Abstract
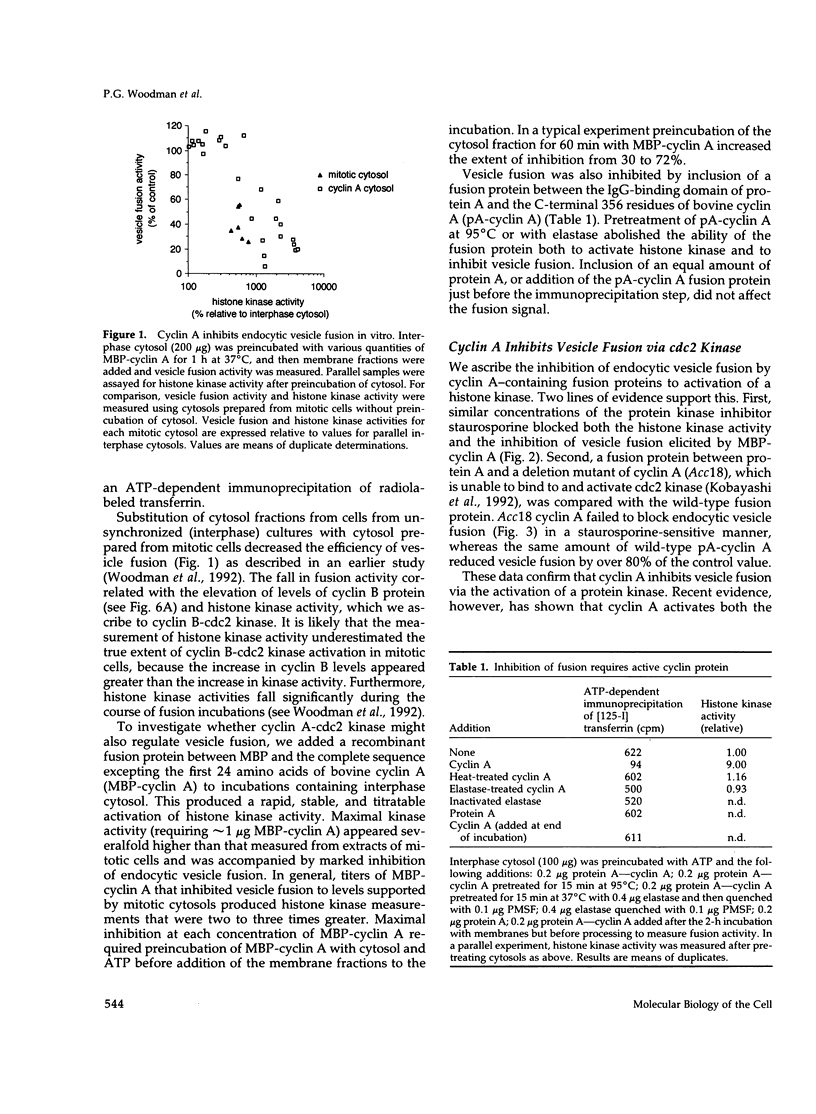
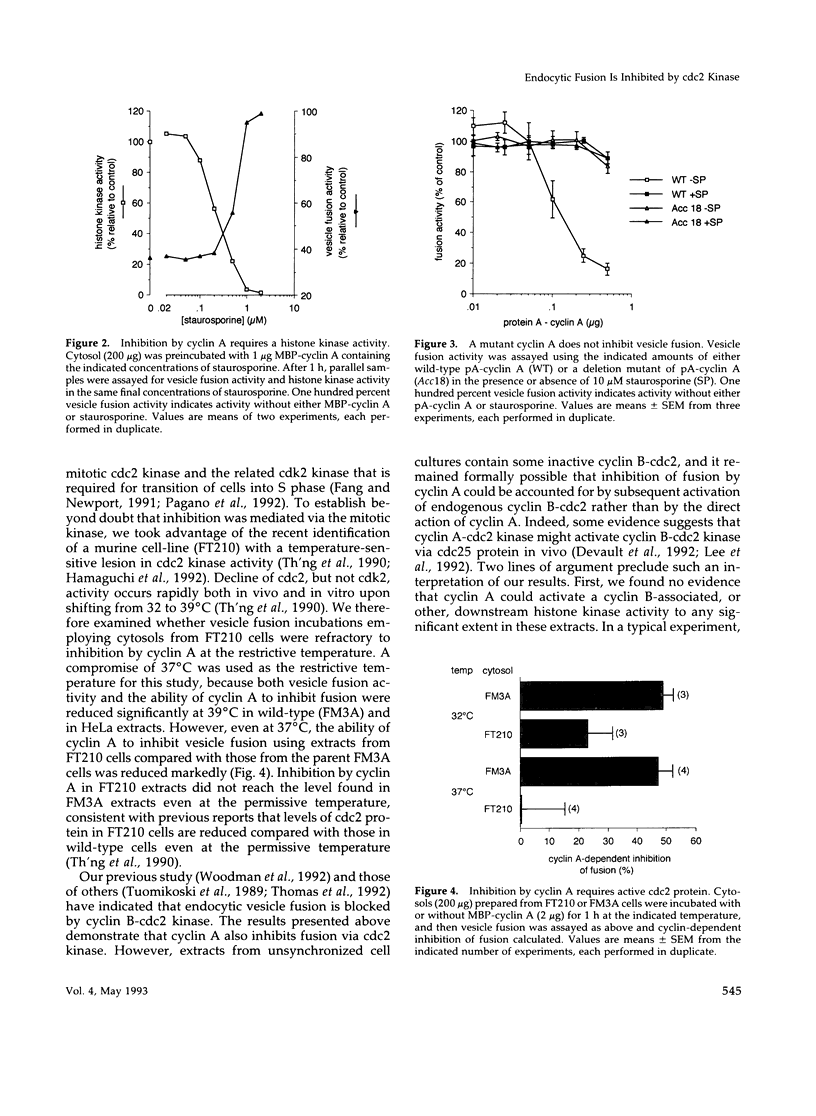
Receptor-mediated endocytosis and recycling are inhibited in mitotic mammalian cells, and previous studies have shown that inhibition of endocytic vesicle fusion in vitro occurs via cyclin B-cdc2 kinase. To test for the ability of cyclin A-cdc2 kinase to inhibit endocytic vesicle fusion, we employed recombinant cyclin A proteins. Addition of cyclin A to interphase extracts activated a histone kinase and markedly reduced the efficiency of endocytic vesicle fusion. By a number of criteria, inhibition of fusion was shown to be due to the action of cyclin A, via the mitosis-specific cdc2 kinase, and not an indirect effect through cyclin B. Two-stage incubations were used to demonstrate that at least one target of cyclin A-cdc2 kinase is a cytosolic component of the fusion apparatus. Reconstitution experiments showed that this component was also modified in mitotic cytosols and was unaffected by N-ethyl maleimide treatment.
Full text
PDF






Images in this article
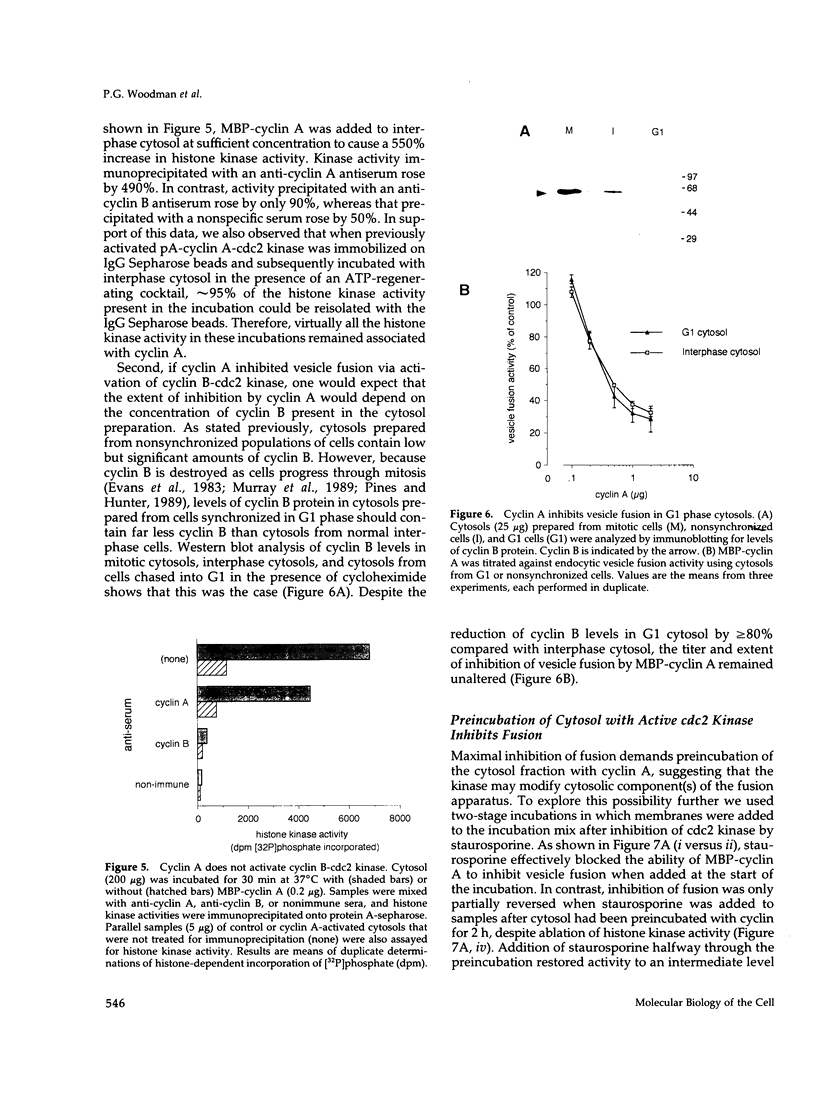
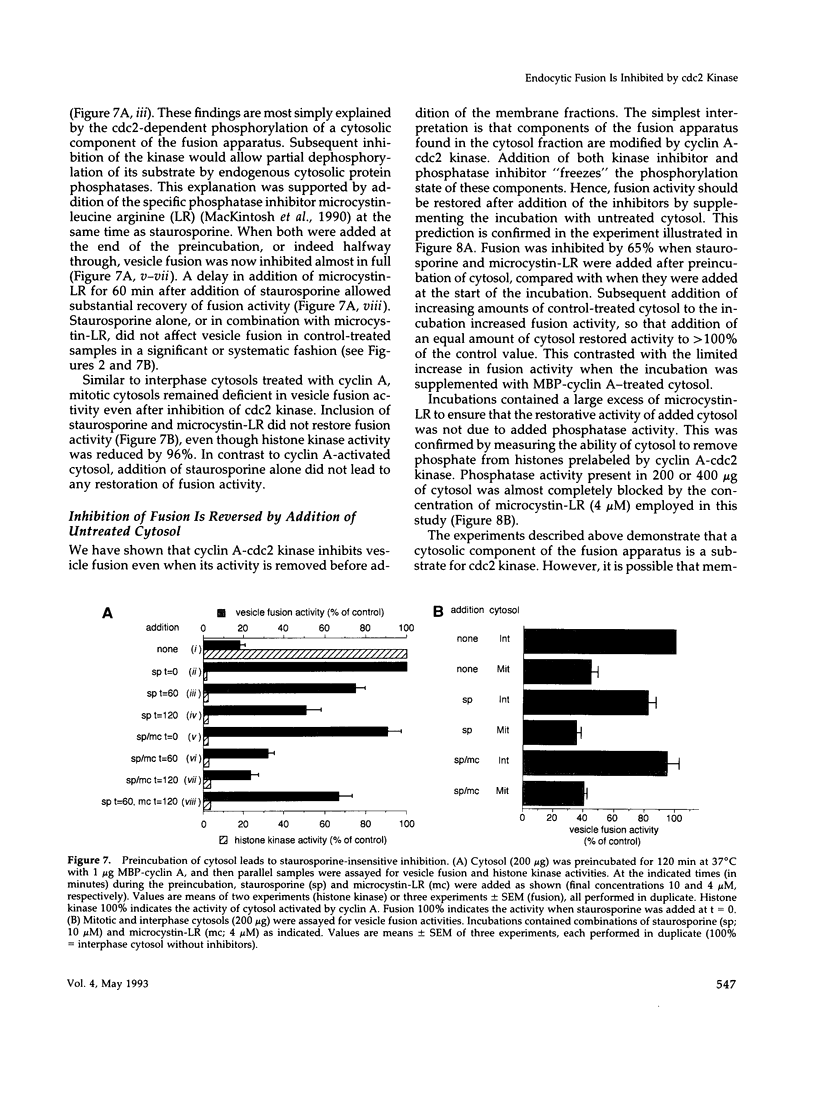
Selected References
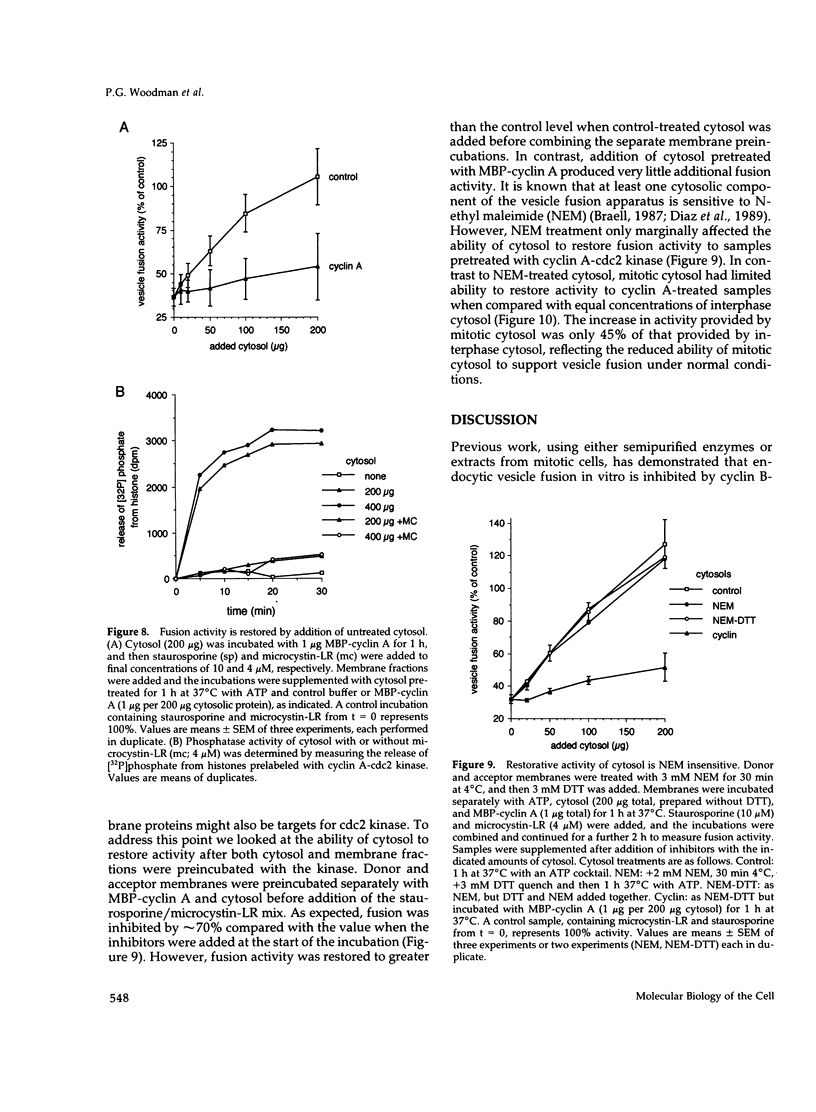
These references are in PubMed. This may not be the complete list of references from this article.
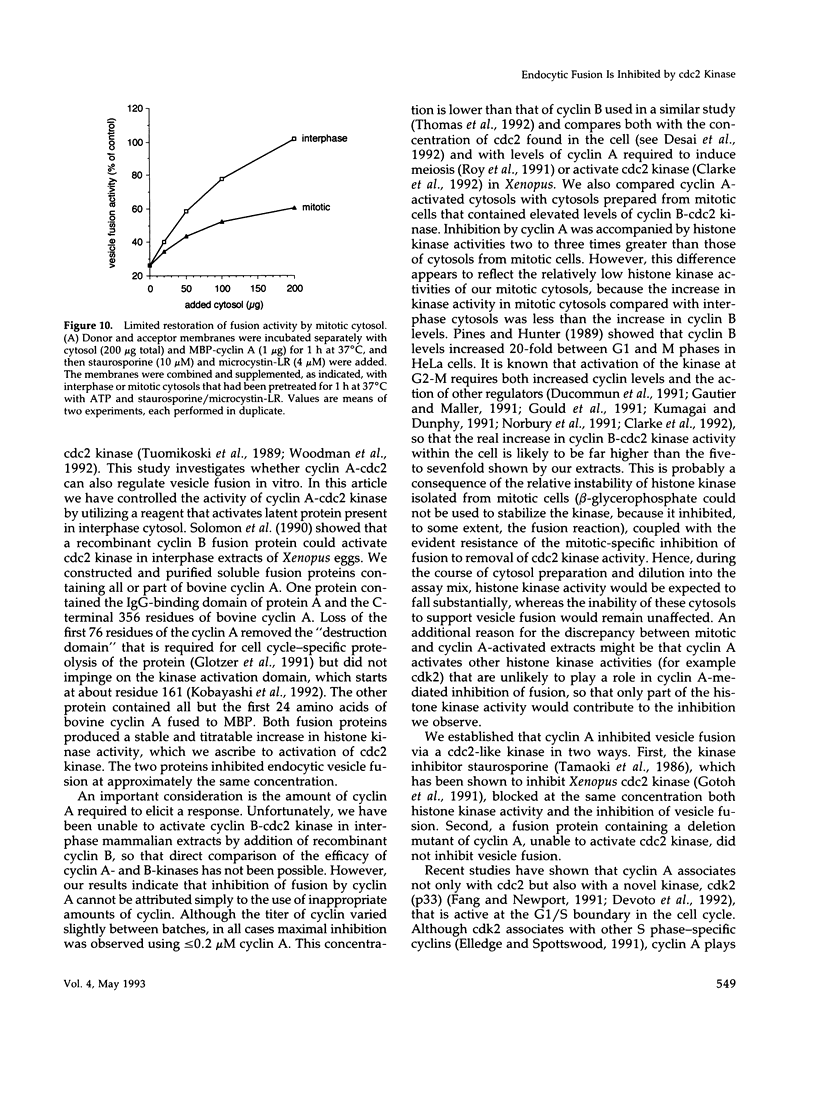
- Berlin R. D., Oliver J. M., Walter R. J. Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Cell. 1978 Oct;15(2):327–341. doi: 10.1016/0092-8674(78)90002-8. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Braell W. A. Fusion between endocytic vesicles in a cell-free system. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1137–1141. doi: 10.1073/pnas.84.5.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brizuela L., Draetta G., Beach D. Activation of human CDC2 protein as a histone H1 kinase is associated with complex formation with the p62 subunit. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4362–4366. doi: 10.1073/pnas.86.12.4362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriotti A., Colman A. Protein transport from endoplasmic reticulum to the Golgi complex can occur during meiotic metaphase in Xenopus oocytes. J Cell Biol. 1989 Oct;109(4 Pt 1):1439–1444. doi: 10.1083/jcb.109.4.