Abstract
Purpose
Del22q11.2, also known as DiGeorge syndrome, has a spectrum of ocular, facial and systemic features. Despite features of T cell dysfunction, infection and autoimmunity (including juvenile idiopathic arthritis), uveitis has not been described in patients with DiGeorge syndrome.
Methods
We describe a case of a 25-year-old male with bilateral granulomatous panuveitis who after initial investigation and treatment for an infectious cause was determined to have autoimmune-related uveitis with evidence on clinical, laboratory and imaging assessments suggestive of ocular sarcoidosis.
Results
The patient was found to have a normal T cell count and T cell proliferative response that was compared to a control patient, and phenotypes determined by flow cytometry were normal. However, the CD4/CD8 ratio in this patient was slightly lower than normal and the number of CD28 negative T cells, in both CD4 and CD8 populations, were significantly higher than a control.
Conclusions
The significance of these T cell abnormalities is unknown in the context of this patient’s uveitis but is suggestive of a role in autoimmunity, which is a known phenomenon in del22q11.2 syndrome, although autoimmune-related uveitis is not a previously described feature.
Keywords: DiGeorge syndrome, Uveitis, Deletion 22q11.2, Velocardiofacial syndrome, Sarcoidosis
INTRODUCTION
Deletion of chromosome 22q11.2, also known as DiGeorge Syndrome, occurs in 1 in 4000 live births: It has been called the most viable chromosomal deletion, and is the second most frequent genetic cause of cardiac defects after Down syndrome.1 DiGeorge syndrome has a wide range of associated anomalies. The most common defects leading to the diagnosis are the cardiac defects, absence of the thymus gland, infections and neonatal hypocalcemia. However, over 180 features have been associated with del22q11.2. The two main causes of mortality in patients are congenital cardiac defects and infections secondary to immune deficiency. Improvements in surgery and prophylactic regimens for opportunistic infections have enabled the survival of patients with del22q11.2 beyond infancy.
Approximately 90% of patients with features of DiGeorge syndrome have a deletion of chromosome 22.2 The deleted region is between 1.5 and 3 megabases long and contains over 30 genes. Deletions in the region of 22q11 are spontaneous, but not random. Certain regions along chromosome 22 have a high propensity for instability during meiosis because of duplicated chromosome segments containing a high degree of nearly identical sequence (> 96%). This predisposes to deletions, recombination and ring chromosomes. The deletion can be heritable as a hemizygous trait, predominantly autosomal dominant, and families have been described.3–5
Patients with only mild reductions in T-cell number and function are termed “partial DiGeorge syndrome.” Complete DiGeorge syndrome denotes patients with thymic aplasia. As with other immune deficient conditions, there is a higher rate of autoimmune disease in del22q11.2 syndrome. Autoimmune cytopenias, juvenile idiopathic arthritis and Graves’ disease are reported, but no autoimmune defects have been linked to specific chromosome 22q11.2 deletions.
It is interesting to note, however, that despite the reported association with autoimmune arthropathy (juvenile idiopathic arthritis, JIA), uveitis is not a known ocular feature of DiGeorge syndrome.6–11 Herein, we report a case of autoimmune uveitis in a patient with del22q11.2 syndrome. The possible etiologies are discussed, as well as the relationship of autoimmune-related ocular inflammation to del22q11.2 syndrome.
CASE
A 25-year-old Caucasian male was assessed at the National Eye Institute (NEI) for a 3 month history of bilateral blurry vision, photophobia, ocular pain, floaters and photopsias. Symptoms included fevers (undocumented), chills, fatigue, headaches, myalgias, arthralgias, dry cough, nasal congestion and sinus pain. The patient was diagnosed with partial DiGeorge syndrome shortly after birth based on features of neonatal hypocalcemia, partial cleft palate, absent thymus gland on imaging, and bicuspid aortic valve. The patient’s medical history and abnormalities associated with del22q11.2 syndrome are included in Table 1.
TABLE 1.
