [PubMed] [Google Scholar]
- 226.Titus-Ernstoff L, Troisi R, Hatch EE, Wise LA, Palmer J, Hyer M, Kaufman R, Adam E, Strohsnitter W, Noller K, Herbst AL, Gibson-Chambers J, Hartge P, Hoover RN. Menstrual and reproductive characteristics of women whose mothers were exposed in utero to diethylstilbestrol (DES) Int J Epidemiol. 2006;35:862–8. doi: 10.1093/ije/dyl106. [DOI] [PubMed] [Google Scholar]
- 227.Herbst AL, Ulfelder H, Poskanzer DC. Adenocarcinoma of the vagina. Association of maternal stilbestrol therapy with tumor appearance in young women. N Engl J Med. 1971;284:878–81. doi: 10.1056/NEJM197104222841604. [DOI] [PubMed] [Google Scholar]
- 228.Blatt J, Van Le L, Weiner T, Sailer S. Ovarian carcinoma in an adolescent with transgenerational exposure to diethylstilbestrol. J Pediatr Hematol Oncol. 2003;25:635–6. doi: 10.1097/00043426-200308000-00009. [DOI] [PubMed] [Google Scholar]
- 229.Palmlund I. Exposure to a xenoestrogen before birth: the diethylstilbestrol experience. J Psychosom Obstet Gynaecol. 1996;17:71–84. doi: 10.3109/01674829609025667. [DOI] [PubMed] [Google Scholar]
- 230.Goldberg JM, Falcone T. Effect of diethylstilbestrol on reproductive function. Fertil Steril. 1999;72:1–7. doi: 10.1016/s0015-0282(99)00153-3. [DOI] [PubMed] [Google Scholar]
- 231.Turusov VS, Trukhanova LS, Parfenov Yu D, Tomatis L. Occurrence of tumours in the descendants of CBA male mice prenatally treated with diethylstilbestrol. Int J Cancer. 1992;50:131–5. doi: 10.1002/ijc.2910500126. [DOI] [PubMed] [Google Scholar]
- 232.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Proliferative lesions and reproductive tract tumors in male descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 2000;21:1355–63. [PubMed] [Google Scholar]
- 233.Newbold RR, Hanson RB, Jefferson WN, Bullock BC, Haseman J, McLachlan JA. Increased tumors but uncompromised fertility in the female descendants of mice exposed developmentally to diethylstilbestrol. Carcinogenesis. 1998;19:1655–63. doi: 10.1093/carcin/19.9.1655. [DOI] [PubMed] [Google Scholar]
- 234.Walker BE, Haven MI. Intensity of multigenerational carcinogenesis from diethylstilbestrol in mice. Carcinogenesis. 1997;18:791–3. doi: 10.1093/carcin/18.4.791. [DOI] [PubMed] [Google Scholar]
- 235.Wordinger RJ, Highman B. Histology and ultrastructure of the adult mouse ovary following a single prenatal exposure to diethylstilbestrol. Virchows Arch B Cell Pathol Incl Mol Pathol. 1984;45:241–53. doi: 10.1007/BF02889867. [DOI] [PubMed] [Google Scholar]
- 236.Haney AF, Newbold RR, McLachlan JA. Prenatal diethylstilbestrol exposure in the mouse: effects on ovarian histology and steroidogenesis in vitro. Biol Reprod. 1984;30:471–8. doi: 10.1095/biolreprod30.2.471. [DOI] [PubMed] [Google Scholar]
- 237.Tenenbaum A, Forsberg JG. Structural and functional changes in ovaries from adult mice treated with diethylstilboestrol in the neonatal period. J Reprod Fertil. 1985;73:465–77. doi: 10.1530/jrf.0.0730465. [DOI] [PubMed] [Google Scholar]
- 238.McLachlan JA, Newbold RR, Shah HC, Hogan MD, Dixon RL. Reduced fertility in female mice exposed transplacentally to diethylstilbestrol (DES) Fertil Steril. 1982;38:364–71. doi: 10.1016/s0015-0282(16)46520-9. [DOI] [PubMed] [Google Scholar]
- 239.Wordinger RJ, Derrenbacker J. In utero exposure of mice to diethylstilbestrol alters neonatal ovarian follicle growth and development. Acta Anat (Basel) 1989;134:312–8. doi: 10.1159/000146708. [DOI] [PubMed] [Google Scholar]
- 240.Halling A, Forsberg JG. Effects of neonatal exposure to diethylstilbestrol on early mouse embryo development in vivo and in vitro. Biol Reprod. 1991;45:157–62. doi: 10.1095/biolreprod45.1.157. [DOI] [PubMed] [Google Scholar]
- 241.Iguchi T, Kamiya K, Uesugi Y, Sayama K, Takasugi N. In vitro fertilization of oocytes from polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol. In Vivo. 1991;5:359–63. [PubMed] [Google Scholar]
- 242.Forsberg JG, Halling A. Failure to detect a second-generation effect in female mice after neonatal treatment with an estrogen (diethylstilbestrol) Acta Anat (Basel) 1992;144:103–6. doi: 10.1159/000147292. [DOI] [PubMed] [Google Scholar]