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke P. R., Leiss D., Pagano M., Karsenti E. Cyclin A- and cyclin B-dependent protein kinases are regulated by different mechanisms in Xenopus egg extracts. EMBO J. 1992 May;11(5):1751–1761. doi: 10.1002/j.1460-2075.1992.tb05227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins R. N., Warren G. Sphingolipid transport in mitotic HeLa cells. J Biol Chem. 1992 Dec 5;267(34):24906–24911. [PubMed] [Google Scholar]
- Davey J., Hurtley S. M., Warren G. Reconstitution of an endocytic fusion event in a cell-free system. Cell. 1985 Dec;43(3 Pt 2):643–652. doi: 10.1016/0092-8674(85)90236-3. [DOI] [PubMed] [Google Scholar]
- Desai D., Gu Y., Morgan D. O. Activation of human cyclin-dependent kinases in vitro. Mol Biol Cell. 1992 May;3(5):571–582. doi: 10.1091/mbc.3.5.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devault A., Fesquet D., Cavadore J. C., Garrigues A. M., Labbé J. C., Lorca T., Picard A., Philippe M., Dorée M. Cyclin A potentiates maturation-promoting factor activation in the early Xenopus embryo via inhibition of the tyrosine kinase that phosphorylates cdc2. J Cell Biol. 1992 Sep;118(5):1109–1120. doi: 10.1083/jcb.118.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devoto S. H., Mudryj M., Pines J., Hunter T., Nevins J. R. A cyclin A-protein kinase complex possesses sequence-specific DNA binding activity: p33cdk2 is a component of the E2F-cyclin A complex. Cell. 1992 Jan 10;68(1):167–176. doi: 10.1016/0092-8674(92)90215-x. [DOI] [PubMed] [Google Scholar]
- Diaz R., Mayorga L. S., Weidman P. J., Rothman J. E., Stahl P. D. Vesicle fusion following receptor-mediated endocytosis requires a protein active in Golgi transport. Nature. 1989 Jun 1;339(6223):398–400. doi: 10.1038/339398a0. [DOI] [PubMed] [Google Scholar]
- Draetta G., Luca F., Westendorf J., Brizuela L., Ruderman J., Beach D. Cdc2 protein kinase is complexed with both cyclin A and B: evidence for proteolytic inactivation of MPF. Cell. 1989 Mar 10;56(5):829–838. doi: 10.1016/0092-8674(89)90687-9. [DOI] [PubMed] [Google Scholar]
- Ducommun B., Brambilla P., Félix M. A., Franza B. R., Jr, Karsenti E., Draetta G. cdc2 phosphorylation is required for its interaction with cyclin. EMBO J. 1991 Nov;10(11):3311–3319. doi: 10.1002/j.1460-2075.1991.tb04895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunphy W. G., Newport J. W. Fission yeast p13 blocks mitotic activation and tyrosine dephosphorylation of the Xenopus cdc2 protein kinase. Cell. 1989 Jul 14;58(1):181–191. doi: 10.1016/0092-8674(89)90414-5. [DOI] [PubMed] [Google Scholar]
