Abstract
Background
Nitric oxide (NO) is a free radical that is involved in carcinogenesis. Endothelial NO, synthesized from L-arginine by endothelial NO synthase (eNOS), inhibits apoptosis and promotes angiogenesis, tumor cell proliferation and metastasis. The aim of this study was to evaluate the influence of polymorphisms in the eNOS gene on prognosis of patients with advanced stage non-small-cell lung cancer (NSCLC).
Methods
Unresectable, chemotherapy naïve stage III or IV NSCLC patients who were treated with standard platinum-containing doublet regimens were analyzed. All individuals were genotyped for the single-nucleotide polymorphism G894T in exon 7 of the eNOS gene and for a variable number of tandem repeats (VNTR) polymorphism in intron 4 that results in a rare smaller allele (a) and a common larger allele (b), to investigate the association between these polymorphisms and clinical outcomes. The primary endpoint was correlation with overall survival.
Results
From October 2004 to December 2007, 108 patients (male/female, 66/42; Stage IIIA/IIIB/IV, 6/30/72) aged 29-77 years (median 63) with good performance status were consecutively enrolled in this study. Using Kaplan-Meier estimates, we showed that 5-year overall survival was significantly increased in patients carrying the VNTR a-allele compared with VNTR b/b patients (P = 0.015). In multivariate Cox proportional hazard analysis, the VNTR polymorphism was an independent prognostic factor for survival.
Conclusions
The results support the role of the VNTR polymorphism in intron 4 as a marker for survival in patients with advanced stage NSCLC who are candidates for standard chemotherapy.
Background
Lung cancer is the leading cause of cancer-related death in most developed countries [1]. Non-small-cell lung cancer (NSCLC) accounts for 80% of all lung cancers, and platinum-based chemotherapy is considered the standard of care worldwide for patients with advanced stage disease [2]. The treatment response is related to various factors, most of which are defined by tumor and clinical characteristics, such as disease stage and performance status. However, recent studies have shown that genetic factors may also influence the effectiveness of treatment [3,4].
Nitric oxide (NO) is a small free radical and is involved in numerous physiologic and pathophysiologic processes, including vasodilation, neurotransmission, immunity and carcinogenesis. NO is synthesized from L-arginine and oxygen by four major isoforms of NO synthase (NOS): neuronal NOS, endothelial NOS (eNOS), inducible NOS, and mitochondrial NOS. Although eNOS produces low levels of NO, many clinical observational studies have shown a dysregulation of eNOS expression in human solid tumors [5].
The number of reported polymorphisms in the gene encoding eNOS (NOS3) exceeds 160. The G894T polymorphism (rs1799983), which is located in exon 7 of the gene and leads to the amino acid change from Glu298Asp, is associated with reduced basal NO production [6]. In addition, a 27-bp variable number of tandem repeat (VNTR) polymorphism in intron 4 has been associated with variations in plasma levels of NO and its metabolites [7,8]. This polymorphism has three alleles: a short 4 repeat allele (a-allele), a more common long 5 repeat allele (b-allele), and a rare allele with 6 repeats. Recent studies have indicated that this repeat polymorphism in intron 4 is the source of a 27 nt-long RNA derived from pre-mRNA splicing. This RNA species inhibits eNOS expression and may represent a new class of small RNA [9,10]. Endothelial cells containing the a-allele produce a lower level of 27-nt small RNA and a higher level of eNOS mRNA compared with cells with the more common b-allele [10]. The association between eNOS mRNA levels and the a-allele appeared to be dose-dependent [11].
To date, very few studies have investigated the association of these polymorphisms with the prognosis of various neoplastic disorders, and no studies have examined them in relation to survival of advanced stage NSCLC patients who are candidates for standard platinum-based chemotherapy. In this study, we evaluated the potential association between these polymorphisms and survival of NSCLC patients.
