Abstract
Th17 cells are major effector T cells in the intestine but the regulation of their tissue tropism within the gut is poorly understood. We investigated here the roles of vitamin A and retinoic acid in generation of inflammatory Th17 cells with distinct tissue tropisms within the intestine. We found that Th17 cells with distinct tissue tropisms and pathogenic activities are generated depending on the available concentration of retinoic acid (RA). In contrast to the widespread perception that RA would suppress the generation of Th17 cells, we provide evidence here that RA is actually required for generation of Th17 cells with specific tissue tropisms within the gut. Th17 cells induced at suboptimal serum concentrations of RA migrated and induced moderate inflammation mainly in the large intestine, whereas the Th17 cells induced with optimal levels of exogenous RA (~10 nM) migrated to the small intestine and induced more severe inflammation. The Th17 cells, induced in the presence or absence of retinoic acid, differentially expressed the trafficking receptors CCR9 and α4β7. CCR9 is required for Th17 cell migration to the small intestine, whileα4β7 is required for the migration of Th17 cells throughout the whole intestine. Our results identified retinoic acid as a major signal that regulates the generation of gut Th17 cells with distinct capacities in migration and inflammatory activities. The results indicate also that specific gut tropism of Th17 cells is determined by the combination of trafficking receptors regulated by the RA signal.
Introduction
Th17 cells are highly enriched in the intestinal lamina propria (1). They are present in all segments of the intestine including the large and small intestine (2). Certain groups of intestinal commensal bacteria provide the major signal for such enrichment of Th17 cells (3, 4). While it is thought that Th17 cells are major effector T cells that provide immunity against potential pathogens (5, 6), the exact roles of Th17 cells in the intestine are incompletely understood. Th17 cells may induce or regulate the inflammation in the gut. Th17 cell cytokines are increased in various types of inflammatory bowel diseases (7–9), and these cytokines have been implicated in the pathogenesis of inflammatory bowel diseases (1, 2, 10, 11). In contrast, another Th17 cell cytokine IL22 has a protective role in colitis (12). It is not well understood how Th17 cells acquire specific tissue tropisms and segment-specific inflammatory activity within the intestine.
The intestine absorbs and metabolizes vitamin A into retinoic acid (RA), and RA is an important regulatory signal in the intestinal environment (13–17). In this regard, RA is particularly associated with imprinting the tissue tropism of T cells and B cells for their migration into the small intestine (18–20). It has been reported that RA can suppress the differentiation of naïve T cells into Th17 cells at least in vitro (19, 21–24). Together with its function in inducing FoxP3+ regulatory T cells, RA is considered a key regulatory signal in inducing immune tolerance in the intestine (25).
We investigated the role of RA in generation of Th17 cells with distinct tissue tropisms within the intestine. RA at physiologically relevant concentrations induced gut homing Th17 cells which are highly inflammatory upon transfer into mice. These cells were particularly active in migrating and inducing inflammation in the small intestine, while Th17 cells, generated in the presence of no or suboptimal concentrations of RA, induced inflammation mainly in the large intestine. We report also that the two gut trafficking receptors CCR9 and α4β7 play distinctive roles in migration and function of the retinoid-induced Th17 cells (hereafter called “RA Th17 cells”) in eliciting inflammation in the intestine. Thus, our results establish that distinct Th17 cell subsets with specific tissue tropism are generated depending on the available concentrations of RA. Our findings are important in understanding of the origin, trafficking and function of Th17 cells in the intestine.
Materials and Methods
Cell isolation and culture
Naïve CD4+ T cells were isolated from pooled mononuclear cells of peripheral lymph nodes (PLN), mesenteric lymph nodes (MLN) and spleen as described before (2). Naïve CD4+ T cells were cultured in complete RPMI 1640 medium (10% FBS) supplemented with concanavalin A (2.5 μg/ml), hTGF-β1 (5 ng/ml; PeproTech), mIL-6 (20 ng/ml; PeproTech), mIL-21 (10 ng/ml; PeproTech), mIL-23 (10 ng/ml; R&D Systems), mIL-1β (10 ng/ml; PeproTech), mTNF-α (20 ng/ml; PeproTech), anti-mIL-4 (11B11, 10 μg/ml; BioLegend), anti-mIFN-γ (XMG2.4, 10 μg/ml; BioLegend) and anti-mIL-2 (S4B6, 2.5 μg/ml; BD Bioscience) for 7 days to generate control Th17 cells that were induced without exogenous all-trans-RA (hereafter called “RA”). RA Th17 cells were prepared using the same condition with RA at physiological concentrations (10–20 nM). In some experiments, naïve T cells were CFSE-labeled and cultured in the Th17 cell induction condition to determine the relationship between cell division and expression of the gut trafficking receptors (Figure S1). The Th17 cells were also re-cultured for another 7 days to determine the stability of trafficking receptor expression (Figure S2).
Animals and generation of vitamin A-deficient or sufficient mice
All the experiments with animals in this study were approved by the Purdue animal care and use committee (PACUC). CCR9-deficient mice were described previously (26). Integrin (Itg)β7-deficient mice (C57BL/6-Itgb7tm1Cgn/J) and Rag1-deficient (B6.129s7-Rag1 tm1Mom/J) mice were purchased from the Jackson laboratory. AKR/J mice (Jackson laboratory) were kept on custom research diets based on AIN-93G containing 25,000 IU/kg or 0 IU/kg levels of retinyl acetate (Harlan Teklad TD-06528 and 07267) immediately following birth. The pups were weaned at 4 weeks of age and maintained on the same diets for additional 9 weeks. Vitamin A deficiency was verified by determining defective CCR9 expression by small intestinal T cells as described previously (2).
Flow cytometry to identify Th17 cells and to determine their expression of trafficking receptors
Intracellular cytokine staining for IL-17 (TC11-18H10.1, BioLegend ) expression was performed as described previously after activation with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μM) in the presence of monensin (10 μg/ml)(2). Before activation, the cells were stained for surface expression of CD4 (clone RM4-5; BioLegend), CD44 (clone IM7; BioLegend), CCR9 (clone 242504; R&D Systems), α4β7 (clone DATK32; BioLegend), and/orαEβ7 (Clone 2E7; BioLegend). Stained cells were analyzed using a BD Canto II (BD Bioscience).