- Elledge S. J., Spottswood M. R. A new human p34 protein kinase, CDK2, identified by complementation of a cdc28 mutation in Saccharomyces cerevisiae, is a homolog of Xenopus Eg1. EMBO J. 1991 Sep;10(9):2653–2659. doi: 10.1002/j.1460-2075.1991.tb07808.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T., Rosenthal E. T., Youngblom J., Distel D., Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983 Jun;33(2):389–396. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- FAWCETT D. W. SURFACE SPECIALIZATIONS OF ABSORBING CELLS. J Histochem Cytochem. 1965 Feb;13:75–91. doi: 10.1177/13.2.75. [DOI] [PubMed] [Google Scholar]
- Fang F., Newport J. W. Evidence that the G1-S and G2-M transitions are controlled by different cdc2 proteins in higher eukaryotes. Cell. 1991 Aug 23;66(4):731–742. doi: 10.1016/0092-8674(91)90117-h. [DOI] [PubMed] [Google Scholar]
- Featherstone C., Griffiths G., Warren G. Newly synthesized G protein of vesicular stomatitis virus is not transported to the Golgi complex in mitotic cells. J Cell Biol. 1985 Dec;101(6):2036–2046. doi: 10.1083/jcb.101.6.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Maller J. L. Cyclin B in Xenopus oocytes: implications for the mechanism of pre-MPF activation. EMBO J. 1991 Jan;10(1):177–182. doi: 10.1002/j.1460-2075.1991.tb07934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautier J., Matsukawa T., Nurse P., Maller J. Dephosphorylation and activation of Xenopus p34cdc2 protein kinase during the cell cycle. Nature. 1989 Jun 22;339(6226):626–629. doi: 10.1038/339626a0. [DOI] [PubMed] [Google Scholar]
- Gerace L., Blobel G. The nuclear envelope lamina is reversibly depolymerized during mitosis. Cell. 1980 Jan;19(1):277–287. doi: 10.1016/0092-8674(80)90409-2. [DOI] [PubMed] [Google Scholar]