- 243.Iguchi T, Takasugi N. Polyovular follicles in the ovary of immature mice exposed prenatally to diethylstilbestrol. Anat Embryol (Berl) 1986;175:53–5. doi: 10.1007/BF00315455. [DOI] [PubMed] [Google Scholar]
- 244.Iguchi T, Fukazawa Y, Uesugi Y, Takasugi N. Polyovular follicles in mouse ovaries exposed neonatally to diethylstilbestrol in vivo and in vitro. Biol Reprod. 1990;43:478–84. doi: 10.1095/biolreprod43.3.478. [DOI] [PubMed] [Google Scholar]
- 245.Dohler KD, Srivastava SS, Shryne JE, Jarzab B, Sipos A, Gorski RA. Differentiation of the sexually dimorphic nucleus in the preoptic area of the rat brain is inhibited by postnatal treatment with an estrogen antagonist. Neuroendocrinology. 1984;38:297–301. doi: 10.1159/000123907. [DOI] [PubMed] [Google Scholar]
- 246.Faber KA, Ayyash L, Dixon S, Hughes CL., Jr Effect of neonatal diethylstilbestrol exposure on volume of the sexually dimorphic nucleus of the preoptic area of the hypothalamus and pituitary responsiveness to gonadotropin-releasing hormone in female rats of known anogenital distance at birth. Biol Reprod. 1993;48:947–51. doi: 10.1095/biolreprod48.5.947. [DOI] [PubMed] [Google Scholar]
- 247.Kitajewski J, Sassoon D. The emergence of molecular gynecology: homeobox and Wnt genes in the female reproductive tract. Bioessays. 2000;22:902–10. doi: 10.1002/1521-1878(200010)22:10<902::AID-BIES5>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 248.McLachlan JA, Newbold RR, Bullock BC. Long-term effects on the female mouse genital tract associated with prenatal exposure to diethylstilbestrol. Cancer Res. 1980;40:3988–99. [PubMed] [Google Scholar]
- 249.Nelson KG, Sakai Y, Eitzman B, Steed T, McLachlan J. Exposure to diethylstilbestrol during a critical developmental period of the mouse reproductive tract leads to persistent induction of two estrogen-regulated genes. Cell Growth Differ. 1994;5:595–606. [PubMed] [Google Scholar]
- 250.Lingham RB, Stancel GM, Loose-Mitchell DS. Estrogen regulation of epidermal growth factor receptor messenger ribonucleic acid. Mol Endocrinol. 1988;2:230–5. doi: 10.1210/mend-2-3-230. [DOI] [PubMed] [Google Scholar]
- 251.Loose-Mitchell DS, Chiappetta C, Stancel GM. Estrogen regulation of c-fos messenger ribonucleic acid. Mol Endocrinol. 1988;2:946–51. doi: 10.1210/mend-2-10-946. [DOI] [PubMed] [Google Scholar]
- 252.Kamiya K, Sato T, Nishimura N, Goto Y, Kano K, Iguchi T. Expression of estrogen receptor and proto-oncogene messenger ribonucleic acids in reproductive tracts of neonatally diethylstilbestrol-exposed female mice with or without post-puberal estrogen administration. Exp Clin Endocrinol Diabetes. 1996;104:111–22. doi: 10.1055/s-0029-1211432. [DOI] [PubMed] [Google Scholar]
- 253.Falck L, Forsberg JG. Immunohistochemical studies on the expression and estrogen dependency of EGF and its receptor and C-fos proto-oncogene in the uterus and vagina of normal and neonatally estrogen-treated mice. Anat Rec. 1996;245:459–71. doi: 10.1002/(SICI)1097-0185(199607)245:3<459::AID-AR2>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 254.Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in mullerian duct by the synthetic estrogen diethylstilbestrol (DES) Dev Biol. 1998;197:141–54. doi: 10.1006/dbio.1998.8907. [DOI] [PubMed] [Google Scholar]
- 255.Block K, Kardana A, Igarashi P, Taylor HS. In utero diethylstilbestrol (DES) exposure alters Hox gene expression in the developing mullerian system. Faseb J. 2000;14:1101–8. doi: 10.1096/fasebj.14.9.1101. [DOI] [PubMed] [Google Scholar]
- 256.Miller C, Degenhardt K, Sassoon DA. Fetal exposure to DES results in deregulation of Wnt7a during uterine morphogenesis. Nat Genet. 1998;20:228–30. doi: 10.1038/3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Huang WW, Yin Y, Bi Q, Chiang TC, Garner N, Vuoristo J, McLachlan JA, Ma L. Developmental diethylstilbestrol exposure alters genetic pathways of uterine cytodifferentiation. Mol Endocrinol. 2005;19:669–82. doi: 10.1210/me.2004-0155. [DOI] [PubMed] [Google Scholar]