Patient’s medical history and clinical features consistent with del22q11.2 syndrome
| Asthma | Hypoplastic thymus gland |
| Eczema | Neonatal hypocalcemia |
| Seasonal allergy | Right-sided aortic arch, bicuspid aortic valve |
| Recurrent pneumonia (childhood) | Partial cleft palate |
| Recurrent cold sores (childhood) | Focal deformity & hypoplasia of the 1st and 2nd ribs |
| Recurrent otitis media | Mild neurosensory hearing loss |
| Tonsillectomy | Atypical facies |
| Measles, chickenpox (childhood) | |
| Chronic sinusitis | |
| Migraine |
The family history was significant for allergies and asthma in the siblings and grandparents, Crohn’s disease in the mother, cervical and brain cancer in the maternal grandmother and diabetes in both grandparents on the maternal side. No other family members were recalled to have features of del22q11.2.
The patient had been evaluated at an outside medical center and treated for bilateral anterior uveitis and optic disc edema with prednisolone acetate 1% drops and prednisone 60 mg a day for 6 weeks with improvement in vision and eye pain while on prednisone. He was investigated with cerebral CT and lumbar puncture for possible idiopathic intracranial hypertension. The CT scan revealed normal brain structures and no masses. The opening pressure of the LP was normal and CSF investigations, including gram stain, culture, VDRL and ELISA for Lyme antibody were negative. A tuberculin skin test was also negative. The patient was prescribed valacyclovir, for presumptive herpes-associated uveitis based on an elevated serum HSV I/II IgG which did not result in improvement of ocular inflammation or systemic symptoms.
On examination at the NEI, best corrected visual acuity was 20/25 in the right eye (OD) and 20/23 + 2 in the left eye (OS). Intraocular pressures were 10 mmHg OD and 12 mmHg OS. There was no relative afferent pupillary defect. The orbital examination, ocular motility, eyelids, lashes and lacrimal system were normal. The conjunctivas were injected bilaterally. The corneas had fine non-granulomatous keratic precipitates inferiorly in both eyes (OU). Posterior synechiae were noted OU (Figure 1). The anterior chambers had 2+ cells and 1+ flare OU. The dilated examination revealed clear lenses, trace vitreous cells OU, no vitreous haze OD and trace haze OS. Snowballs were present in the inferior vitreous OU. There were no pars plana exudates. There were well-circumscribed punched-out chorioretinal lesions and ill-defined small cream-colored deep chorioretinal infiltrates scattered inferiorly and in the mid-periphery, reminiscent of Dalen-Fuchs nodules (Figure 2A). Optic disc edema was present bilaterally (Figure 2B). The fluorescein angiogram showed no retinal vascular leakage, however, a small area of leakage in the left macula was confirmed on OCT to be an intraretinal cyst (Figure 2C).
FIGURE 1.
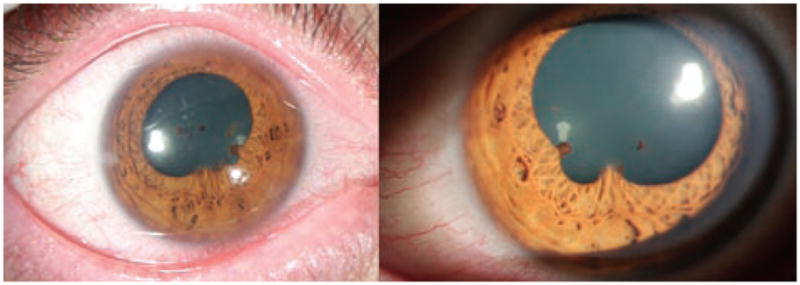
Anterior segment photos of the right and left eyes demonstrate conjunctival injection in both eyes, irregular pupils due to posterior synechiae and mild flare in the anterior chamber.
FIGURE 2.