- Glotzer M., Murray A. W., Kirschner M. W. Cyclin is degraded by the ubiquitin pathway. Nature. 1991 Jan 10;349(6305):132–138. doi: 10.1038/349132a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Moriyama K., Matsuda S., Okumura E., Kishimoto T., Kawasaki H., Suzuki K., Yahara I., Sakai H., Nishida E. Xenopus M phase MAP kinase: isolation of its cDNA and activation by MPF. EMBO J. 1991 Sep;10(9):2661–2668. doi: 10.1002/j.1460-2075.1991.tb07809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Moreno S., Owen D. J., Sazer S., Nurse P. Phosphorylation at Thr167 is required for Schizosaccharomyces pombe p34cdc2 function. EMBO J. 1991 Nov;10(11):3297–3309. doi: 10.1002/j.1460-2075.1991.tb04894.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gould K. L., Nurse P. Tyrosine phosphorylation of the fission yeast cdc2+ protein kinase regulates entry into mitosis. Nature. 1989 Nov 2;342(6245):39–45. doi: 10.1038/342039a0. [DOI] [PubMed] [Google Scholar]
- Gruenberg J., Howell K. E. Membrane traffic in endocytosis: insights from cell-free assays. Annu Rev Cell Biol. 1989;5:453–481. doi: 10.1146/annurev.cb.05.110189.002321. [DOI] [PubMed] [Google Scholar]
- Gurley L. R., D'Anna J. A., Barham S. S., Deaven L. L., Tobey R. A. Histone phosphorylation and chromatin structure during mitosis in Chinese hamster cells. Eur J Biochem. 1978 Mar;84(1):1–15. doi: 10.1111/j.1432-1033.1978.tb12135.x. [DOI] [PubMed] [Google Scholar]
- Hamaguchi J. R., Tobey R. A., Pines J., Crissman H. A., Hunter T., Bradbury E. M. Requirement for p34cdc2 kinase is restricted to mitosis in the mammalian cdc2 mutant FT210. J Cell Biol. 1992 Jun;117(5):1041–1053. doi: 10.1083/jcb.117.5.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanki J. P., Newport J. W. The cell cycle dependence of the secretory pathway in developing Xenopus laevis. Dev Biol. 1991 Jul;146(1):214–227. doi: 10.1016/0012-1606(91)90461-b. [DOI] [PubMed] [Google Scholar]
- Klevecz R. R. Automated cell cycle analysis. Methods Cell Biol. 1975;10:157–172. doi: 10.1016/s0091-679x(08)60735-9. [DOI] [PubMed] [Google Scholar]
- Knoblich J. A., Lehner C. F. Synergistic action of Drosophila cyclins A and B during the G2-M transition. EMBO J. 1993 Jan;12(1):65–74. doi: 10.1002/j.1460-2075.1993.tb05632.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H., Stewart E., Poon R., Adamczewski J. P., Gannon J., Hunt T. Identification of the domains in cyclin A required for binding to, and activation of, p34cdc2 and p32cdk2 protein kinase subunits. Mol Biol Cell. 1992 Nov;3(11):1279–1294. doi: 10.1091/mbc.3.11.