- 258.Yin Y, Lin C, Ma L. MSX2 promotes vaginal epithelial differentiation and wolffian duct regression and dampens the vaginal response to diethylstilbestrol. Mol Endocrinol. 2006;20:1535–46. doi: 10.1210/me.2005-0451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50:7677–81. [PubMed] [Google Scholar]
- 260.Li S, Washburn KA, Moore R, Uno T, Teng C, Newbold RR, McLachlan JA, Negishi M. Developmental exposure to diethylstilbestrol elicits demethylation of estrogen-responsive lactoferrin gene in mouse uterus. Cancer Res. 1997;57:4356–9. [PubMed] [Google Scholar]
- 261.Li S, Hansman R, Newbold R, Davis B, McLachlan JA, Barrett JC. Neonatal diethylstilbestrol exposure induces persistent elevation of c-fos expression and hypomethylation in its exon-4 in mouse uterus. Mol Carcinog. 2003;38:78–84. doi: 10.1002/mc.10147. [DOI] [PubMed] [Google Scholar]
- 262.Sato K, Fukata H, Kogo Y, Ohgane J, Shiota K, Mori C. Neonatal exposure to diethylstilbestrol alters expression of DNA methyltransferases and methylation of genomic DNA in the mouse uterus. Endocr J. 2009;56:131–9. doi: 10.1507/endocrj.k08e-239. [DOI] [PubMed] [Google Scholar]
- 263.Li S, Chiang TC, Richard-Davis G, Barrett JC, McLachlan JA. DNA hypomethylation and imbalanced expression of DNA methyltransferases (DNMT1, 3A, and 3B) in human uterine leiomyoma. Gynecol Oncol. 2003;90:123–30. doi: 10.1016/s0090-8258(03)00194-x. [DOI] [PubMed] [Google Scholar]
- 264.Bromer JG, Wu J, Zhou Y, Taylor HS. Hypermethylation of homeobox A10 by in utero diethylstilbestrol exposure: an epigenetic mechanism for altered developmental programming. Endocrinology. 2009;150:3376–82. doi: 10.1210/en.2009-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Li S, Ma L, Chiang T, Burow M, Newbold RR, Negishi M, Barrett JC, McLachlan JA. Promoter CpG methylation of Hox-a10 and Hox-a11 in mouse uterus not altered upon neonatal diethylstilbestrol exposure. Mol Carcinog. 2001;32:213–9. doi: 10.1002/mc.10015. [DOI] [PubMed] [Google Scholar]
- 266.Alworth LC, Howdeshell KL, Ruhlen RL, Day JK, Lubahn DB, Huang TH, Besch-Williford CL, vom Saal FS. Uterine responsiveness to estradiol and DNA methylation are altered by fetal exposure to diethylstilbestrol and methoxychlor in CD-1 mice: effects of low versus high doses. Toxicol Appl Pharmacol. 2002;183:10–22. doi: 10.1006/taap.2002.9459. [DOI] [PubMed] [Google Scholar]
- 267.Couse JF, Korach KS. Estrogen receptor-alpha mediates the detrimental effects of neonatal diethylstilbestrol (DES) exposure in the murine reproductive tract. Toxicology. 2004;205:55–63. doi: 10.1016/j.tox.2004.06.046. [DOI] [PubMed] [Google Scholar]
- 268.Yamashita S, Newbold RR, McLachlan JA, Korach KS. The role of the estrogen receptor in uterine epithelial proliferation and cytodifferentiation in neonatal mice. Endocrinology. 1990;127:2456–63. doi: 10.1210/endo-127-5-2456. [DOI] [PubMed] [Google Scholar]
- 269.Ruden DM, Xiao L, Garfinkel MD, Lu X. Hsp90 and environmental impacts on epigenetic states: a model for the trans-generational effects of diethylstibesterol on uterine development and cancer. Hum Mol Genet. 2005;14(Spec No 1):R149–55. doi: 10.1093/hmg/ddi103. [DOI] [PubMed] [Google Scholar]
- 270.Bennetts HW, Underwood EJ. The oestrogenic effects of subterranean clover (trifolium subterraneum); uterine maintenance in the ovariectomised ewe on clover grazing. Aust J Exp Biol Med Sci. 1951;29:249–53. doi: 10.1038/icb.1951.29. [DOI] [PubMed] [Google Scholar]
- 271.Peeters PH, Keinan-Boker L, van der Schouw YT, Grobbee DE. Phytoestrogens and breast cancer risk. Review of the epidemiological evidence. Breast Cancer Res Treat. 2003;77:171–83. doi: 10.1023/a:1021381101632. [DOI] [PubMed] [Google Scholar]
- 272.Setchell KD, Zimmer-Nechemias L, Cai J, Heubi JE. Isoflavone content of infant formulas and the metabolic fate of these phytoestrogens in early life. Am J Clin Nutr. 1998;68:1453S–1461S. doi: 10.1093/ajcn/68.6.1453S. [DOI] [PubMed] [Google Scholar]
- 273.Johns P, Dowlati L, Wargo W. Determination of isoflavones in ready-to-feed soy-based infant formula. J AOAC Int. 2003;86:72–8. [PubMed] [Google Scholar]