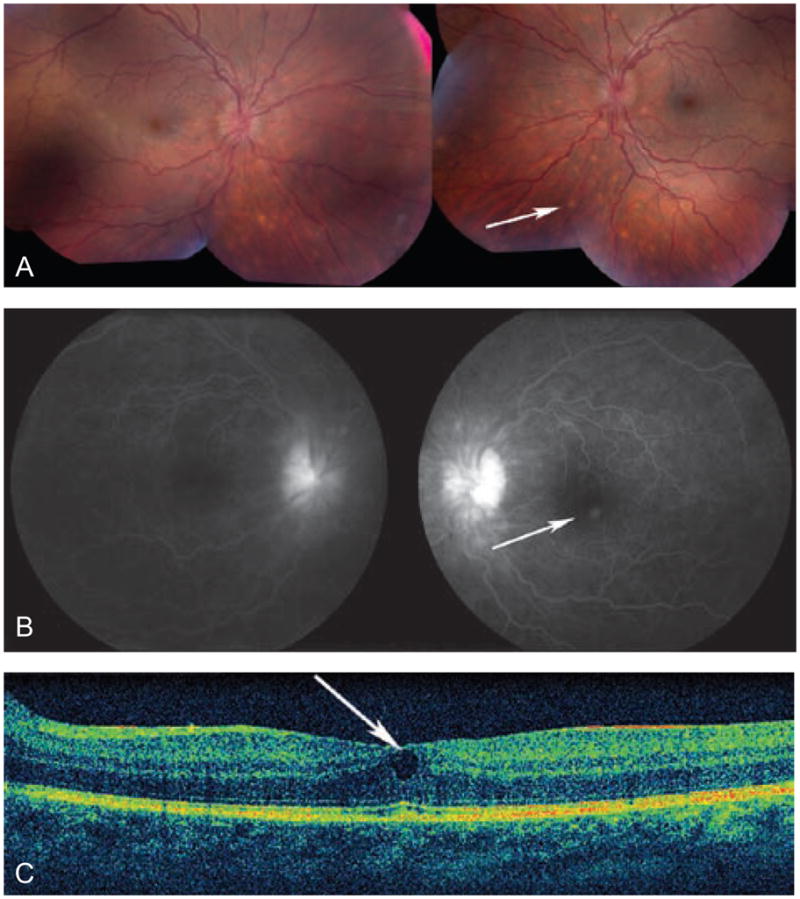
(A) Color photos of the right and left eyes demonstrate optic disc edema and splinter hemorrhages, as well as tortuous vessels and deep choroidal lesions (arrow, most easily visible inferior to the nerve heads). There is trace vitreous haze OD. (B) Fluorescein angiogram of the right and left eyes showing hyperfluorescence of the optic nerves and an area of leakage near the fovea corresponding to a retinal cyst seen by OCT (C)
Consultation with internal medicine, neurology, infectious disease, audiology, rheumatology, dermatology and immunogenetics specialists confirmed the phenotype of DiGeorge syndrome and did not reveal signs of infection, arthritis or other associated disease. Fluorescence in-situ hybridization (FISH) analysis confirmed a deletion of chromosome 22q11.2 (Figure 3). Laboratory workup for infectious causes was negative (Table 2). Abnormal tests included elevated erythrocyte sedimentation rate (ESR, 29 mm/hr, normal 0–25 mm/hr), C-reactive protein (CRP, 3.86 mg/dL, normal < 0.8 mg/dL), elevated C3 complement (192 mg/dL, normal 69–175 mg/dL), high-normal C4 complement (37 mg/dL, normal 13–38 mg/dL), and an elevated angiotensin converting enzyme (ACE, 79 U/L, normal 16–52 U/L). Mild-to-moderate hearing loss was confirmed by audiology testing. An echocardiogram revealed normal systolic function, thickened mitral valve leaflets and mild pulmonic insufficiency. Radiography of the chest showed a right-sided aortic arch, normal heart size, clear lungs and hypoplasia of the first and second ribs. CT of the chest demonstrated finely nodular lung infiltrates. CT and MRI of the head and orbits with contrast were unremarkable. Radiography of the peripheral joints revealed neither degenerative nor arthritic changes. A gallium scan showed significant uptake of lacrimal glands, parotid glands and nasal region.
FIGURE 3.