Homing experiment
In vitro-generated Th17 cells (1 × 107 cells /mouse) were labeled with carboxyfluorescein diacetate succinimidyl ester (CFSE) or tetramethylrhodamine isothiocyanate (TRITC) and co-injected into wild type C57BL/6 mice via a tail vein. ~20 hrs later, the mice were sacrificed and single cell suspension was prepared from selected organs after collagenase digestion (2). The numbers of injected CFSE+ or TRITC+ Th17 cells migrated into each organ were determined with flow cytometry. The relative homing index to a organ/tissue was determined according to the formula: %Homing index (HI) for organ A= [(# of CFSE+ cells in organ A) / (# of TRITC+ cells in organ A) ÷ ( # of CFSE+ cells in input) / (# of TRITC+ cells in input) × 100. The % distribution of injected Th17 cells (Figure 1E) was calculated based on the numbers of migrated cells into the indicated organs.
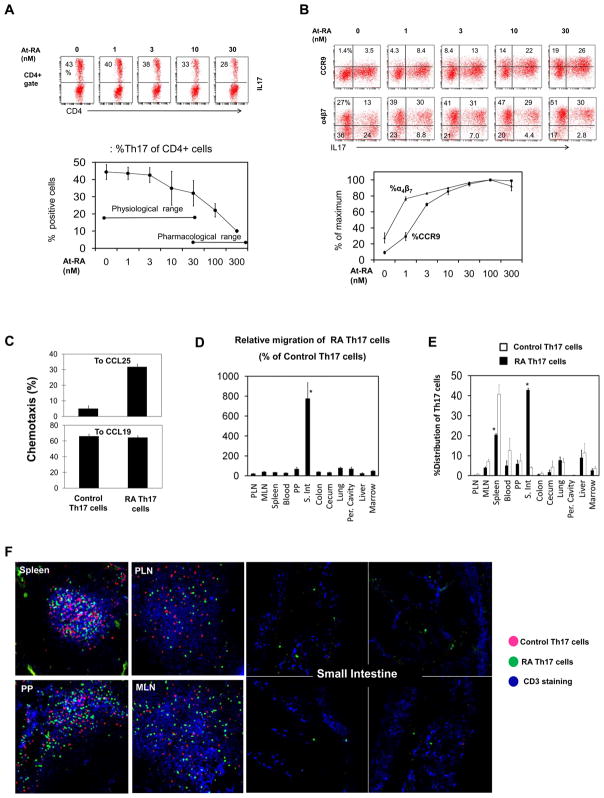
Figure 1. Gut homing CCR9+α4β7+ Th17 cells are induced in the presence of physiological concentrations of RA.
(A) Naïve T cells were cultured in a Th17 cell induction condition for 7 days at indicated concentrations of RA. (B) Expression of gut homing receptors (CCR9 and α4β7) by Th17 cells was examined. (C) The chemotactic ability of RA Th17 cells and control Th17 cells to CCL25 and CCL19. (D) Twenty hour short-term homing capacity of RA Th17 cells and control Th17 cells was compared in C57BL/6 mice. The Th17 cells were injected i.v. Homing index of RA Th17 cells is shown as % of control Th17 cells. *A significant increase in migration of RA Th17 cells over control Th17 cells. (E) Migration of RA Th17 cells is shown as % distribution among the indicated organs. *Significant differences from control Th17 cells. (F) In situ visualization of the RA Th17 cells and control Th17 cells migrated into indicated organs. All experiments were performed at least 3 times and combined (A-E) or representative data (F) are shown. Averages and SEM are shown in the combined data.
Chemotaxis
Chemotaxis was performed using Transwells (Corning) with 5 μm pores as described previously (27). Optimal concentrations of CCL25 (2.5 μg/ml) and CCL19 (1 μg/ml), determined by a preliminary titration experiment, were used.
In vivo intestinal inflammation study to assess the effector function of Th17 cells
B6.129s7-Rag1 tm1Mom/J mice were injected i.p. with 1 × 106 control or RA-induced Th17 cells. Weight change was monitored, and the mice were sacrificed on day 30–32 post injection when some mice became moribund. Intestinal inflammation in Rag1-deficient mice was scored as previously described with some modification (27). Briefly, we scored the degree of inflammation on a scale of 0–4 and the degree of mucosal hyperplasia and loss of villi also on a scale of 0–4. The two scores were combined to obtain the final inflammation index. The histological images were obtained with a brightfield Leica microscope equipped with a color camera at 200 × magnification.
Confocal analysis to determine localization of injected Th17 cells
Indicated tissues (spleen, peripheral lymph nodes, mesenteric lymph nodes, and Peyer’s patches (PP)) were harvested from euthanized mice that were previously injected with cells stained with TRITIC and CFSE, and frozen in Tissue-Tek (Sakura). 6 μm frozen tissue sections were fixed in acetone and stained with ant-CD3-APC. A Zeiss LSM 710 confocal microscope system was used to image the cells within the tissues.
Statistical analyses
Student’s paired and unpaired 2-tailed t tests were used to compare the significance of differences in means of two groups of related or unrelated data. P values ≤ 0.05 were considered significant.
Results
Naïve T cells differentiate into CCR9+α4β7+ Th17 cells at physiological concentrations of RA
RA (i.e. At-RA) is the major biologically active metabolite of vitamin A. In order to determine the role of RA in induction of gut homing Th17 cells, we activated naïve T cells in a Th17 cell-inducing condition in the presence of increasing concentrations of RA. The induction of Th17 cells was reduced but still occurred at significant levels in the presence of RA lower than 30 nM (Figure 1A). Severe reduction occurred only at high concentrations of RA (≥ 100 nM). An interesting feature of these retinoid-induced Th17 cells (RA Th17 cells) was the expression of CCR9 and α4β7 (Figure 1B). However, they poorly expressed CD103 (or αEβ7), another mucosal homing receptor (Figure S3). Compared to CCR9, α4β7 was more readily expressed by the Th17 cells at low (~1 nM) RA concentrations (Figure 1B). α4β7 was induced at a medium level even in the absence of exogenous RA perhaps in response to the basal level of RA present in the serum. The induction of CCR9 and α4β7 on Th17 cells by RA was very prompt, occurring after 1–2 cell divisions following T cell activation (Figure S1). Only RA Th17 cells were able to migrate to the CCR9 ligand CCL25 (Figure 1C). Expression of α4β7 induced by RA was relatively more stable than that of CCR9 on Th17 cells upon subsequent T cell activation in the absence of RA (Figure S2). In contrast, CCR9, once induced on Th17 cells by RA, quickly disappeared upon restimulation of Th17 cells in the absence of exogenous RA.