- 274.Patisaul HB, Adewale HB. Long-term effects of environmental endocrine disruptors on reproductive physiology and behavior. Front Behav Neurosci. 2009;3:10. doi: 10.3389/neuro.08.010.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jefferson WN, Padilla-Banks E, Goulding EH, Eddy EM. Neonatal exposure to the endocrine disruptor genistein adversely affects fertilization rate and oocyte quality. Endocrine Society’s 88th Annual Meeting; Boston, MA. 2006. p. 78. [Google Scholar]
- 276.Nagao T, Yoshimura S, Saito Y, Nakagomi M, Usumi K, Ono H. Reproductive effects in male and female rats of neonatal exposure to genistein. Reprod Toxicol. 2001;15:399–411. doi: 10.1016/s0890-6238(01)00141-1. [DOI] [PubMed] [Google Scholar]
- 277.Todaka E, Sakurai K, Fukata H, Miyagawa H, Uzuki M, Omori M, Osada H, Ikezuki Y, Tsutsumi O, Iguchi T, Mori C. Fetal exposure to phytoestrogens--the difference in phytoestrogen status between mother and fetus. Environ Res. 2005;99:195–203. doi: 10.1016/j.envres.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 278.Nikaido Y, Yoshizawa K, Danbara N, Tsujita-Kyutoku M, Yuri T, Uehara N, Tsubura A. Effects of maternal xenoestrogen exposure on development of the reproductive tract and mammary gland in female CD-1 mouse offspring. Reprod Toxicol. 2004;18:803–11. doi: 10.1016/j.reprotox.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 279.Jefferson WN, Doerge D, Padilla-Banks E, Woodling KA, Kissling GE, Newbold R. Oral exposure to genistin, the glycosylated form of genistein, during neonatal life adversely affects the female reproductive system. Environ Health Perspect. 2009;117:1883–9. doi: 10.1289/ehp.0900923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Dickerson SM, Gore AC. Estrogenic environmental endocrine-disrupting chemical effects on reproductive neuroendocrine function and dysfunction across the life cycle. Rev Endocr Metab Disord. 2007;8:143–59. doi: 10.1007/s11154-007-9048-y. [DOI] [PubMed] [Google Scholar]
- 281.Bateman HL, Patisaul HB. Disrupted female reproductive physiology following neonatal exposure to phytoestrogens or estrogen specific ligands is associated with decreased GnRH activation and kisspeptin fiber density in the hypothalamus. Neurotoxicology. 2008;29:988–97. doi: 10.1016/j.neuro.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Patisaul HB, Fortino AE, Polston EK. Differential disruption of nuclear volume and neuronal phenotype in the preoptic area by neonatal exposure to genistein and bisphenol-A. Neurotoxicology. 2007;28:1–12. doi: 10.1016/j.neuro.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 283.Li AA, Baum MJ, McIntosh LJ, Day M, Liu F, Gray LE., Jr Building a scientific framework for studying hormonal effects on behavior and on the development of the sexually dimorphic nervous system. Neurotoxicology. 2008;29:504–19. doi: 10.1016/j.neuro.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 284.Jefferson WN, Padilla-Banks E, Clark G, Newbold RR. Assessing estrogenic activity of phytochemicals using transcriptional activation and immature mouse uterotrophic responses. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;777:179–89. doi: 10.1016/s1570-0232(02)00493-2. [DOI] [PubMed] [Google Scholar]
- 285.Jefferson WN, Padilla-Banks E, Newbold RR. Disruption of the developing female reproductive system by phytoestrogens: genistein as an example. Mol Nutr Food Res. 2007;51:832–44. doi: 10.1002/mnfr.200600258. [DOI] [PubMed] [Google Scholar]
- 286.Strom BL, Schinnar R, Ziegler EE, Barnhart KT, Sammel MD, Macones GA, Stallings VA, Drulis JM, Nelson SE, Hanson SA. Exposure to soy-based formula in infancy and endocrinological and reproductive outcomes in young adulthood. Jama. 2001;286:807–14. doi: 10.1001/jama.286.7.807. [DOI] [PubMed] [Google Scholar]
- 287.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–31. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Calafat AM, Kuklenyik Z, Reidy JA, Caudill SP, Ekong J, Needham LL. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ Health Perspect. 2005;113:391–5. doi: 10.1289/ehp.7534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Brede C, Fjeldal P, Skjevrak I, Herikstad H. Increased migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit Contam. 2003;20:684–9. doi: 10.1080/0265203031000119061. [DOI] [PubMed] [Google Scholar]