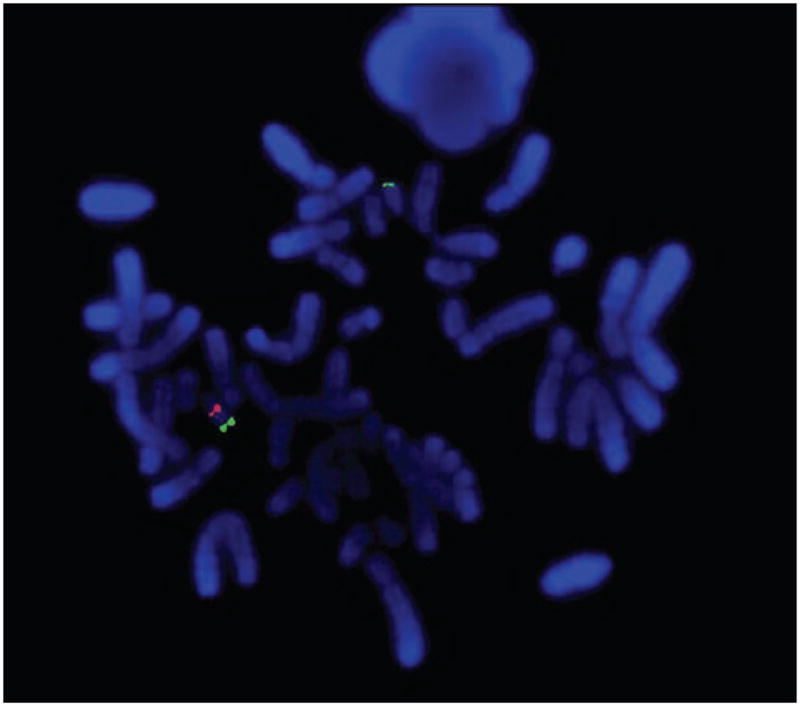
Cytogenetic analysis by fluorescence in-situ hybridization (FISH). The DiGeorge critical region is detected by the red probe (lower left), a distal 22q gene (ARSA) is detected by the green probe (lower left and upper). There is one normal #22 chromosome (both red and green signals, lower left) and one abnormal #22 chromosome deleted for the DGS/VCFS region with only a green signal (upper).
TABLE 2.
Patient’s laboratory test results, negative or normal range
| Blood chemistries, renal function tests, liver function tests |
| Blood counts |
| Blood cultures |
| Prealbumin serum level |
| INR, PTT, aPTT |
| Urinalysis |
| TSH, antibody testing (anti-thyroglobulin antibody, anti-thyroid peroxidase) |
| CSF: cell count, gram stain, culture, glucose, myelin basic protein, protein electrophoresis, VDRL, immune globulins, PCR (CMV, EBV, HSV, VZV, toxoplasmosis, Lyme), Lyme ELISA |
| Aqueous: PCR CMV, HSV, Lyme |
| HIV 1/2 |
| Syphilis IgG, RPR |
| Lyme PCR, ELISA, Western blot |
| Toxocara IgG, IgM |
| Toxoplasma IgG, IgM |
| Bartonella henselae and quintana IgG, IgM |
| CMV PCR |
| HSV 1/2, HSV 1 IgG positive, HSV 2 IgG negative, HSV IgM negative |
| EBV PCR negative, EBVCA-IgG positive, EBVCA-IgM negative, EBNA positive |
| Parvovirus B19 IgG, IgM |
| Plasma lysozyme |
| HLA-B27 |
| HLA-A29 |
| ANCA |
| Anti-dsDNA antibody |
| ANA |
| Anti-ENA screen |
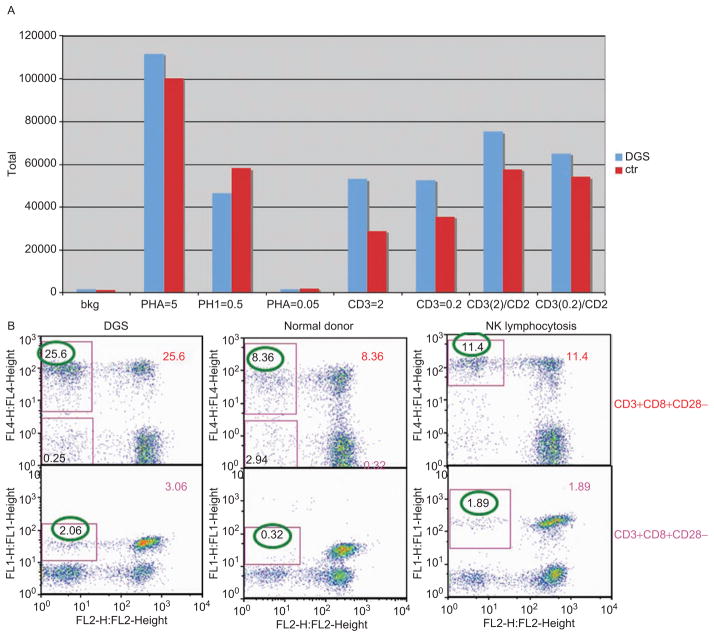
T cell counts and serum IgM fluctuated from normal to slightly below normal. Additional testing was performed in the Laboratory of Immunology at NEI and through the immunogenetics laboratory of NIAID (National Institute of Allergy and Infectious Diseases): The patient had a normal number of recent thymic immigrants (CD4+/CD45RA+/CD31+). Although T cell proliferative response to polyclonal and T cell receptor stimulation was normal (Figure 4), the antibody titers of tetanus, rubeola, mumps, rubella, and varicella zoster were undetectable, despite having received childhood vaccines and boosters, and having had chickenpox and rubeola infection in childhood. Phenotypic analysis by flow cytometry revealed no apparent changes on major leukocyte sub-populations, for example, CD4+ and CD8+ T cells, CD3+CD4−CD8− (double negative T cells), and monocytes/macrophages were all within normal range. However, CD4/CD8 ratio in this patient was slightly lower than normal range (1:1 instead of 2:1). Detailed analysis of CD28 expression on T cells revealed that CD28 negative T cells, in both CD4 and CD8 populations, were significantly higher than that of a normal control donor and a patient with presumed diagnosis of NK lymphocytosis (Figure 4). The number of recent thymic emigrants can be investigated by a count of T cell receptor excision circles (TREC). A low count indicates few recent thymic emigrants and this was the case in our patient (CD4 subset: 43.49 TREC/10,000 cells and CD8 subset: 58.97 TREC/10,000 cells). Our patient also had normal numbers of peripheral blood regulatory T cells and TCRVβ distribution.
FIGURE 4.