Methods
One hundred and eight patients who were judged to have inoperable stage III or IV NSCLC at the time of diagnosis were consecutively enrolled in our study between October 2004 and December 2007. All patients were native-Japanese of Asian ethnicity. Staging was based upon the 6th tumor node metastasis (TNM) staging system [12]. Patients with symptomatic brain metastasis, malignancies other than NSCLC within the last 5 years, presence of acute or chronic infections rendering them unsuitable for chemotherapy, or an Eastern Cooperative Oncology Group (ECOG) performance status (PS) of between 2 and 4 were excluded from the study. Patients with operable disease were also excluded, because surgery was considered to be a substantial confounding factor that masks the genetic effect on survival. Tumor response was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) [13], and all responses were evaluated at least 4 weeks after initial assessment. The local ethical committee (Kyoto University Graduate School and Faculty of Medicine, Ethics Committee) approved the study, and written informed consent was obtained from each patient before enrollment. The study was conducted in accordance with the Helsinki declaration.
The patients received one of the following three chemotherapy treatments: carboplatin/paclitaxel, carboplatin/gemcitabine or cisplatin/gemcitabine. With regard to the carboplatin/paclitaxel regimen, paclitaxel was administered either at a lower dose (70 mg/m2) on a weekly basis for 3 out of 4 weeks, or at 180 mg/m2 on day 1 (carboplatin dose was area under the curve (AUC) = 5 or 6 mg/mL • min on day 1). The combination of carboplatin and gemcitabine consisted of carboplatin AUC 5 mg/mL • min on day 1 plus gemcitabine 1000 mg/m2 on days 1 and 8 every 3 weeks. Carboplatin was replaced with cisplatin (80 mg/m2 on day 1) in the cisplatin/gemcitabine regimen. Each treatment was repeated for three to six cycles, unless unacceptable toxicity or disease progression was apparent. Patients who are candidate of definite thoracic irradiation therapy received carboplatin AUC 5 mg/mL • min on day 1 plus paclitaxel 40 mg/m2 on days 1, 8 and 15 every 4 weeks in a concurrent fashion. Two to four cycles of carboplatin/paclitaxel were administered subsequently for consolidation.
Blood samples were collected and DNA was isolated from whole blood cell fractions using the QIAamp DNA Blood Mini Kit (Qiagen, Tokyo, Japan). The single nucleotide polymorphism (SNP) of G894T (a G-to-T transversion at nucleotide 894 in exon 7) was analyzed with TaqMan genotyping assays, using the ABI 7500 real-time PCR system (Applied Biosystems, Inc., Tokyo, Japan). Probes, primers and TaqMan universal PCR master mix were those from ABI and TaqMan assays were performed according to the manufacturer's instructions. The intronic 27 bp insertion/deletion VNTR was genotyped using PCR amplification and agarose gel electrophoresis with ethidium bromide staining [14].
The distribution of genotypes was tested for Hardy-Weinburg equilibrium with the goodness-of-fit χ2 test. The linkage disequilibrium of the two polymorphisms was analyzed. The association between overall survival times was estimated using the Kaplan-Meier method and assessed using the log-rank test. The effects of different genotypes on overall survival were estimated using hazard ratios (HRs), and 95% confidence intervals (95%CIs) were estimated using the multivariate Cox proportional hazards regression model, with adjustment for gender, age (<70 vs. older), ECOG PS (0 vs. 1), smoking status (never-smoker vs. former/current smoker), clinical stage (III vs. IV), histology (adenocarcinoma vs. others), thoracic radiotherapy (yes vs. no) and the two genotypes examined. We used Akaike's information criterion for entering and removing a variable in a stepwise Cox regression [15]. Survival time was calculated from diagnosis to death or last follow-up. A P value of <0.05 was considered statistically significant.
We estimated the false-positive report probability for the observed statistically significant associations using the methods described by Wacholder et al [16]. HR values from 2.0 to 4.0 were considered likely threshold values. The prior probability used was 0.1 for these polymorphisms and the false-positive report probability value for noteworthiness was set at 0.5.
Statistical analyses were performed using JMP version 6 (SAS Institute, Cary, NC, USA.), R software (R Foundation for Statistical Computing., Vienna, Austria.), and Haploview version 4.0 [17].
Results
Patients' characteristics are summarized in Table 1. Eighty percent of patients had a cytological or histological diagnosis of adenocarcinoma. Treatment characteristics are shown in Table 2. By July 31, 2009, 73 (68%) of the 108 patients had died. The median follow-up period was 21.5 months.