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreiner T., Moore H. P. Membrane traffic between secretory compartments is differentially affected during mitosis. Cell Regul. 1990 Apr;1(5):415–424. doi: 10.1091/mbc.1.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leaf D. S., Roberts S. J., Gerhart J. C., Moore H. P. The secretory pathway is blocked between the trans-Golgi and the plasma membrane during meiotic maturation in Xenopus oocytes. Dev Biol. 1990 Sep;141(1):1–12. doi: 10.1016/0012-1606(90)90097-3. [DOI] [PubMed] [Google Scholar]
- Lee M. S., Ogg S., Xu M., Parker L. L., Donoghue D. J., Maller J. L., Piwnica-Worms H. cdc25+ encodes a protein phosphatase that dephosphorylates p34cdc2. Mol Biol Cell. 1992 Jan;3(1):73–84. doi: 10.1091/mbc.3.1.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner C. F., O'Farrell P. H. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990 May 4;61(3):535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorca T., Labbé J. C., Devault A., Fesquet D., Strausfeld U., Nilsson J., Nygren P. A., Uhlen M., Cavadore J. C., Dorée M. Cyclin A-cdc2 kinase does not trigger but delays cyclin degradation in interphase extracts of amphibian eggs. J Cell Sci. 1992 May;102(Pt 1):55–62. doi: 10.1242/jcs.102.1.55. [DOI] [PubMed] [Google Scholar]
- Luca F. C., Ruderman J. V. Control of programmed cyclin destruction in a cell-free system. J Cell Biol. 1989 Nov;109(5):1895–1909. doi: 10.1083/jcb.109.5.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luca F. C., Shibuya E. K., Dohrmann C. E., Ruderman J. V. Both cyclin A delta 60 and B delta 97 are stable and arrest cells in M-phase, but only cyclin B delta 97 turns on cyclin destruction. EMBO J. 1991 Dec;10(13):4311–4320. doi: 10.1002/j.1460-2075.1991.tb05009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Berger E. G., Warren G. Mitotic Golgi fragments in HeLa cells and their role in the reassembly pathway. J Cell Biol. 1989 Aug;109(2):463–474. doi: 10.1083/jcb.109.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucocq J. M., Pryde J. G., Berger E. G., Warren G. A mitotic form of the Golgi apparatus in HeLa cells. J Cell Biol. 1987 Apr;104(4):865–874. doi: 10.1083/jcb.104.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKintosh C., Beattie K. A., Klumpp S., Cohen P., Codd G. A. Cyanobacterial microcystin-LR is a potent and specific inhibitor of protein phosphatases 1 and 2A from both mammals and higher plants. FEBS Lett. 1990 May 21;264(2):187–192. doi: 10.1016/0014-5793(90)80245-e. [DOI] [PubMed] [Google Scholar]