- 290.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Exposure of the U.S. population to bisphenol A and 4-tertiary-octylphenol: 2003–2004. Environ Health Perspect. 2008;116:39–44. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Calafat AM, Weuve J, Ye X, Jia LT, Hu H, Ringer S, Huttner K, Hauser R. Exposure to bisphenol A and other phenols in neonatal intensive care unit premature infants. Environ Health Perspect. 2009;117:639–44. doi: 10.1289/ehp.0800265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Carwile JL, Luu HT, Bassett LS, Driscoll DA, Yuan C, Chang JY, Ye X, Calafat AM, Michels KB. Polycarbonate bottle use and urinary bisphenol A concentrations. Environ Health Perspect. 2009;117:1368–72. doi: 10.1289/ehp.0900604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Gould JC, Leonard LS, Maness SC, Wagner BL, Conner K, Zacharewski T, Safe S, McDonnell DP, Gaido KW. Bisphenol A interacts with the estrogen receptor alpha in a distinct manner from estradiol. Mol Cell Endocrinol. 1998;142:203–14. doi: 10.1016/s0303-7207(98)00084-7. [DOI] [PubMed] [Google Scholar]
- 294.Howdeshell KL, Hotchkiss AK, Thayer KA, Vandenbergh JG, vom Saal FS. Exposure to bisphenol A advances puberty. Nature. 1999;401:763–4. doi: 10.1038/44517. [DOI] [PubMed] [Google Scholar]
- 295.Honma S, Suzuki A, Buchanan DL, Katsu Y, Watanabe H, Iguchi T. Low dose effect of in utero exposure to bisphenol A and diethylstilbestrol on female mouse reproduction. Reprod Toxicol. 2002;16:117–22. doi: 10.1016/s0890-6238(02)00006-0. [DOI] [PubMed] [Google Scholar]
- 296.Markey CM, Wadia PR, Rubin BS, Sonnenschein C, Soto AM. Long-term effects of fetal exposure to low doses of the xenoestrogen bisphenol-A in the female mouse genital tract. Biol Reprod. 2005;72:1344–51. doi: 10.1095/biolreprod.104.036301. [DOI] [PubMed] [Google Scholar]
- 297.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24:253–8. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Adewale HB, Jefferson WN, Newbold RR, Patisaul HB. Neonatal bisphenol-a exposure alters rat reproductive development and ovarian morphology without impairing activation of gonadotropin-releasing hormone neurons. Biol Reprod. 2009;81:690–9. doi: 10.1095/biolreprod.109.078261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Suzuki A, Sugihara A, Uchida K, Sato T, Ohta Y, Katsu Y, Watanabe H, Iguchi T. Developmental effects of perinatal exposure to bisphenol-A and diethylstilbestrol on reproductive organs in female mice. Reprod Toxicol. 2002;16:107–16. doi: 10.1016/s0890-6238(02)00005-9. [DOI] [PubMed] [Google Scholar]
- 300.Hunt PA, Koehler KE, Susiarjo M, Hodges CA, Ilagan A, Voigt RC, Thomas S, Thomas BF, Hassold TJ. Bisphenol a exposure causes meiotic aneuploidy in the female mouse. Curr Biol. 2003;13:546–53. doi: 10.1016/s0960-9822(03)00189-1. [DOI] [PubMed] [Google Scholar]
- 301.Susiarjo M, Hassold TJ, Freeman E, Hunt PA. Bisphenol A exposure in utero disrupts early oogenesis in the mouse. PLoS Genet. 2007;3:e5. doi: 10.1371/journal.pgen.0030005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Toda K, Miyaura C, Okada T, Shizuta Y. Dietary bisphenol A prevents ovarian degeneration and bone loss in female mice lacking the aromatase gene (Cyp19 ) Eur J Biochem. 2002;269:2214–22. doi: 10.1046/j.1432-1033.2002.02879.x. [DOI] [PubMed] [Google Scholar]
- 303.Patisaul HB, Fortino AE, Polston EK. Neonatal genistein or bisphenol-A exposure alters sexual differentiation of the AVPV. Neurotoxicol Teratol. 2006;28:111–8. doi: 10.1016/j.ntt.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 304.Rubin BS, Lenkowski JR, Schaeberle CM, Vandenberg LN, Ronsheim PM, Soto AM. Evidence of Altered Brain Sexual Differentiation in Mice Exposed Perinatally to Low, Environmentally Relevant Levels of Bisphenol A. Endocrinology. 2006 doi: 10.1210/en.2006-0189. [DOI] [PubMed] [Google Scholar]