Table 1.
Characteristics of the 108 patients with non-small-cell lung cancer enrolled in the study
| Patients | ||
|---|---|---|
| N | % | |
| Age, Years | ||
| Median | 63 | |
| Range | 29 - 77 | |
| Gender | ||
| Male | 66 | 61.1 |
| Female | 42 | 38.9 |
| Stage | ||
| IIIA | 6 | 5.5 |
| IIIB | 30 | 27.8 |
| IV | 72 | 66.7 |
| PS | ||
| 0 | 23 | 21.3 |
| 1 | 85 | 78.7 |
| Chemotherapy regimen | ||
| CBDCA+PAC | 89 | 82.4 |
| CBDCA+GEM | 17 | 15.7 |
| CDDP+GEM | 2 | 1.9 |
| Definitive thoracic radiotherapy | ||
| Yes | 20 | 18.5 |
| No | 88 | 81.5 |
Abbreviations: CBDCA, carboplatin; PAC, paclitaxel; CDDP, cisplatin; GEM, gemcitabine
Table 2.
Treatment characteristics of the enrolled patients
| No. of patients | |||
|---|---|---|---|
| Stage IIIA | Stage IIIB | Stage IV | |
| CBDCA + PAC + TRT | 6 | 14 | 0 |
| CBDCA + PAC | 0 | 13 | 56 |
| CBDCA + GEM | 0 | 3 | 14 |
| CDDP + GEM | 0 | 0 | 2 |
Abbreviations: TRT, thoracic radiotherapy
Median survival for all 108 patients was 26.8 months (95%CI, range 21.9 - 30.7 months). Median survival was 23.3 months for male patients and 32.3 months for female patients (HR, 1.72; 95%CI, 1.04-2.85; P = 0.034). Median survival was 24.6 months for smokers and 35.2 months for never-smokers (HR, 1.69; 95%CI, 1.00-2.85; P = 0.048). There was substantial agreement between gender and smoking status (Cohen's kappa coefficient, 0.64). Age, ECOG PS, disease stage, histology, and thoracic radiotherapy were not significantly associated with overall survival in the univariate analysis. Regarding epidermal growth factor receptor (EGFR) gene mutations, tumor samples of 34 patients (31.5%) were analyzed and 21 were positive for mutations. Eleven tumors had an L858R mutation in exon 21 and nine contained in-frame nucleotide deletions (including E746-A750) in exon 19. A G719S mutation was found in one tumor. EGFR mutation status (positive vs. negative/unknown) was not prognostic in the univariate analysis.
The distribution of all genotypes and allelic frequencies is shown in Table 3. These frequencies were in agreement with previous reports, and with those expected according to the Hardy-Weinburg equilibrium model. Linkage disequilibrium between these polymorphisms was not observed. Since there was no TT homozygote carrier with respect to G894T, and analyses were performed between the GG homozygote group and the GT heterozygote group. In addition, because the frequency of intron 4 short repeat (a-allele) homozygotes was low (0.9%; n = 1), b/a heterozygotes and a/a homozygotes were combined into an a-allele carrier group for analyses.
Table 3.