- Minshull J., Golsteyn R., Hill C. S., Hunt T. The A- and B-type cyclin associated cdc2 kinases in Xenopus turn on and off at different times in the cell cycle. EMBO J. 1990 Sep;9(9):2865–2875. doi: 10.1002/j.1460-2075.1990.tb07476.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray A. W., Solomon M. J., Kirschner M. W. The role of cyclin synthesis and degradation in the control of maturation promoting factor activity. Nature. 1989 May 25;339(6222):280–286. doi: 10.1038/339280a0. [DOI] [PubMed] [Google Scholar]
- Norbury C., Blow J., Nurse P. Regulatory phosphorylation of the p34cdc2 protein kinase in vertebrates. EMBO J. 1991 Nov;10(11):3321–3329. doi: 10.1002/j.1460-2075.1991.tb04896.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurse P. Universal control mechanism regulating onset of M-phase. Nature. 1990 Apr 5;344(6266):503–508. doi: 10.1038/344503a0. [DOI] [PubMed] [Google Scholar]
- Ohkura H., Kinoshita N., Miyatani S., Toda T., Yanagida M. The fission yeast dis2+ gene required for chromosome disjoining encodes one of two putative type 1 protein phosphatases. Cell. 1989 Jun 16;57(6):997–1007. doi: 10.1016/0092-8674(89)90338-3. [DOI] [PubMed] [Google Scholar]
- Pagano M., Pepperkok R., Verde F., Ansorge W., Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992 Mar;11(3):961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunt T. Molecular cloning and characterization of the mRNA for cyclin from sea urchin eggs. EMBO J. 1987 Oct;6(10):2987–2995. doi: 10.1002/j.1460-2075.1987.tb02604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Human cyclins A and B1 are differentially located in the cell and undergo cell cycle-dependent nuclear transport. J Cell Biol. 1991 Oct;115(1):1–17. doi: 10.1083/jcb.115.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J., Hunter T. Isolation of a human cyclin cDNA: evidence for cyclin mRNA and protein regulation in the cell cycle and for interaction with p34cdc2. Cell. 1989 Sep 8;58(5):833–846. doi: 10.1016/0092-8674(89)90936-7. [DOI] [PubMed] [Google Scholar]