The RA-induced Th17 cells preferentially migrate to the small intestine
We performed a short term (20 h) homing assay to determine if RA Th17 cells (induced in the presence of exogenous RA), indeed, has the homing potential to the intestine. Upon injection through the intravenous route, the Th17 cells preferentially migrated to the small intestine (Figure 1D and E). RA Th17 cells, when compared to control Th17 cells (induced in the absence of exogenous RA), were somewhat less efficient in migration to non-small intestinal tissues (Figure 1D). Within the small intestine, the lamina propria is the preferential destination of RA Th17 cells (Figure 1F). In spleen, PP and lymph nodes, both the injected RA and control Th17 cells were found in the T cell area of the lymphoid tissues.
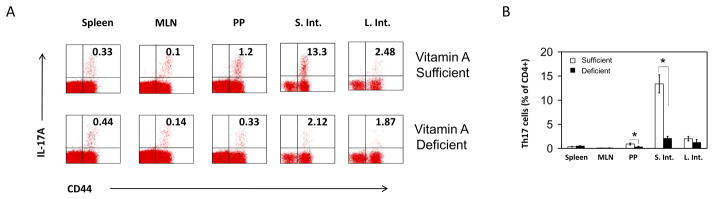
Th17 cells are decreased specifically in the small intestine of vitamin A-deficient mice
The results shown in Figure 1 that RA-induced gut homing Th17 cells is surprising as it has been reported that RA suppresses the induction of Th17 cells from naïve T cells in vitro. To determine if vitamin A is required for the presence of Th17 cells in vivo, we induced vitamin A deficiency in mice and examined the frequency of Th17 cells in different organs including the intestine. We found that the frequencies of Th17 cells were decreased in the small intestine and PP of vitamin A-deficient mice (Figure 2A and B). However, the numbers in spleen and mesenteric lymph nodes were unchanged. The data suggest that vitamin A has a tissue-specific role in population of Th17 cells and support the role of RA in generating small intestine-homing Th17 cells.
Figure 2. Th17 cells are decreased specifically in the small intestine of vitamin A-deficient animals.
The frequencies of Th17 cells in various organs of vitamin A-sufficient and deficient AKR/J mice were determined. Representative (A) and combined (B) data of 3 independent experiments are shown. *Significant differences between RA and control Th17 cells.
Differential roles of α4β7 and CCR9 in migration of gut-homing Th17 cells
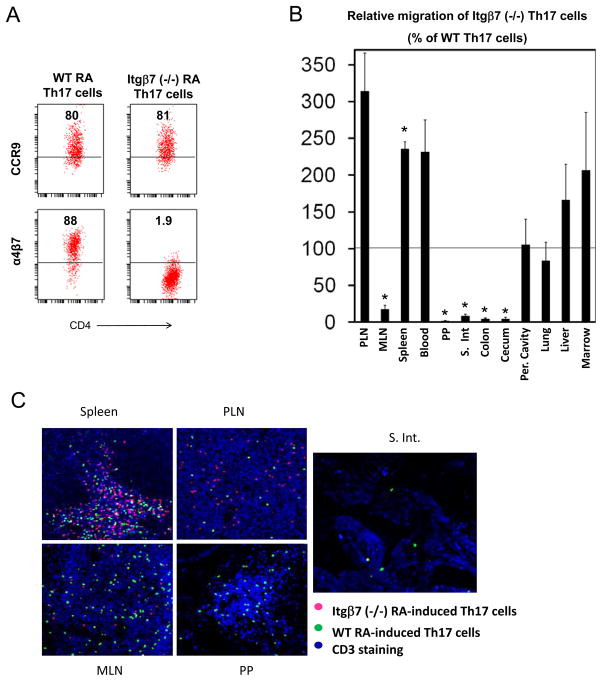
We, next, investigated the function of α4β7 and CCR9 in migration of Th17 cells. First, we compared Th17 cells derived from wild type and integrin β7 (Itgβ7)-deficient mice (Figure 3A). Itgβ7 is a subunit for both αEβ7 and α4β7. Since RA Th17 cells have low expression of ItgαE (Figure S3), most of the Itgβ7 subunit would be paired with the α4 subunit. We performed a 20 h short-term homing assay to compare wild type and Itgβ7-deficient RA Th17 cells in migration to various organs. The migration of Itgβ7-deficient Th17 cells to the intestine including MLN, PP, small intestine, colon and cecum was defective (Figure 3B). Instead, the migration of these Th17 cells to other non-gut tissue sites such as PLN (313% ± 52), spleen (235% ± 9.5), liver (166% ± 48), and marrow (206% ± 78) was generally enhanced. The Itgβ7-deficient Th17 cells failed to enter MLN, PP, and the lamina propria of the intestine (Figure 3C).
Figure 3. RA Th17 cells use Itgβ7 to migrate to the whole intestine including the lamina propria and gut-associated lymphoid tissues.
(A) Expression of CCR9 and α4β7 by wild type and Itgβ7 (−/−) RA Th17 cells. (B) The Th17 cells were injected i.v. and the migration of Th17 cells to the indicated organs were determined 20 h post cell-injection. (C) The localization of the injected Th17 cells in the indicated tissues was determined with a confocal microscope. The experiments were performed 3 times, and combined data (B) or representative data (C) are shown. *Significant changes.
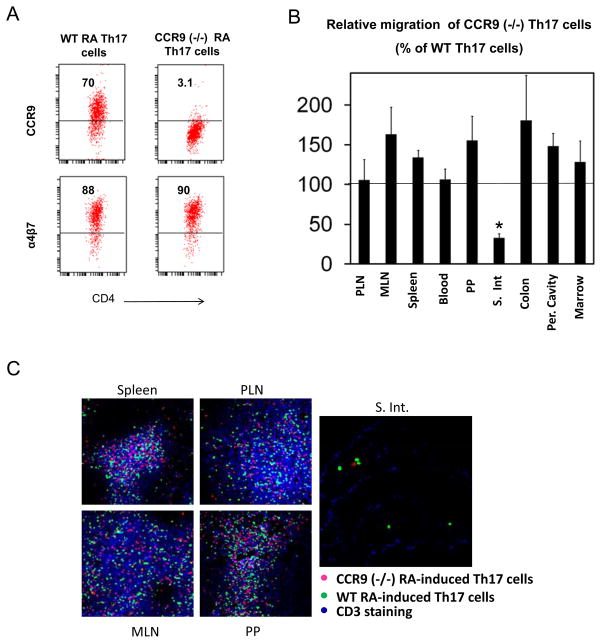
When CCR9-deficient RA Th17 cells were compared with wild type Th17 cells (Figure 4A), they were defective in migration only to the small intestinal lamina propria (Figure 4B and C). Although not statistically significant, their migration to other mucosal tissues such as MLN (163% ± 34), colon (180% ± 56), PP (155% ± 30) and peritoneal cavity (148% ± 16) was increased (Figure 4B). Overall, compared to α4β7, CCR9 had a more specific role in migration of Th17 cells to the small intestinal lamina propria.