Genotypic and allelic frequencies of the G894T SNP in exon 7 and the VNTR in intron 4
| Genotypic frequencies (%) | Allelic frequencies (%) | ||||
|---|---|---|---|---|---|
| G894T | GluGlu | GluAsp | AspAsp | Glu | Asp |
| 84.3 | 15.7 | 0 | 92.1 | 7.9 | |
| VNTR in intron 4 | bb | ba | aa | b | a |
| 80.6 | 18.5 | 0.9 | 89.8 | 10.2 | |
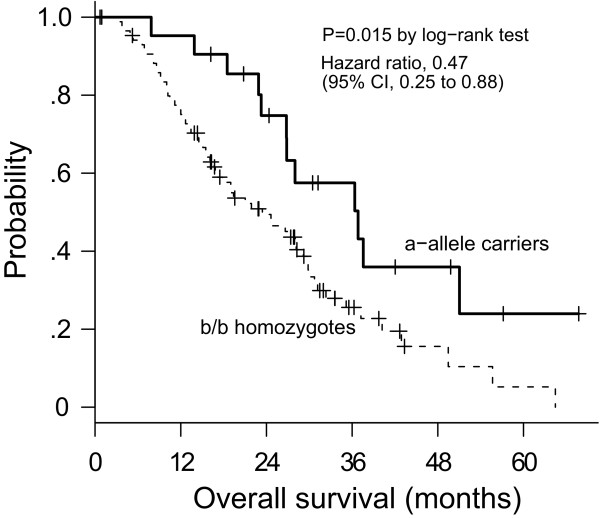
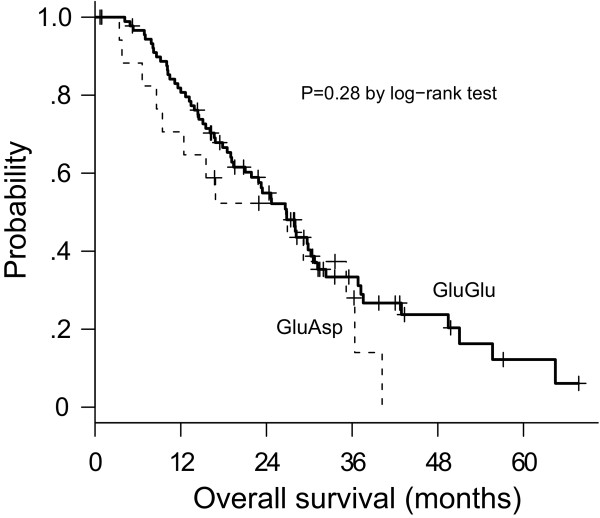
Median survival was 36.8 months for a-allele carriers and 23.4 months for b/b homozygotes (Figure 1). The univariate Cox regression model showed that presence of the a-allele significantly correlated with prolonged survival (HR, 0.47; 95% CI, 0.25-0.88; P = 0.015). Correspondingly, carriers of the common b/b genotype had a higher risk of death than carriers of the a-allele (HR, 2.14). The false-positive report probability for the VNTR was 0.16, indicating noteworthiness. No significant difference in survival was observed according to the G894T SNP (P = 0.28) (Figure 2).
Figure 1.
Kaplan-Meier plot of overall survival in relation to the VNTR genotype
Figure 2.
Kaplan-Meier plot of overall survival in relation to the G894T genotype in exon 7
EGFR mutation status was not included in a Cox proportional hazards model because the number of analyzed patients was small and statistically significant results were not observed in the univariate analysis. Including gender, age, ECOG PS, smoking status, clinical stage, histology, thoracic radiotherapy and two genotypes in a model, the common b/b genotype (HR, 2.33; 95% CI, 1.24 - 4.36; P = 0.0085), male gender (HR, 2.02; 95% CI, 1.20-3.43; P = 0.0084) and stage IV disease (HR, 1.76; 95% CI, 1.04-2.99; P = 0.036) were selected as the predictors for overall survival. Correspondingly, HRs of female gender and stage III disease were 0.50 and 0.57, respectively.
With regard to clinical and pathological parameters, there were no discrepancies between carriers of the VNTR common b/b genotype and the VNTR a-allele carriers (Table 4). To investigate whether the eNOS intron 4 VNTR was associated with clinical benefit of chemotherapy, we analyzed the treatment response among patients without definitive thoracic radiotherapy. The overall response rate in 82 evaluable patients was 27%, with 22 (27%) partial responders (PR), 50 patients (61%) with stable disease (SD), and 10 patients (12%) with disease progression (PD). There was no significant distribution of a-allele carriers when patients were separated into two groups (PR+SD group vs. PD group; Table 5), indicating that the effect of the polymorphism on survival was not associated with the clinical benefit of first-line platinum-containing chemotherapy. We restricted the population to patients who received carboplatin plus paclitaxel without definitive thoracic radiotherapy and re-analyzed. The PR+SD rate was 81% in a-allele carriers and 86% in b/b genotype patients, respectively. Again there was no significant difference between the groups (P = 0.69).