- Pypaert M., Lucocq J. M., Warren G. Coated pits in interphase and mitotic A431 cells. Eur J Cell Biol. 1987 Dec;45(1):23–29. [PubMed] [Google Scholar]
- ROBBINS E., GONATAS N. K. THE ULTRASTRUCTURE OF A MAMMALIAN CELL DURING THE MITOTIC CYCLE. J Cell Biol. 1964 Jun;21:429–463. doi: 10.1083/jcb.21.3.429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy L. M., Swenson K. I., Walker D. H., Gabrielli B. G., Li R. S., Piwnica-Worms H., Maller J. L. Activation of p34cdc2 kinase by cyclin A. J Cell Biol. 1991 May;113(3):507–514. doi: 10.1083/jcb.113.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sager P. R., Brown P. A., Berlin R. D. Analysis of transferrin recycling in mitotic and interphase HeLa cells by quantitative fluorescence microscopy. Cell. 1984 Dec;39(2 Pt 1):275–282. doi: 10.1016/0092-8674(84)90005-9. [DOI] [PubMed] [Google Scholar]
- Smythe E., Pypaert M., Lucocq J., Warren G. Formation of coated vesicles from coated pits in broken A431 cells. J Cell Biol. 1989 Mar;108(3):843–853. doi: 10.1083/jcb.108.3.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon M. J., Glotzer M., Lee T. H., Philippe M., Kirschner M. W. Cyclin activation of p34cdc2. Cell. 1990 Nov 30;63(5):1013–1024. doi: 10.1016/0092-8674(90)90504-8. [DOI] [PubMed] [Google Scholar]
- Stuart R. A., Mackay D., Adamczewski J., Warren G. Inhibition of intra-Golgi transport in vitro by mitotic kinase. J Biol Chem. 1993 Feb 25;268(6):4050–4054. [PubMed] [Google Scholar]
- Swenson K. I., Farrell K. M., Ruderman J. V. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986 Dec 26;47(6):861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Tamaoki T., Nomoto H., Takahashi I., Kato Y., Morimoto M., Tomita F. Staurosporine, a potent inhibitor of phospholipid/Ca++dependent protein kinase. Biochem Biophys Res Commun. 1986 Mar 13;135(2):397–402. doi: 10.1016/0006-291x(86)90008-2. [DOI] [PubMed] [Google Scholar]
- Th'ng J. P., Wright P. S., Hamaguchi J., Lee M. G., Norbury C. J., Nurse P., Bradbury E. M. The FT210 cell line is a mouse G2 phase mutant with a temperature-sensitive CDC2 gene product. Cell. 1990 Oct 19;63(2):313–324. doi: 10.1016/0092-8674(90)90164-a. [DOI] [PubMed] [Google Scholar]
- Thomas B. J., Rothstein R. Sex, maps, and imprinting. Cell. 1991 Jan 11;64(1):1–3. doi: 10.1016/0092-8674(91)90199-9. [DOI] [PubMed] [Google Scholar]