Figure 4. RA Th17 cells use CCR9 to migrate to the small intestinal lamina propria.
(A) Expression of CCR9 and α4β7 by wild type and CCR9 (−/−) RA Th17 cells. (B) The Th17 cells were injected i.v. and the short-term (20 h) migration of Th17 cells was determined. (C) The localization of the Th17 cells was determined with confocal microscopy. Combined data (B) or representative data (C) of 3 independent experiments are shown. *Significant changes.
RA regulates the gut tissue tropism of Th17 cells in pathogenesis
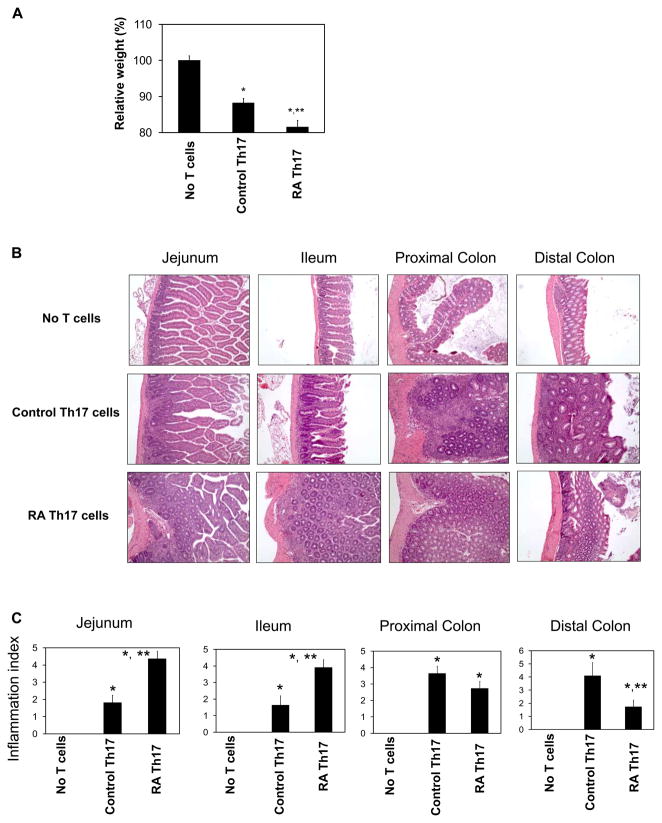
We hypothesized that RA Th17 cells and control Th17 cells would induce inflammation in different locations of the intestine because these two T cell populations have different tissue tropisms. To test this hypothesis, the RA Th17 cells and control Th17 cells were separately injected i.p. into Rag1-deficient mice. We found that RA Th17 cells induced greater weight loss compared to control Th17 cells that were induced in the absence of RA (Figure 5A). Mice injected with control Th17 cells had more severe inflammation in the distal colon than in the small intestine. In contrast, mice injected with RA Th17 cells had the most severe inflammation in the small intestine (Figure 5B and C). The inflammation in the small intestine was accompanied by mucosa/crypt hyperplasia and loss of villi (Figure 5B and C). The inflammation consisted primarily of infiltration by lymphocytes and macrophages with fewer neutrophils and occasional eosinophils. In support of the tissue tropism of RA Th17 cells, many more Th17 cells were found in the Rag1-deficient mice injected with RA Th17 cells compared to control Th17 cells even ~30 days after the injection of the Th17 cells (Figure S4). In contrast, there was no statistically significant difference in numbers of Th1 and Treg cells in the small intestine. Overall, the RA Th17 cells and control Th17 cells induce inflammation largely in distinct segments of the intestine.
Figure 5. The inflammatory activities of RA Th17 cells and control Th17 cells.
RA Th17 cells and control Th17 cells were separately injected i.p. into Rag1-deficient mice. (A) Relative weight change due to intestinal inflammation. (B) H&E staining of the intestinal tissues (200 × original magnification). (C) Inflammation scores. Data obtained from 10–11 mice are shown. Significant differences from the no T cell group (*) or control Th17 cell group (**).
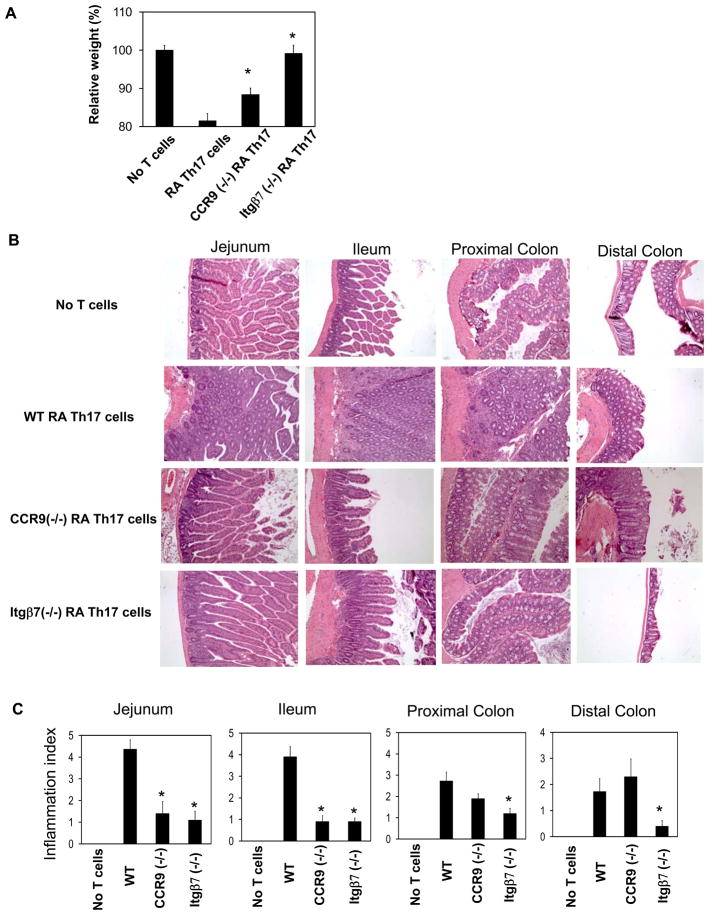
The higher inflammatory activity of RA Th17 cells could be due to their differences in expression of trafficking receptors and migration ability. Alternatively, this is due to other non-migratory features of the cells. To clarify this, we compared the inflammatory activities of wild type and CCR9 or Itgβ7-deficient RA Th17 cells (Figure 6A). We found that both CCR9 (−/−) RA Th17 cells and Itgβ7 (−/−) RA Th17 cells had decreased inflammatory activities compared to wild type RA Th17 cells. Particularly, the Itgβ7 deficiency completely blocked the inflammatory activity of RA Th17 cells in all segments of the intestine (Figure 6B). In contrast, CCR9 deficiency led to blockade of inflammation largely in the small intestine (Figure 6C).