- 305.Fernandez M, Bianchi M, Lux-Lantos V, Libertun C. Neonatal exposure to bisphenol a alters reproductive parameters and gonadotropin releasing hormone signaling in female rats. Environ Health Perspect. 2009;117:757–62. doi: 10.1289/ehp.0800267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Newbold RR, Jefferson WN, Padilla-Banks E. Prenatal exposure to bisphenol a at environmentally relevant doses adversely affects the murine female reproductive tract later in life. Environ Health Perspect. 2009;117:879–85. doi: 10.1289/ehp.0800045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Varayoud J, Ramos JG, Bosquiazzo VL, Munoz-de-Toro M, Luque EH. Developmental exposure to Bisphenol a impairs the uterine response to ovarian steroids in the adult. Endocrinology. 2008;149:5848–60. doi: 10.1210/en.2008-0651. [DOI] [PubMed] [Google Scholar]
- 308.Youngson NA, Whitelaw E. Transgenerational epigenetic effects. Annu Rev Genomics Hum Genet. 2008;9:233–57. doi: 10.1146/annurev.genom.9.081307.164445. [DOI] [PubMed] [Google Scholar]
- 309.Skinner MK, Manikkam M, Guerrero-Bosagna C. Epigenetic transgenerational actions of environmental factors in disease etiology. Trends Endocrinol Metab. doi: 10.1016/j.tem.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 310.Lumey LH. Decreased birthweights in infants after maternal in utero exposure to the Dutch famine of 1944–1945. Paediatr Perinat Epidemiol. 1992;6:240–53. doi: 10.1111/j.1365-3016.1992.tb00764.x. [DOI] [PubMed] [Google Scholar]
- 311.Kaati G, Bygren LO, Edvinsson S. Cardiovascular and diabetes mortality determined by nutrition during parents’ and grandparents’ slow growth period. Eur J Hum Genet. 2002;10:682–8. doi: 10.1038/sj.ejhg.5200859. [DOI] [PubMed] [Google Scholar]
- 312.Pembrey ME, Bygren LO, Kaati G, Edvinsson S, Northstone K, Sjostrom M, Golding J. Sex-specific, male-line transgenerational responses in humans. Eur J Hum Genet. 2006;14:159–66. doi: 10.1038/sj.ejhg.5201538. [DOI] [PubMed] [Google Scholar]
- 313.Holliday R. The inheritance of epigenetic defects. Science. 1987;238:163–70. doi: 10.1126/science.3310230. [DOI] [PubMed] [Google Scholar]
- 314.Holliday R. The possibility of epigenetic transmission of defects induced by teratogens. Mutat Res. 1998;422:203–5. doi: 10.1016/s0027-5107(98)00219-x. [DOI] [PubMed] [Google Scholar]
- 315.Skinner MK. What is an epigenetic transgenerational phenotype? F3 or F2. Reprod Toxicol. 2008;25:2–6. doi: 10.1016/j.reprotox.2007.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Waterland RA, Travisano M, Tahiliani KG. Diet-induced hypermethylation at agouti viable yellow is not inherited transgenerationally through the female. Faseb J. 2007;21:3380–5. doi: 10.1096/fj.07-8229com. [DOI] [PubMed] [Google Scholar]
- 317.John RM, Surani MA. Agouti germ line gets acquisitive. Nat Genet. 1999;23:254–6. doi: 10.1038/15425. [DOI] [PubMed] [Google Scholar]
- 318.Morgan HD, Sutherland HG, Martin DI, Whitelaw E. Epigenetic inheritance at the agouti locus in the mouse. Nat Genet. 1999;23:314–8. doi: 10.1038/15490. [DOI] [PubMed] [Google Scholar]
- 319.Cubas P, Vincent C, Coen E. An epigenetic mutation responsible for natural variation in floral symmetry. Nature. 1999;401:157–61. doi: 10.1038/43657. [DOI] [PubMed] [Google Scholar]
- 320.Roemer I, Reik W, Dean W, Klose J. Epigenetic inheritance in the mouse. Curr Biol. 1997;7:277–80. doi: 10.1016/s0960-9822(06)00124-2. [DOI] [PubMed] [Google Scholar]
- 321.Rakyan VK, Chong S, Champ ME, Cuthbert PC, Morgan HD, Luu KV, Whitelaw E. Transgenerational inheritance of epigenetic states at the murine Axin(Fu) allele occurs after maternal and paternal transmission. Proc Natl Acad Sci U S A. 2003;100:2538–43. doi: 10.1073/pnas.0436776100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 322.Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003;23:5293–300. doi: 10.1128/MCB.23.15.5293-5300.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.Dolinoy DC, Huang D, Jirtle RL. Maternal nutrient supplementation counteracts bisphenol A-induced DNA hypomethylation in early development. Proc Natl Acad Sci U S A. 2007;104:13056–61. doi: 10.1073/pnas.0703739104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Chong S, Youngson NA, Whitelaw E. Heritable germline epimutation is not the same as transgenerational epigenetic inheritance. Nat Genet. 2007;39:574–5. doi: 10.1038/ng0507-574. author reply 575–6. [DOI] [PubMed] [Google Scholar]