(A) T cell proliferative response to phytohemaglutinin (PHA) and anti-TCR stimulation. Peripheral blood mononuclear cells (PBMCs) were isolated from fresh blood from the patient and a healthy donor. PBMCs were stimulated with PHA or anti-CD3 with without anti-CD28 at different dosages as depicted in the figure. Tritium labeled thymidine incorporation was used to measure proliferative response of T cells to the stimuli. There was no difference in T cell proliferation between the patient and a healthy control. (B) Increased CD28 negative T cells in patient compared to that in normal control. Whole blood samples were analyzed by a 4-color flow cytometry staining using FITC-CD4, PE-CD28, PE-CY7-CD3 and APC-CD8 antibodies (BD Biosciences, CA). The T cell staining was analyzed using FlowJo software (TriStar, San Jose, CA). CD28 negative T cells were gated based on CD3+CD4+CD28- and CD3+CD8+CD28- triple staining. CD28 negative T cells in both CD3+CD4+ and CD3+CD8+ T cells were much higher in DGS (DiGeorge syndrome) patient than in a normal control donor. A patient with a diagnosis of NK (natural killer cell) lymphocytosis and multiple autoimmune diseases also showed increased CD28 negative T cells.
From an ocular perspective, the patient was diagnosed with bilateral chronic granulomatous panuveitis. However, due to the presence of risk factors for exposure to Borrelia burgdoferi, and a history of chronic fatigue, arthralgias, myalgias, headaches and rash, a repeat LP was performed, despite a previously negative Lyme PCR and ELISA of the serum, and this was negative for Borrelia (gram stain, ELISA, PCR and culture), herpes-family viruses, EBV, toxoplasmosis, syphilis and bacteria. The opening pressure was normal. A biopsy of the skin rash did not reveal spirochetes. An aqueous humor tap was similarly unrevealing following PCR for Lyme and herpes viruses, and culture for microorganisms. Despite an extensive negative work-up, the patient was treated empirically with IV ceftriaxone for 3 weeks with no significant change in ocular inflammation. It was felt that the patient’s aberrant immune system and, in general, the low sensitivity of Borrelia testing, may have been factors preventing a definitive diagnosis. Subsequently, the patient was treated with prednisone (0.4 mg/kg) and cyclosporine (4 mg/kg) with an improvement in ocular inflammation.
DISCUSSION
To the best of our knowledge, this is the first case of uveitis in a patient with DiGeorge syndrome (DGS). A search of the literature was performed using PubMed (Medline) and search headings “DiGeorge” and “deletion 22q11.2.”
In patients with systemic disorders and impaired immune function, caution is emphasized when immunosuppressive therapy is introduced for treatment of ocular inflammation. This case is a key example of the need for thorough investigation and treatment of possible infectious causes of uveitis in a patient with immune dysregulation prior to the administration of immunosuppressive therapy that may potentiate systemic infection. Improvement of the ocular inflammation followed a course of prednisone and cyclosporine, supporting the presupposition that the inflammation was autoimmune in nature.