- Thomas L., Clarke P. R., Pagano M., Gruenberg J. Inhibition of membrane fusion in vitro via cyclin B but not cyclin A. J Biol Chem. 1992 Mar 25;267(9):6183–6187. [PubMed] [Google Scholar]
- Tuomikoski T., Felix M. A., Dorée M., Gruenberg J. Inhibition of endocytic vesicle fusion in vitro by the cell-cycle control protein kinase cdc2. Nature. 1989 Dec 21;342(6252):942–945. doi: 10.1038/342942a0. [DOI] [PubMed] [Google Scholar]
- Verde F., Dogterom M., Stelzer E., Karsenti E., Leibler S. Control of microtubule dynamics and length by cyclin A- and cyclin B-dependent kinases in Xenopus egg extracts. J Cell Biol. 1992 Sep;118(5):1097–1108. doi: 10.1083/jcb.118.5.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Davoust J., Cockcroft A. Recycling of transferrin receptors in A431 cells is inhibited during mitosis. EMBO J. 1984 Oct;3(10):2217–2225. doi: 10.1002/j.1460-2075.1984.tb02119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G., Woodman P., Pypaert M., Smythe E. Cell-free assays and the mechanism of receptor-mediated endocytosis. Trends Biochem Sci. 1988 Dec;13(12):462–465. doi: 10.1016/0968-0004(88)90229-0. [DOI] [PubMed] [Google Scholar]
- Whitfield W. G., Gonzalez C., Maldonado-Codina G., Glover D. M. The A- and B-type cyclins of Drosophila are accumulated and destroyed in temporally distinct events that define separable phases of the G2-M transition. EMBO J. 1990 Aug;9(8):2563–2572. doi: 10.1002/j.1460-2075.1990.tb07437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman P. G., Mundy D. I., Cohen P., Warren G. Cell-free fusion of endocytic vesicles is regulated by phosphorylation. J Cell Biol. 1992 Jan;116(2):331–338. doi: 10.1083/jcb.116.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodman P. G., Warren G. Fusion between vesicles from the pathway of receptor-mediated endocytosis in a cell-free system. Eur J Biochem. 1988 Apr 5;173(1):101–108. doi: 10.1111/j.1432-1033.1988.tb13972.x. [DOI] [PubMed] [Google Scholar]
- Woodman P. G., Warren G. Fusion of endocytic vesicles in a cell-free system. Methods Cell Biol. 1989;31:197–206. doi: 10.1016/s0091-679x(08)61610-6. [DOI] [PubMed] [Google Scholar]
- Woodman P. G., Warren G. Isolation of functional, coated, endocytic vesicles. J Cell Biol. 1991 Mar;112(6):1133–1141. doi: 10.1083/jcb.112.6.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]