Figure 6. Roles of CCR9 and Itgβ7 for the inflammatory activity of RA Th17 cells.
RA Th17 cells that are sufficient or deficient in expression of CCR9 or Itgβ7 were separately injected i.p. into Rag1-deficient mice to induce inflammation. (A) Weight change due to intestinal inflammation (n=10–11). (B) H&E staining of the intestinal tissues (200 × original magnification). (C) Histological scores (n=10–11). Significant differences from the wild type RA Th17 cell group (*).
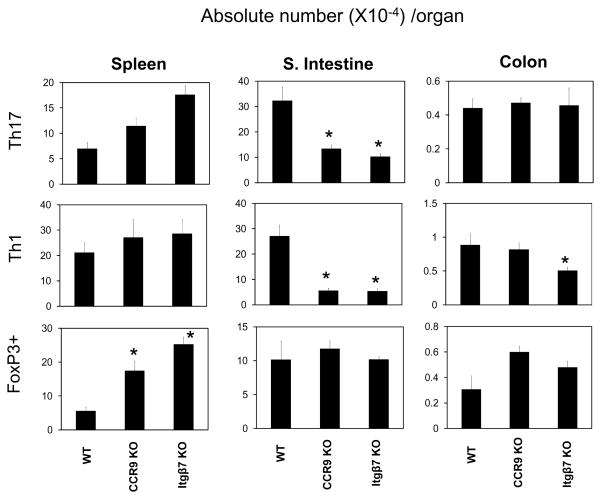
The decreased inflammation by CCR9 (−/−) RA Th17 cells and Itgβ7 (−/−) RA Th17 cells was consistent with decreased numbers of Th17 and Th1 cells in the small intestine (Figure 7). Moreover, the number of Th1 cells was decreased in the large intestine of the mice injected with Itgβ7 (−/−) RA Th17 cells. FoxP3+ T cells were increased in the spleen and large intestine of the mice injected with CCR9 (−/−) or Itgβ7 (−/−) Th17 cells. Taken together, these results show that the two trafficking receptors, CCR9 and α4β7 that are induced by RA, have essential functions in induction of the intestinal inflammation initiated by RA Th17 cells.
Figure 7. Roles of CCR9 and Itgβ7 in long term population of Th17 cells and effector T cell balance in the intestine.
RA Th17 cells that are sufficient or deficient in expression of CCR9 or Itgβ7 were injected i.p. into Rag1-deficient mice, and absolute numbers of Th1 and Th17 cells in the spleen, small intestine, and large intestine were determined ~30 days later. Significant differences from the wild type RA Th17 cell group (*). Absolute numbers of the T cells per organ detected by the method are shown. Combined data with averages and SEM are shown (n=10–12/group).
Discussion
We report the presence of Th17 cell sub-populations with distinct in vivo tissue tropism which is regulated by the RA signal. The tissue tropism of these Th17 cells is determined by differential expression of gut homing receptors. Strikingly, the Th17 cell sub-populations with different tissue tropism induced inflammation in different parts of the intestine. We believe that this is the first demonstration of the presence of Th17 cell subsets with inflammatory activities in distinct compartments of the intestine.
What concentration of RA is really available for T cells undergoing antigen activation in vivo? The RA concentration in normal human blood plasma is ~4.9 ng/ml (28). Comparably, ~2 ng/ml of RA was detected in rat serum. It is estimated that 50–75% of the RA in serum or plasma is At-RA. Therefore, the concentration of At-RA in normal blood plasma is estimated to be ~5 nM. The balance between RA-synthesizing retinaldehyde dehydrogenases (RALDHs) and RA-inactivating cytochrome P450RAI (CYP26) determines the availability of RA in a given tissue microenvironment. The expression patterns of CYP26 and RALDH2 are largely complementary in developing embryos (29–31), and it is expected that a similar pattern of complementary expression of the two enzymes would occur in adults. Based on the sensitivity of the expression of CCR9 and α4β7 in response to RA, it is estimated that the intestinal antigen priming environment would have at least 2–3 nM and potentially higher levels of active RA (up to 30 nM). The concentration of RA in peripheral lymph nodes, where CCR9 is not induced, is expected to be < 1 nM. We estimate that RA levels between 0.1 and 30 nM are within the physiological range. RA concentrations higher than this range could be found in vivo but the need for that high RA signal is unclear.
A most characteristic feature of the Th17 cells induced at physiological levels (10–20 nM) of RA is the expression of both CCR9 and α4β7. These two trafficking receptors are somewhat different from each other in requirement for RA for persistent expression. α4β7 initially induced by RA persists even after subsequent antigen priming in the absence of exogenous RA. In contrast, expression of CCR9 on Th17 cells is transient requiring continued presence of RA. Moreover, CCR9 is not induced well in response to RA on the Th17 cells that were previously activated in the presence of suboptimal concentrations of RA. We found that α4β7 is a general homing receptor of Th17 cells for the intestine and associated lymphoid tissues, while CCR9 is a receptor more specific for the small intestine. This suggests that at least several populations of Th17 cells (CCR9+ α4β7+, CCR9− α4β7+, and CCR9− α4β7−) with different homing behaviors can be generated in different sites of the intestine depending on the RA availability during repetitive priming processes.