This patient also had elevated ACE, ESR and CRP, as well as lacrimal gland uptake on the gallium scan. Although one cannot make an absolute diagnosis without a tissue biopsy, these laboratory and imaging findings in the context of granulomatous panuveitis may be compatible with ocular sarcoidosis. Additionally, our patient’s HLA typing included DQB1*0602, which has been shown to be associated with chronic disease in sarcoidosis.12,13 Autoimmune disease and allergy are more frequent in del22q11.2 than in the general population. JIA and autoimmune cytopenias are the most commonly reported autoimmune associations in DGS.1 Sarcoidosis, however, has not been described in DiGeorge syndrome. The immune dysregulation in sarcoidosis is primarily a T cell-mediated process. The T cell activation and abnormal oligoclonal expansion of CD4+ T cells seen in sarcoidosis, as well as the postulated relationship between environmental factors (allergens) viruses and bacteria as causes of sarcoidosis, are similar factors involved in prompting autoimmune phenomena in DiGeorge syndrome, that has as its central features, thymic dysregulation, recurrent infections and allergy.14 Repeated infections (antigenic stimulation) may predispose these patients to autoimmunity.15
It has been shown that T cell numbers in 75–80% of infants with del22q11.2 are low, however, 20% of adult patients have no evidence of decreased T cells.1 We found that our patient had low-normal CD3+ and CD4+ T cell numbers and CD8+ T cell counts consistently in the mid-to-upper range of normal. The CD4/CD8 ratio was accordingly lower than normal. This has been reported as a common feature of patients with partial DGS.15 The finding of normal T cell numbers, despite abnormal thymus development, has been proposed to result from either a homeostatic proliferation of cells over time by peripheral expansion of T cell clones or an increase in memory or effector cells produced by the thymus.16, 17 Our patient’s TREC numbers were low but not unusual for DGS, indicating multiple rounds of cell division have occurred and the peripheral expansion of T cells may have continued to maintain normal T cell counts. Low TREC counts have been shown to correlate with immunologic function in DGS patients, lower TREC being associated with poorer lymphocyte proliferation response to mitogens and antigens.18 CD28-CD8+ T cells have been linked to autoimmune disease; these cells were found in high numbers in granulomatous lesions of Wegener’s disease and related to the tissue damage in hepatitis C patients.19, 20 CD28- T cells, especially CD28-CD8+ T cells, were also significantly higher in this patient compared to both a normal control and another patient with known multiple autoimmune diseases, and is of particular interest. Further studies will be required to confirm and elucidate the contribution of this unique sub-population of cytotoxic T cells to DiGeorge syndrome and their link to autoimmune disease.
CONCLUSION
We report the first known case of autoimmune-mediated bilateral granulomatous uveitis in a 25-year-old male with partial DiGeorge syndrome with confirmed del22q11.2 by FISH analysis. The patient’s features, medical history and immunologic abnormalities are consistent with prior reports. Uveitis should be considered among the autoimmune features of patients with DiGeorge syndrome.
Footnotes
Declaration of interest: The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
This article is not subject to United States copyright law.