Table 4.
Comparative analysis of demographic and pathological information
| No. of bb genotype pts. | No. of a-allele carriers | P | |
|---|---|---|---|
| Gender | |||
| Female | 35 | 7 | P = 0.63 |
| Male | 52 | 14 | |
| Age | |||
| <70 | 64 | 15 | P > 0.9 |
| 70 or older | 23 | 6 | |
| ECOG PS | |||
| 0 | 17 | 6 | P = 0.38 |
| 1 | 70 | 15 | |
| Smoking Status | |||
| Never or Light | 29 | 7 | P > 0.9 |
| Current or Former | 58 | 14 | |
| Clinical Stage | |||
| III | 31 | 5 | P = 0.44 |
| IV | 56 | 16 | |
| Histology | |||
| Adenocarcinoma | 68 | 18 | P = 0.56 |
| Non-adenocarcinoma | 19 | 3 | |
| EGFR mutation | |||
| Activating mutation | 19 | 2 | P = 0.30 |
| Wild type | 9 | 4 | |
| Unknown | 59 | 15 | |
| Thoracic radiotherapy | |||
| Yes | 17 | 3 | P = 0.76 |
| No | 70 | 18 |
Table 5.
Frequency and distribution of eNOS intron 4 VNTR alleles in 72 patients with partial response or stable disease and 10 patients with progressive disease
| PR+SD | PD | ||||
|---|---|---|---|---|---|
| No. of Patients | % | No. of Patients | % | P | |
| a-allele carriers | 15 | 83 | 3 | 17 | 0.68 |
| bb genotype | 57 | 89 | 7 | 11 | |
Discussion
Our results show that polymorphism of the 27-bp VNTR in intron 4 of the eNOS gene is strongly associated with survival in advanced stage NSCLC patients treated with standard platinum-based chemotherapy. Median survival was significantly prolonged in patients carrying the shorter a-allele (i.e., those with a higher level of eNOS mRNA production).
Published studies indicate a procarcinogenic role of eNOS. In solid tumors, eNOS plays an essential role in VEGF-induced angiogenesis and vascular permeability [18]. A recent study showed that blocking phosphorylation of eNOS inhibited tumor initiation and maintenance through inactivation of the PI3K-AKT-eNOS-RAS pathway [19]. Moreover, a higher eNOS level was shown to correlate with cisplatin resistance in ovarian cancer cell lines [20]. Our findings were unexpected and in contrast to these observations.
However, our results can be explained by considering the following factors. NO derived from eNOS or eNOS itself can be cytotoxic to cancer cells through direct DNA damage and can be antitumorigenic [21]. The cytotoxic effects of NO can also be mediated through the generation of peroxynitrite (ONOO-) in the presence of superoxide anion (O∙2-) [22]. Moreover, clearance of metastatic cells from organ microvasculature is regulated in part by NO. NO has been reported to reduce the attachment of tumor cells to the endothelium and NO-mediated vasodilation may also contribute to the clearance of tumor cells [23,24]. Using an eNOS knockout mice model and the B16F1 melanoma cell line, Wang and colleagues have shown that arrest of tumor cell metastasis in the portal and pulmonary circulation can trigger the immediate release of NO in an eNOS-dependent manner, and subsequently induce apoptosis in these tumor cells [25,26]. Our observations were consistent with these reports. Furthermore, Mortensen et al. showed that expression of immunoreactive eNOS in the peritumoral microvasculature is a favorable prognostic indicator in premenopausal breast cancer patients, providing clinical evidence for a defensive role of eNOS against cancer cells [27]. With respect to treatment modality, NO was shown to increase the efficacy of chemotherapy and improve survival of patients with NSCLC in one phase II trial. Yasuda et al. randomized 120 patients with advanced stage NSCLC to receive standard chemotherapy (cisplatin and vinorelbine) alone, or the same regimen with nitroglycerin, a NO-donating drug [28]. The median time to progression was longer (10.9 vs. 6.2 months) in the nitroglycerin treatment group. Thus, in certain situations NO appears to act as a defense against the progression and/or metastasis of malignant tumors.