The potent activity of retinoid-induced Th17 cells in inducing small intestinal inflammation is notable and is associated with their expression ofα4β7 and CCR9. α4β7 is widely expressed by intestinal lymphocytes and serves as the major homing receptor (32, 33).α4β7 is an adhesion molecule for MAdCAM-1 (32, 34) and can guide the Th17 cells to the entire gut system. It appears that even basal levels of α4β7, induced in the presence of low (serum) levels of RA, is required for migration of Th17 cells into the intestine and associated lymphoid tissues. Thus, higher expression of α4β7, induced at optimal levels of RA over the physiological range, confers Th17 cells with enhanced ability to stay within the intestine and to induce inflammation. CCR9 is expressed more specifically by the T cells in the small intestine and acts as a homing receptor specific for this tissue (35–38). CCR9 can guide the Th17 cells to the small intestinal lamina propria compartment close to epithelial cells where CCL25 is expressed (35, 39, 40), and therefore would cause the more focused migration and inflammation in the small intestine. Consistently, in our study, Itgβ7 deficiency had a broader impact on Th17 cell migration to the intestine, while CCR9 deficiency had a specific effect on their homing to the small intestine. In line with the differential roles of these trafficking receptors in determining the tissue tropism of Th17 cells, Itgβ7 deficiency had greater impact than CCR9 deficiency on the inflammatory activity of the retinoid-induced Th17 cells. This is supported by the fact that without proper expression of Itgβ7 (or α4β7), Th17 cells fail to migrate to any site of the entire intestinal system including the small intestine despite their normal expression of CCR9. This finding is in line with the established roles of these trafficking receptors for other T cells (32, 33, 35, 39, 40), and suggests that Th17 cells do not deviate from the general trafficking behavior of gut T cells. Some reported that IL-22 has a protective role in intestinal inflammation (12, 41) while others reported pro-inflammatory roles of IL-22 in intestinal inflammation (42, 43). We would like to point out that there was no significant difference in expression of IL-22 by control and RA Th17 cells (Figure S5).
Because of the previously reported in vitro phenomenon that induction of Th17 cells is suppressed by RA (19, 22, 23), it has been assumed that vitamin A antagonized the population of Th17 cells in the intestine. Our finding with vitamin A-deficient mice indicates that the RA signal is actually important for presence of Th17 cells in the small intestine. A similar decrease of small intestinal Th17 cells in vitamin A deficiency was observed in a mouse model of chronic intestinal inflammation (44), suggesting that it occurs broadly in both normal and inflammatory conditions. We don’t rule out the possibility that induction of Th17 cells could be somewhat suppressed by the RA in the small intestine in vivo. However, this small decrease due to the presence of RA would be greatly compensated by the increase of gut homing receptor-expressing Th17 cells. Thus, there would be an overall increase in numbers of gut Th17 cells in the intestine in response to RA.
Our results provide useful targets of intervention in regulation of the inflammatory activities of Th17 cells in the intestine. It is possible to regulate the inflammatory activity of Th17 cells in the small versus other compartments of the intestine by selective blocking of CCR9 or Itgβ7. This projection is well supported by the U.S. Food and Drug Administration (FDA)-approved and other proposed therapies targetingα4 or α4β7 integrin. For examples, blocking of the Itgα4 using monoclonal antibodies to integrin α4 is a FDA-approved treatment for Crohn’s disease (45). It was shown effective in amelioration of colitis in animal models too (45–47). While this therapy targets all α4 integrins including α4β7 and α4β1, monoclonal antibodies more specifically blocking Itgβ7 or MAdCAM-1 were also effective in inhibiting colitis in animal models (48, 49). We propose that these reagents can act on the α4 integrins of Th17 cells and, thus, can block their migration and inflammatory activities in the intestine. If the inflammatory activity of Th17 cells is localized to the small intestine, blocking of CCR9 would be more specific in treating the localized inflammation without the potential side effects of broad immune-suppression in the body.
Supplementary Material
Acknowledgments
The authors thank JS Chang, JH Lee and Jeongho Park (Purdue University) for their helpful inputs.
Abbreviations
- RA
all-trans retinoic acid
- Th17 cells
T helper cells expressing IL-17
- Itg
integrin
- PP
Peyer’s patches
Footnotes
Grant support: This study was supported, in part, from grants from NIH (1R01AI074745, 1R56AI080769, and 1R01DK076616) and Crohn’s and Colitis Foundation of America to CHK.
References
- 1.Ivanov, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 2.Wang C, Kang SG, Lee J, Sun Z, Kim CH. The roles of CCR6 in migration of Th17 cells and regulation of effector T-cell balance in the gut. Mucosal immunology. 2009;2:173–183. doi: 10.1038/mi.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaph C, Du Y, Saenz SA, Nair MG, Perrigoue JG, Taylor BC, Troy AE, Kobuley DE, Kastelein RA, Cua DJ, Yu Y, Artis D. Commensal-dependent expression of IL-25 regulates the IL-23-IL-17 axis in the intestine. The Journal of experimental medicine. 2008;205:2191–2198. doi: 10.1084/jem.20080720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ivanov, Frutos Rde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4:337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hung LY, Velichko S, Huang F, Thai P, Wu R. Regulation of airway innate and adaptive immune responses: the IL-17 paradigm. Crit Rev Immunol. 2008;28:269–279. doi: 10.1615/critrevimmunol.v28.i4.10. [DOI] [PubMed] [Google Scholar]
- 6.Iwakura Y, Nakae S, Saijo S, Ishigame H. The roles of IL-17A in inflammatory immune responses and host defense against pathogens. Immunol Rev. 2008;226:57–79. doi: 10.1111/j.1600-065X.2008.00699.x. [DOI] [PubMed] [Google Scholar]