- 325.Druker R, Whitelaw E. Retrotransposon-derived elements in the mammalian genome: a potential source of disease. J Inherit Metab Dis. 2004;27:319–30. doi: 10.1023/B:BOLI.0000031096.81518.66. [DOI] [PubMed] [Google Scholar]
- 326.Morgan DK, Whitelaw E. The case for transgenerational epigenetic inheritance in humans. Mamm Genome. 2008;19:394–7. doi: 10.1007/s00335-008-9124-y. [DOI] [PubMed] [Google Scholar]
- 327.Anway MD, Leathers C, Skinner MK. Endocrine disruptor vinclozolin induced epigenetic transgenerational adult-onset disease. Endocrinology. 2006;147:5515–23. doi: 10.1210/en.2006-0640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 328.Skinner MK, Anway MD, Savenkova MI, Gore AC, Crews D. Transgenerational epigenetic programming of the brain transcriptome and anxiety behavior. PLoS ONE. 2008;3:e3745. doi: 10.1371/journal.pone.0003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Crews D, Gore AC, Hsu TS, Dangleben NL, Spinetta M, Schallert T, Anway MD, Skinner MK. Transgenerational epigenetic imprints on mate preference. Proc Natl Acad Sci U S A. 2007;104:5942–6. doi: 10.1073/pnas.0610410104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Nilsson EE, Anway MD, Stanfield J, Skinner MK. Transgenerational epigenetic effects of the endocrine disruptor vinclozolin on pregnancies and female adult onset disease. Reproduction. 2008;135:713–21. doi: 10.1530/REP-07-0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Inawaka K, Kawabe M, Takahashi S, Doi Y, Tomigahara Y, Tarui H, Abe J, Kawamura S, Shirai T. Maternal exposure to anti-androgenic compounds, vinclozolin, flutamide and procymidone, has no effects on spermatogenesis and DNA methylation in male rats of subsequent generations. Toxicol Appl Pharmacol. 2009;237:178–87. doi: 10.1016/j.taap.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 332.Schneider S, Kaufmann W, Buesen R, van Ravenzwaay B. Vinclozolin--the lack of a transgenerational effect after oral maternal exposure during organogenesis. Reprod Toxicol. 2008;25:352–60. doi: 10.1016/j.reprotox.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 333.Stouder C, Paoloni-Giacobino A. Transgenerational effects of the endocrine disruptor vinclozolin on the methylation pattern of imprinted genes in the mouse sperm. Reproduction. 139:373–9. doi: 10.1530/REP-09-0340. [DOI] [PubMed] [Google Scholar]
- 334.Salian S, Doshi T, Vanage G. Impairment in protein expression profile of testicular steroid receptor coregulators in male rat offspring perinatally exposed to Bisphenol A. Life Sci. 2009;85:11–8. doi: 10.1016/j.lfs.2009.04.005. [DOI] [PubMed] [Google Scholar]
- 335.Kim KH. Transgenerational changes after embryonic exposure to plasticizer phthalates PPTOXII: Role of Environmental Stressors in the Developmental Origins of Disease. Vol. 45 Miami, Florida, USA: 2009. [Google Scholar]
- 336.Osteen KG. PPTOXII: Role of Environmental Stressors in the Developmental Origins of Disease. Miami, Florida, USA: 2009. Transgenerational effect of dioxin on the endometriosis phenotype; pp. V.43–44. [Google Scholar]
- 337.Falls JG, Pulford DJ, Wylie AA, Jirtle RL. Genomic imprinting: implications for human disease. Am J Pathol. 1999;154:635–47. doi: 10.1016/S0002-9440(10)65309-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Feinberg AP, Cui H, Ohlsson R. DNA methylation and genomic imprinting: insights from cancer into epigenetic mechanisms. Semin Cancer Biol. 2002;12:389–98. doi: 10.1016/s1044-579x(02)00059-7. [DOI] [PubMed] [Google Scholar]
- 339.Wood AJ, Oakey RJ. Genomic imprinting in mammals: emerging themes and established theories. PLoS Genet. 2006;2:e147. doi: 10.1371/journal.pgen.0020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Lewis A, Reik W. How imprinting centres work. Cytogenet Genome Res. 2006;113:81–9. doi: 10.1159/000090818. [DOI] [PubMed] [Google Scholar]
- 341.Trasler JM. Gamete imprinting: setting epigenetic patterns for the next generation. Reprod Fertil Dev. 2006;18:63–9. doi: 10.1071/rd05118. [DOI] [PubMed] [Google Scholar]