References
- 1.Sullivan KE. Chromosome 22q11.2 deletion syndrome: DiGeorge syndrome/velocardiofacial Syndrome. Immunol Allergy Clin North Am. 2008;28(2):353–366. doi: 10.1016/j.iac.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Martin Mateos MA, Perez Duenas BP, Iriondo M, et al. Clinical and immunological spectrum of partial DiGeorge syndrome. J Investig Allergol Clin Immunol. 2000;10(6):352–360. [PubMed] [Google Scholar]
- 3.Lichtner P, Konig R, Hasegawa T, et al. An HDR (hypopara-thyroidism, deafness, renal dysplasia) syndrome locus maps distal to the DiGeorge syndrome region on 10p13/14. J Med Genet. 2000;37(1):33–37. doi: 10.1136/jmg.37.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenberg F, Courtney KB, Wessels RA, et al. Prenatal diagnosis of deletion 17p13 associated with DiGeorge anomaly. Am J Med Genet. 1988;31(1):1–4. doi: 10.1002/ajmg.1320310102. [DOI] [PubMed] [Google Scholar]
- 5.DeBerardinis RJ, Medne L, Spinner NB, et al. DiGeorge anomaly in a patient with isochromosome 18p born to a diabetic mother. Am J Med Genet A. 2005;138A(2):155–159. doi: 10.1002/ajmg.a.30913. [DOI] [PubMed] [Google Scholar]
- 6.Cron RQ, Sullivan KE. Chronic arthritis without uveitis in velocardiofacial syndrome. J Pediatr. 2006;149(2):281. doi: 10.1016/j.jpeds.2005.09.017. [DOI] [PubMed] [Google Scholar]
- 7.Pelkonen P, Lahdenne P, Lantto R, et al. Chronic arthritis associated with chromosome deletion 22q11.2 syndrome. J Rheumatol. 2002;29(12):2648–2650. [PubMed] [Google Scholar]
- 8.Davies K, Stiehm ER, Woo P, et al. Juvenile idiopathic polyarticular arthritis and IgA deficiency in the 22q11 deletion syndrome. J Rheumatol. 2001;28(10):2326–2334. [PubMed] [Google Scholar]
- 9.Verloes A, Curry C, Jamar M, et al. Juvenile rheumatoid arthritis and del(22q11) syndrome: a non-random association. J Med Genet. 1998;35(11):943–947. doi: 10.1136/jmg.35.11.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Keenan GF, Sullivan KE, McDonald-McGinn DM, et al. Arthritis associated with deletion of 22q11.2: more common than previously suspected. Am J Med Genet. 1997;71(4):488. [PubMed] [Google Scholar]
- 11.Rasmussen SA, Williams CA, Ayoub EM, et al. Juvenile rheumatoid arthritis in velo-cardio-facial syndrome: coincidence or unusual complication? Am J Med Genet. 1996;64(4):546–550. doi: 10.1002/(SICI)1096-8628(19960906)64:4<546::AID-AJMG4>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 12.Grunewald J, Eklund A, Olerup O. Human leukocyte antigen class I alleles and the disease course in sarcoidosis patients. Am J Respir Crit Care Med. 2004;169(6):696–702. doi: 10.1164/rccm.200303-459OC. [DOI] [PubMed] [Google Scholar]
- 13.Smith G, Brownell I, Sanchez M, et al. Advances in the genetics of sarcoidosis. Clin Genet. 2008;73(5):401–412. doi: 10.1111/j.1399-0004.2008.00970.x. [DOI] [PubMed] [Google Scholar]
- 14.Gerke AK, Hunninghake G. The immunology of sarcoidosis. Clin Chest Med. 2008;29(3):379–390. vii. doi: 10.1016/j.ccm.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 15.McLean-Tooke A, Spickett GP, Gennery AR. Immunodeficiency and autoimmunity in 22q11.2 deletion syndrome. Scand J Immunol. 2007;66(1):1–7. doi: 10.1111/j.1365-3083.2007.01949.x. [DOI] [PubMed] [Google Scholar]
- 16.Finocchi A, Di Cesare S, Romiti ML, et al. Humoral immune responses and CD27+ B cells in children with DiGeorge syndrome (22q11.2 deletion syndrome) Pediatr Allergy Immunol. 2006;17(5):382–388. doi: 10.1111/j.1399-3038.2006.00409.x. [DOI] [PubMed] [Google Scholar]
- 17.McLean-Tooke A, Barge D, Spickett GP, et al. Immunologic defects in 22q11.2 deletion syndrome. J Allergy Clin Immunol. 2008;122(2):362–367. 367, e361–364. doi: 10.1016/j.jaci.2008.03.033. [DOI] [PubMed] [Google Scholar]
- 18.Lavi RF, Kamchaisatian W, Sleasman JW, et al. Thymic output markers indicate immune dysfunction in DiGeorge syndrome. J Allergy Clin Immunol. 2006;118(5):1184–1186. doi: 10.1016/j.jaci.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 19.Kurokohchi K, Masaki T, Arima K, et al. CD28-negative CD8-positive cytotoxic T lymphocytes mediate hepatocellular damage in hepatitis C virus infection. J Clin Immunol. 2003;23(6):518–527. doi: 10.1023/b:joci.0000010428.98823.02. [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht P, Moosig F, Csernok E, et al. CD28 negative T cells are enriched in granulomatous lesions of the respiratory tract in Wegener’s granulomatosis. Thorax. 2001;56(10):751–757. doi: 10.1136/thorax.56.10.751. [DOI] [PMC free article] [PubMed] [Google Scholar]