- 7.Seiderer J, Elben I, Diegelmann J, Glas J, Stallhofer J, Tillack C, Pfennig S, Jurgens M, Schmechel S, Konrad A, Goke B, Ochsenkuhn T, Muller-Myhsok B, Lohse P, Brand S. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflammatory bowel diseases. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 8.Fujino S, Andoh A, Bamba S, Ogawa A, Hata K, Araki Y, Bamba T, Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dambacher J, Beigel F, Zitzmann K, De Toni EN, Goke B, Diepolder HM, Auernhammer CJ, Brand S. The role of the novel Th17 cytokine IL-26 in intestinal inflammation. Gut. 2009;58:1207–1217. doi: 10.1136/gut.2007.130112. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z, Zheng M, Bindas J, Schwarzenberger P, Kolls JK. Critical role of IL-17 receptor signaling in acute TNBS-induced colitis. Inflammatory bowel diseases. 2006;12:382–388. doi: 10.1097/01.MIB.0000218764.06959.91. [DOI] [PubMed] [Google Scholar]
- 11.Leppkes M, Becker C, Ivanov, Hirth S, Wirtz S, Neufert C, Pouly S, Murphy AJ, Valenzuela DM, Yancopoulos GD, Becher B, Littman DR, Neurath MF. RORgamma-expressing Th17 cells induce murine chronic intestinal inflammation via redundant effects of IL-17A and IL-17F. Gastroenterology. 2009;136:257–267. doi: 10.1053/j.gastro.2008.10.018. [DOI] [PubMed] [Google Scholar]
- 12.Zenewicz LA, Yancopoulos GD, Valenzuela DM, Murphy AJ, Stevens S, Flavell RA. Innate and adaptive interleukin-22 protects mice from inflammatory bowel disease. Immunity. 2008;29:947–957. doi: 10.1016/j.immuni.2008.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agace WW. T-cell recruitment to the intestinal mucosa. Trends in immunology. 2008;29:514–522. doi: 10.1016/j.it.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 14.Iwata M. Retinoic acid production by intestinal dendritic cells and its role in T-cell trafficking. Seminars in immunology. 2009;21:8–13. doi: 10.1016/j.smim.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Kim CH. Roles of retinoic acid in induction of immunity and immune tolerance. Endocr Metab Immune Disord Drug Targets. 2008;8:289–294. doi: 10.2174/187153008786848312. [DOI] [PubMed] [Google Scholar]
- 16.Mora JR, von Andrian UH. Role of retinoic acid in the imprinting of gut-homing IgA-secreting cells. Seminars in immunology. 2009;21:28–35. doi: 10.1016/j.smim.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sigmundsdottir H, Butcher EC. Environmental cues, dendritic cells and the programming of tissue-selective lymphocyte trafficking. Nat Immunol. 2008;9:981–987. doi: 10.1038/ni.f.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 19.Kang SG, Lim HW, Andrisani OM, Broxmeyer HE, Kim CH. Vitamin A Metabolites Induce Gut-Homing FoxP3+ Regulatory T Cells. J Immunol. 2007;179:3724–3733. doi: 10.4049/jimmunol.179.6.3724. [DOI] [PubMed] [Google Scholar]
- 20.Mora JR, Iwata M, Eksteen B, Song SY, Junt T, Senman B, Otipoby KL, Yokota A, Takeuchi H, Ricciardi-Castagnoli P, Rajewsky K, Adams DH, von Andrian UH. Generation of gut-homing IgA-secreting B cells by intestinal dendritic cells. Science (New York, NY) 2006;314:1157–1160. doi: 10.1126/science.1132742. [DOI] [PubMed] [Google Scholar]
- 21.Xiao S, Jin H, Korn T, Liu SM, Oukka M, Lim B, Kuchroo VK. Retinoic acid increases Foxp3+ regulatory T cells and inhibits development of Th17 cells by enhancing TGF-beta-driven Smad3 signaling and inhibiting IL-6 and IL-23 receptor expression. J Immunol. 2008;181:2277–2284. doi: 10.4049/jimmunol.181.4.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schambach F, Schupp M, Lazar MA, Reiner SL. Activation of retinoic acid receptor-alpha favours regulatory T cell induction at the expense of IL-17-secreting T helper cell differentiation. European journal of immunology. 2007;37:2396–2399. doi: 10.1002/eji.200737621. [DOI] [PubMed] [Google Scholar]
- 23.Mucida D, Park Y, Kim G, Turovskaya O, Scott I, Kronenberg M, Cheroutre H. Reciprocal TH17 and regulatory T cell differentiation mediated by retinoic acid. Science (New York, NY) 2007;317:256–260. doi: 10.1126/science.1145697. [DOI] [PubMed] [Google Scholar]
- 24.Elias KM, Laurence A, Davidson TS, Stephens G, Kanno Y, Shevach EM, O’Shea JJ. Retinoic acid inhibits Th17 polarization and enhances FoxP3 expression through a Stat-3/Stat-5 independent signaling pathway. Blood. 2008;111:1013–1020. doi: 10.1182/blood-2007-06-096438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim CH. Regulation of FoxP3 regulatory T cells and Th17 cells by retinoids. Clin Dev Immunol. 2008;2008:416910. doi: 10.1155/2008/416910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uehara S, Grinberg A, Farber JM, Love PE. A role for CCR9 in T lymphocyte development and migration. J Immunol. 2002;168:2811–2819. doi: 10.4049/jimmunol.168.6.2811. [DOI] [PubMed] [Google Scholar]
- 27.Kang SG, Piniecki RJ, Hogenesch H, Lim HW, Wiebke E, Braun SE, Matsumoto S, Kim CH. Identification of a chemokine network that recruits FoxP3(+) regulatory T cells into chronically inflamed intestine. Gastroenterology. 2007;132:966–981. doi: 10.1053/j.gastro.2007.01.008. [DOI] [PubMed] [Google Scholar]
- 28.Napoli JL. Quantification of physiological levels of retinoic acid. Methods in enzymology. 1986;123:112–124. doi: 10.1016/s0076-6879(86)23015-3. [DOI] [PubMed] [Google Scholar]
- 29.Niederreither K, McCaffery P, Drager UC, Chambon P, Dolle P. Restricted expression and retinoic acid-induced downregulation of the retinaldehyde dehydrogenase type 2 (RALDH-2) gene during mouse development. Mechanisms of development. 1997;62:67–78. doi: 10.1016/s0925-4773(96)00653-3. [DOI] [PubMed] [Google Scholar]
- 30.McCaffery P, Wagner E, O’Neil J, Petkovich M, Drager UC. Dorsal and ventral retinal territories defined by retinoic acid synthesis, break-down and nuclear receptor expression. Mechanisms of development. 1999;82:119–130. doi: 10.1016/s0925-4773(99)00022-2. [DOI] [PubMed] [Google Scholar]