The median survival time of 26.8 months observed in the present study was favorable compared with that described in other reports [29,30]. This is probably due to the inclusion of a heterogeneous treatment group (including patients who received definitive chemoradiation) and the relatively young population (median 63 years of age).
Our study has several limitations. The single-institutional study design contains the potential to introduce a selection bias. Although we estimated the false-positive report probability to confirm the statistical significance obtained by established methods, analysis based upon a small number of enrolled patients could potentially lead to false-positive results. Two recent Japanese trials confirmed that EGFR mutation status is a predictive factor for response to EGFR-TKI treatment with a progression free survival of 10 months [31,32] and this finding paves the way for the personalized therapy for NSCLC. EGFR mutation status was evaluated in only a small proportion of the enrolled patients (31.5%) and the favorable effect of the mutations on prognosis was not observed in our study. Finally, because we restricted the study subjects to those of East-Asian ethnicity, it is uncertain whether these results can be generalized to other populations.
Conclusions
Our results indicate that the VNTR polymorphism in intron 4 of the eNOS gene may be associated with the progression of NSCLC and shed light on the association between polymorphisms in the eNOS gene and survival of patients with NSCLC.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SF participated in the design of the study, carried out the molecular genetic analysis and performed the statistical analysis. KM carried out the molecular genetic analysis, contributed to acquisition of data and helped to draft the manuscript. YH, AF, YHK and TM have made substantial contribution to acquisition of data. AH and RK performed the statistical analysis and helped to draft the manuscript. MM participated in the design of the study and helped to draft the manuscript. NK participated in the design of the study and conceived of the study. All authors read and approved the final manuscript.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Shiro Fujita, Email: jp.shirofujita@gmail.com.
Katsuhiro Masago, Email: masago@kuhp.kyoto-u.ac.jp.
Yukimasa Hatachi, Email: yukimasa@kuhp.kyoto-u.ac.jp.
Akiko Fukuhara, Email: afuku53@kuhp.kyoto-u.ac.jp.
Akito Hata, Email: a-hata@fbri.org.
Reiko Kaji, Email: naya@fbri.org.
Young Hak Kim, Email: ekim@kuhp.kyoto-u.ac.jp.
Tadashi Mio, Email: mio@kuhp.kyoto-u.ac.jp.
Michiaki Mishima, Email: mishima@kuhp.kyoto-u.ac.jp.
Nobuyuki Katakami, Email: katakami@fbri.org.
Acknowledgements
We thank Ms. Miyuki Sasaki for her technical assistance. This work is supported by Health Promoting Association for Respiratory Medicine of Nishi-Nippon.
References
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- Pfister DG, Johnson DH, Azzoli CG, Sause W, Smith TJ, Baker S Jr, Olak J, Stover D, Strawn JR, Turrisi AT, Somerfield MR. American Society of Clinical Oncology treatment of unresectable non-small-cell lung cancer guideline: update 2003. J Clin Oncol. 2004;22:330–353. doi: 10.1200/JCO.2004.09.053. [DOI] [PubMed] [Google Scholar]
- de las Penas R, Sanchez-Ronco M, Alberola V, Taron M, Camps C, Garcia-Carbonero R, Massuti B, Queralt C, Botia M, Garcia-Gomez R, Isla D, Cobo M, Santarpia M, Cecere F, Mendez P, Sanchez JJ, Rosell R. Polymorphisms in DNA repair genes modulate survival in cisplatin/gemcitabine-treated non-small-cell lung cancer patients. Ann Oncol. 2006;17:668–675. doi: 10.1093/annonc/mdj135. [DOI] [PubMed] [Google Scholar]