- 342.Lane N, Dean W, Erhardt S, Hajkova P, Surani A, Walter J, Reik W. Resistance of IAPs to methylation reprogramming may provide a mechanism for epigenetic inheritance in the mouse. Genesis. 2003;35:88–93. doi: 10.1002/gene.10168. [DOI] [PubMed] [Google Scholar]
- 343.Schulz WA, Steinhoff C, Florl AR. Methylation of endogenous human retroelements in health and disease. Curr Top Microbiol Immunol. 2006;310:211–50. doi: 10.1007/3-540-31181-5_11. [DOI] [PubMed] [Google Scholar]
- 344.Roman-Gomez J, Jimenez-Velasco A, Agirre X, Castillejo JA, Navarro G, San Jose-Eneriz E, Garate L, Cordeu L, Cervantes F, Prosper F, Heiniger A, Torres A. Repetitive DNA hypomethylation in the advanced phase of chronic myeloid leukemia. Leuk Res. 2008;32:487–90. doi: 10.1016/j.leukres.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 345.Dolinoy DC, Weidman JR, Waterland RA, Jirtle RL. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ Health Perspect. 2006;114:567–72. doi: 10.1289/ehp.8700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Skinner MK. Endocrine disruptors and epigenetic transgenerational disease etiology. Pediatr Res. 2007;61:48R–50R. doi: 10.1203/pdr.0b013e3180457671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Henderson IR, Jacobsen SE. Epigenetic inheritance in plants. Nature. 2007;447:418–24. doi: 10.1038/nature05917. [DOI] [PubMed] [Google Scholar]
- 348.Rassoulzadegan M, Grandjean V, Gounon P, Vincent S, Gillot I, Cuzin F. RNA-mediated non-mendelian inheritance of an epigenetic change in the mouse. Nature. 2006;441:469–74. doi: 10.1038/nature04674. [DOI] [PubMed] [Google Scholar]
- 349.Li S, Hursting SD, Davis BJ, McLachlan JA, Barrett JC. Environmental exposure, DNA methylation, and gene regulation: lessons from diethylstilbesterol-induced cancers. Ann N Y Acad Sci. 2003;983:161–9. doi: 10.1111/j.1749-6632.2003.tb05971.x. [DOI] [PubMed] [Google Scholar]
- 350.Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes J, Bulun SE. Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biol Reprod. 2007;77:681–7. doi: 10.1095/biolreprod.107.061804. [DOI] [PubMed] [Google Scholar]
- 351.Sasaki M, Tanaka Y, Perinchery G, Dharia A, Kotcherguina I, Fujimoto S, Dahiya R. Methylation and inactivation of estrogen, progesterone, and androgen receptors in prostate cancer. J Natl Cancer Inst. 2002;94:384–90. doi: 10.1093/jnci/94.5.384. [DOI] [PubMed] [Google Scholar]
- 352.Suzuki F, Akahira J, Miura I, Suzuki T, Ito K, Hayashi S, Sasano H, Yaegashi N. Loss of estrogen receptor beta isoform expression and its correlation with aberrant DNA methylation of the 5′-untranslated region in human epithelial ovarian carcinoma. Cancer Sci. 2008;99:2365–72. doi: 10.1111/j.1349-7006.2008.00988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Zhao C, Lam EW, Sunters A, Enmark E, De Bella MT, Coombes RC, Gustafsson JA, Dahlman-Wright K. Expression of estrogen receptor beta isoforms in normal breast epithelial cells and breast cancer: regulation by methylation. Oncogene. 2003;22:7600–6. doi: 10.1038/sj.onc.1207100. [DOI] [PubMed] [Google Scholar]
- 354.Roll JD, Rivenbark AG, Jones WD, Coleman WB. DNMT3b overexpression contributes to a hypermethylator phenotype in human breast cancer cell lines. Mol Cancer. 2008;7:15. doi: 10.1186/1476-4598-7-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Coser KR, Chesnes J, Hur J, Ray S, Isselbacher KJ, Shioda T. Global analysis of ligand sensitivity of estrogen inducible and suppressible genes in MCF7/BUS breast cancer cells by DNA microarray. Proc Natl Acad Sci U S A. 2003;100:13994–9. doi: 10.1073/pnas.2235866100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Shioda T, Chesnes J, Coser KR, Zou L, Hur J, Dean KL, Sonnenschein C, Soto AM, Isselbacher KJ. Importance of dosage standardization for interpreting transcriptomal signature profiles: evidence from studies of xenoestrogens. Proc Natl Acad Sci U S A. 2006;103:12033–8. doi: 10.1073/pnas.0605341103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Hunt EA, Goulding AM, Deo SK. Direct detection and quantification of microRNAs. Anal Biochem. 2009;387:1–12. doi: 10.1016/j.ab.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.