- 31.Chen Y, Huang L, Russo AF, Solursh M. Retinoic acid is enriched in Hensen’s node and is developmentally regulated in the early chicken embryo. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:10056–10059. doi: 10.1073/pnas.89.21.10056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Berlin C, Berg EL, Briskin MJ, Andrew DP, Kilshaw PJ, Holzmann B, Weissman IL, Hamann A, Butcher EC. Alpha 4 beta 7 integrin mediates lymphocyte binding to the mucosal vascular addressin MAdCAM-1. Cell. 1993;74:185–185. doi: 10.1016/0092-8674(93)90305-a. [DOI] [PubMed] [Google Scholar]
- 33.Shaw SK, Brenner MB. The beta 7 integrins in mucosal homing and retention. Seminars in immunology. 1995;7:335–342. doi: 10.1016/1044-5323(95)90014-4. [DOI] [PubMed] [Google Scholar]
- 34.Hamann A, Andrew DP, Jablonski-Westrich D, Holzmann B, Butcher EC. Role of alpha 4-integrins in lymphocyte homing to mucosal tissues in vivo. J Immunol. 1994;152:3282–3293. [PubMed] [Google Scholar]
- 35.Kunkel EJ, Campbell JJ, Haraldsen G, Pan J, Boisvert J, Roberts AI, Ebert EC, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Greenberg HB, Parker CM, Butcher EC, Andrew DP, Agace WW. Lymphocyte CC chemokine receptor 9 and epithelial thymus-expressed chemokine (TECK) expression distinguish the small intestinal immune compartment: Epithelial expression of tissue-specific chemokines as an organizing principle in regional immunity. The Journal of experimental medicine. 2000;192:761–768. doi: 10.1084/jem.192.5.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pabst O, Ohl L, Wendland M, Wurbel MA, Kremmer E, Malissen B, Forster R. Chemokine receptor CCR9 contributes to the localization of plasma cells to the small intestine. The Journal of experimental medicine. 2004;199:411–416. doi: 10.1084/jem.20030996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Svensson M, Marsal J, Ericsson A, Carramolino L, Broden T, Marquez G, Agace WW. CCL25 mediates the localization of recently activated CD8alphabeta(+) lymphocytes to the small-intestinal mucosa. The Journal of clinical investigation. 2002;110:1113–1121. doi: 10.1172/JCI15988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wurbel MA, Malissen M, Guy-Grand D, Meffre E, Nussenzweig MC, Richelme M, Carrier A, Malissen B. Mice lacking the CCR9 CC-chemokine receptor show a mild impairment of early T- and B-cell development and a reduction in T-cell receptor gammadelta(+) gut intraepithelial lymphocytes. Blood. 2001;98:2626–2632. doi: 10.1182/blood.v98.9.2626. [DOI] [PubMed] [Google Scholar]
- 39.Papadakis KA, Prehn J, Nelson V, Cheng L, Binder SW, Ponath PD, Andrew DP, Targan SR. The role of thymus-expressed chemokine and its receptor CCR9 on lymphocytes in the regional specialization of the mucosal immune system. J Immunol. 2000;165:5069–5076. doi: 10.4049/jimmunol.165.9.5069. [DOI] [PubMed] [Google Scholar]
- 40.Wurbel MA, Philippe JM, Nguyen C, Victorero G, Freeman T, Wooding P, Miazek A, Mattei MG, Malissen M, Jordan BR, Malissen B, Carrier A, Naquet P. The chemokine TECK is expressed by thymic and intestinal epithelial cells and attracts double- and single-positive thymocytes expressing the TECK receptor CCR9. European journal of immunology. 2000;30:262–271. doi: 10.1002/1521-4141(200001)30:1<262::AID-IMMU262>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 41.Sugimoto K, Ogawa A, Mizoguchi E, Shimomura Y, Andoh A, Bhan AK, Blumberg RS, Xavier RJ, Mizoguchi A. IL-22 ameliorates intestinal inflammation in a mouse model of ulcerative colitis. The Journal of clinical investigation. 2008;118:534–544. doi: 10.1172/JCI33194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brand S, Beigel F, Olszak T, Zitzmann K, Eichhorst ST, Otte JM, Diepolder H, Marquardt A, Jagla W, Popp A, Leclair S, Herrmann K, Seiderer J, Ochsenkuhn T, Goke B, Auernhammer CJ, Dambacher J. IL-22 is increased in active Crohn’s disease and promotes proinflammatory gene expression and intestinal epithelial cell migration. American journal of physiology. 2006;290:G827–838. doi: 10.1152/ajpgi.00513.2005. [DOI] [PubMed] [Google Scholar]
- 43.Andoh A, Zhang Z, Inatomi O, Fujino S, Deguchi Y, Araki Y, Tsujikawa T, Kitoh K, Kim-Mitsuyama S, Takayanagi A, Shimizu N, Fujiyama Y. Interleukin-22, a member of the IL-10 subfamily, induces inflammatory responses in colonic subepithelial myofibroblasts. Gastroenterology. 2005;129:969–984. doi: 10.1053/j.gastro.2005.06.071. [DOI] [PubMed] [Google Scholar]
- 44.Kang SG, Wang C, Matsumoto S, Kim CH. High and low vitamin A therapies induce distinct FoxP3+ T-cell subsets and effectively control intestinal inflammation. Gastroenterology. 2009;137:1391–1402. e1391–1396. doi: 10.1053/j.gastro.2009.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandborn WJ, Colombel JF, Enns R, Feagan BG, Hanauer SB, Lawrance IC, Panaccione R, Sanders M, Schreiber S, Targan S, van Deventer S, Goldblum R, Despain D, Hogge GS, Rutgeerts P. Natalizumab induction and maintenance therapy for Crohn’s disease. N Engl J Med. 2005;353:1912–1925. doi: 10.1056/NEJMoa043335. [DOI] [PubMed] [Google Scholar]
- 46.Burns RC, Rivera-Nieves J, Moskaluk CA, Matsumoto S, Cominelli F, Ley K. Antibody blockade of ICAM-1 and VCAM-1 ameliorates inflammation in the SAMP-1/Yit adoptive transfer model of Crohn’s disease in mice. Gastroenterology. 2001;121:1428–1436. doi: 10.1053/gast.2001.29568. [DOI] [PubMed] [Google Scholar]
- 47.Podolsky DK, Lobb R, King N, Benjamin CD, Pepinsky B, Sehgal P, deBeaumont M. Attenuation of colitis in the cotton-top tamarin by anti-alpha 4 integrin monoclonal antibody. The Journal of clinical investigation. 1993;92:372–380. doi: 10.1172/JCI116575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Picarella D, Hurlbut P, Rottman J, Shi X, Butcher E, Ringler DJ. Monoclonal antibodies specific for beta 7 integrin and mucosal addressin cell adhesion molecule-1 (MAdCAM-1) reduce inflammation in the colon of scid mice reconstituted with CD45RBhigh CD4+ T cells. J Immunol. 1997;158:2099–2106. [PubMed] [Google Scholar]
- 49.Apostolaki M, Manoloukos M, Roulis M, Wurbel MA, Muller W, Papadakis KA, Kontoyiannis DL, Malissen B, Kollias G. Role of beta7 integrin and the chemokine/chemokine receptor pair CCL25/CCR9 in modeled TNF-dependent Crohn’s disease. Gastroenterology. 2008;134:2025–2035. doi: 10.1053/j.gastro.2008.02.085. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.