- Rosell R, Cecere F, Santarpia M, Reguart N, Taron M. Predicting the outcome of chemotherapy for lung cancer. Curr Opin Pharmacol. 2006;6:323–331. doi: 10.1016/j.coph.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Ying L, Hofseth LJ. An emerging role for endothelial nitric oxide synthase in chronic inflammation and cancer. Cancer Res. 2007;67:1407–1410. doi: 10.1158/0008-5472.CAN-06-2149. [DOI] [PubMed] [Google Scholar]
- Veldman BA, Spiering W, Doevendans PA, Vervoort G, Kroon AA, de Leeuw PW, Smits P. The Glu298Asp polymorphism of the NOS 3 gene as a determinant of the baseline production of nitric oxide. J Hypertens. 2002;20:2023–2027. doi: 10.1097/00004872-200210000-00022. [DOI] [PubMed] [Google Scholar]
- Tsukada T, Yokoyama K, Arai T, Takemoto F, Hara S, Yamada A, Kawaguchi Y, Hosoya T, Igari J. Evidence of association of the ecNOS gene polymorphism with plasma NO metabolite levels in humans. Biochem Biophys Res Commun. 1998;245:190–193. doi: 10.1006/bbrc.1998.8267. [DOI] [PubMed] [Google Scholar]
- Wang XL, Mahaney MC, Sim AS, Wang J, Blangero J, Almasy L, Badenhop RB, Wilcken DE. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17:3147–3153. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]
- Zhang MX, Zhang C, Shen YH, Wang J, Li XN, Chen L, Zhang Y, Coselli JS, Wang XL. Effect of 27nt small RNA on endothelial nitric-oxide synthase expression. Mol Biol Cell. 2008;19:3997–4005. doi: 10.1091/mbc.E07-11-1186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang MX, Zhang C, Shen YH, Wang J, Li XN, Zhang Y, Coselli J, Wang XL. Biogenesis of short intronic repeat 27-nucleotide small RNA from endothelial nitric-oxide synthase gene. J Biol Chem. 2008;283:14685–14693. doi: 10.1074/jbc.M801933200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senthil D, Raveendran M, Shen YH, Utama B, Dudley D, Wang J, Wang XL. Genotype-dependent expression of endothelial nitric oxide synthase (eNOS) and its regulatory proteins in cultured endothelial cells. DNA Cell Biol. 2005;24:218–224. doi: 10.1089/dna.2005.24.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mountain CF. Revisions in the International System for Staging Lung Cancer. Chest. 1997;111:1710–1717. doi: 10.1378/chest.111.6.1710. [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. J Natl Cancer Inst. Vol. 92. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada; 2000. New guidelines to evaluate the response to treatment in solid tumors; pp. 205–216. [DOI] [PubMed] [Google Scholar]
- Miyahara K, Kawamoto T, Sase K, Yui Y, Toda K, Yang LX, Hattori R, Aoyama T, Yamamoto Y, Doi Y, Ogoshi S, Hashimoto K, Kawai C, Sasayama S, Shizuta Y. Cloning and structural characterization of the human endothelial nitric-oxide-synthase gene. Eur J Biochem. 1994;223:719–726. doi: 10.1111/j.1432-1033.1994.tb19045.x. [DOI] [PubMed] [Google Scholar]
- Akaike H. In: Second International Symposium on Information Theory. Petrov BN, Czáki F, editor. Budapest: Akademiai Kiadó; 1973. Information Theory and Extension of the Maximam Likelifood Principle; pp. 267–281. [Google Scholar]
- Wacholder S, Chanock S, Garcia-Closas M, El Ghormli L, Rothman N. Assessing the probability that a positive report is false: an approach for molecular epidemiology studies. J Natl Cancer Inst. 2004;96:434–442. doi: 10.1093/jnci/djh075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- Duda DG, Fukumura D, Jain RK. Role of eNOS in neovascularization: NO for endothelial progenitor cells. Trends Mol Med. 2004;10:143–145. doi: 10.1016/j.molmed.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Lim KH, Ancrile BB, Kashatus DF, Counter CM. Tumour maintenance is mediated by eNOS. Nature. 2008;452:646–649. doi: 10.1038/nature06778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung EL, Fraser M, Fiscus RR, Tsang BK. Cisplatin alters nitric oxide synthase levels in human ovarian cancer cells: involvement in p53 regulation and cisplatin resistance. Br J Cancer. 2008;98:1803–1809. doi: 10.1038/sj.bjc.6604375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonveaux P, Jordan BF, Gallez B, Feron O. Nitric oxide delivery to cancer: why and how? Eur J Cancer. 2009;45:1352–1369. doi: 10.1016/j.ejca.2008.12.018. [DOI] [PubMed] [Google Scholar]
- Reiter CD, Teng RJ, Beckman JS. Superoxide reacts with nitric oxide to nitrate tyrosine at physiological pH via peroxynitrite. J Biol Chem. 2000;275:32460–32466. doi: 10.1074/jbc.M910433199. [DOI] [PubMed] [Google Scholar]
- Kong L, Dunn GD, Keefer LK, Korthuis RJ. Nitric oxide reduces tumor cell adhesion to isolated rat postcapillary venules. Clin Exp Metastasis. 1996;14:335–343. doi: 10.1007/BF00123392. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Terada N, Seiyama A, Nishizawa Y, Akedo H, Kosaka H. Increase in experimental pulmonary metastasis in mice by L-arginine under inhibition of nitric oxide production by NG-nitro-L-arginine methyl ester. Int J Cancer. 1998;75:140–144. doi: 10.1002/(SICI)1097-0215(19980105)75:1<140::AID-IJC21>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Qiu H, Orr FW, Jensen D, Wang HH, McIntosh AR, Hasinoff BB, Nance DM, Pylypas S, Qi K, Song C, Muschel RJ, Al-Mehdi AB. Arrest of B16 melanoma cells in the mouse pulmonary microcirculation induces endothelial nitric oxide synthase-dependent nitric oxide release that is cytotoxic to the tumor cells. Am J Pathol. 2003;162:403–412. doi: 10.1016/S0002-9440(10)63835-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HH, McIntosh AR, Hasinoff BB, Rector ES, Ahmed N, Nance DM, Orr FW. B16 melanoma cell arrest in the mouse liver induces nitric oxide release and sinusoidal cytotoxicity: a natural hepatic defense against metastasis. Cancer Res. 2000;60:5862–5869. [PubMed] [Google Scholar]
- Mortensen K, Holck S, Christensen IJ, Skouv J, Hougaard DM, Blom J, Larsson LI. Endothelial cell nitric oxide synthase in peritumoral microvessels is a favorable prognostic indicator in premenopausal breast cancer patients. Clin Cancer Res. 1999;5:1093–1097. [PubMed] [Google Scholar]
- Yasuda H, Yamaya M, Nakayama K, Sasaki T, Ebihara S, Kanda A, Asada M, Inoue D, Suzuki T, Okazaki T, Takahashi H, Yoshida M, Kaneta T, Ishizawa K, Yamanda S, Tomita N, Yamasaki M, Kikuchi A, Kubo H, Sasaki H. Randomized phase II trial comparing nitroglycerin plus vinorelbine and cisplatin with vinorelbine and cisplatin alone in previously untreated stage IIIB/IV non-small-cell lung cancer. J Clin Oncol. 2006;24:688–694. doi: 10.1200/JCO.2005.04.0436. [DOI] [PubMed] [Google Scholar]
- Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A, Lilenbaum R, Johnson DH. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med. 2006;355:2542–2550. doi: 10.1056/NEJMoa061884. [DOI] [PubMed] [Google Scholar]
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. 2008;26:3543–3551. doi: 10.1200/JCO.2007.15.0375. [DOI] [PubMed] [Google Scholar]
- Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H, Gemma A, Harada M, Yoshizawa H, Kinoshita I, Fujita Y, Okinaga S, Hirano H, Yoshimori K, Harada T, Ogura T, Ando M, Miyazawa H, Tanaka T, Saijo Y, Hagiwara K, Morita S, Nikiwa T. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362:2380–2388. doi: 10.1056/NEJMoa0909530. [DOI] [PubMed] [Google Scholar]
- Mitsudomi T, Morita S, Yatabe Y, Negoro S, Okamoto I, Tsurutani J, Seto T, Satouchi M, Tada H, Hirashima T, Asami K, Katakami N, Takada M, Yoshioka H, Shibata K, Kudoh S, Shimizu E, Saito H, Toyooka S, Nakagawa K, Fukuoka M. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11:121–128. doi: 10.1016/S1470-2045(09)70364-X. [DOI] [PubMed] [Google Scholar]