Abstract
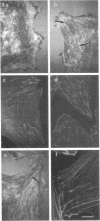
Cell adhesion to extracellular matrix molecules such as fibronectin involves complex transmembrane signaling processes. Attachment and spreading of primary fibroblasts can be promoted by interactions of cell surface integrins with RGD-containing fragments of fibronectin, but the further process of focal adhesion and stress fiber formation requires additional interactions. Heparin-binding fragments of fibronectin can provide this signal. The COOH-terminal heparin-binding domain of fibronectin contains five separate heparin-binding amino acid sequences. We show here that all five sequences, as synthetic peptides coupled to ovalbumin, can support cell attachment. Only three of these sequences can promote focal adhesion formation when presented as multicopy complexes, and only one of these (WQPPRARI) retains this activity as free peptide. The major activity of this peptide resides in the sequence PRARI. The biological response to this peptide and to the COOH-terminal fragment may be mediated through cell surface heparan sulfate proteoglycans because treatment of cells with heparinase II and III, or competition with heparin, reduces the response. Treatment with chondroitinase ABC or competition with chondroitin sulfate does not.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akiyama S. K., Nagata K., Yamada K. M. Cell surface receptors for extracellular matrix components. Biochim Biophys Acta. 1990 Feb 28;1031(1):91–110. doi: 10.1016/0304-4157(90)90004-v. [DOI] [PubMed] [Google Scholar]
- Ankel E. G., Homandberg G., Tooney N. M., Lai C. S. Heparin modulates conformational states of plasma fibronectin: an electron spin resonance spin label approach. Arch Biochem Biophys. 1986 Jan;244(1):50–56. doi: 10.1016/0003-9861(86)90093-7. [DOI] [PubMed] [Google Scholar]
- Aota S., Nagai T., Yamada K. M. Characterization of regions of fibronectin besides the arginine-glycine-aspartic acid sequence required for adhesive function of the cell-binding domain using site-directed mutagenesis. J Biol Chem. 1991 Aug 25;266(24):15938–15943. [PubMed] [Google Scholar]
- Austria M. R., Couchman J. R. Enhanced assembly of basement membrane matrix by endodermal cells in response to fibronectin substrata. J Cell Sci. 1991 Jun;99(Pt 2):443–451. doi: 10.1242/jcs.99.2.443. [DOI] [PubMed] [Google Scholar]
- Barkalow F. J., Schwarzbauer J. E. Localization of the major heparin-binding site in fibronectin. J Biol Chem. 1991 Apr 25;266(12):7812–7818. [PubMed] [Google Scholar]
- Baron M., Main A. L., Driscoll P. C., Mardon H. J., Boyd J., Campbell I. D. 1H NMR assignment and secondary structure of the cell adhesion type III module of fibronectin. Biochemistry. 1992 Feb 25;31(7):2068–2073. doi: 10.1021/bi00122a025. [DOI] [PubMed] [Google Scholar]
- Bernfield M., Kokenyesi R., Kato M., Hinkes M. T., Spring J., Gallo R. L., Lose E. J. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin A. D., Weintraub H. J. Molecular modeling of protein-glycosaminoglycan interactions. Arteriosclerosis. 1989 Jan-Feb;9(1):21–32. doi: 10.1161/01.atv.9.1.21. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Austria R., Woods A., Hughes R. C. Adhesion defective BHK cell mutant has cell surface heparan sulfate proteoglycan of altered properties. J Cell Physiol. 1988 Aug;136(2):226–236. doi: 10.1002/jcp.1041360204. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Hök M., Rees D. A., Timpl R. Adhesion, growth, and matrix production by fibroblasts on laminin substrates. J Cell Biol. 1983 Jan;96(1):177–183. doi: 10.1083/jcb.96.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake S. L., Klein D. J., Mickelson D. J., Oegema T. R., Furcht L. T., McCarthy J. B. Cell surface phosphatidylinositol-anchored heparan sulfate proteoglycan initiates mouse melanoma cell adhesion to a fibronectin-derived, heparin-binding synthetic peptide. J Cell Biol. 1992 Jun;117(6):1331–1341. doi: 10.1083/jcb.117.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Hynes R. O. Lymphoid cells recognize an alternatively spliced segment of fibronectin via the integrin receptor alpha 4 beta 1. Cell. 1990 Jan 12;60(1):53–61. doi: 10.1016/0092-8674(90)90715-q. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Haugen P. K., McCarthy J. B., Skubitz A. P., Furcht L. T., Letourneau P. C. Recognition of the A chain carboxy-terminal heparin binding region of fibronectin involves multiple sites: two contiguous sequences act independently to promote neural cell adhesion. J Cell Biol. 1990 Dec;111(6 Pt 1):2733–2745. doi: 10.1083/jcb.111.6.2733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iida J., Skubitz A. P., Furcht L. T., Wayner E. A., McCarthy J. B. Coordinate role for cell surface chondroitin sulfate proteoglycan and alpha 4 beta 1 integrin in mediating melanoma cell adhesion to fibronectin. J Cell Biol. 1992 Jul;118(2):431–444. doi: 10.1083/jcb.118.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izzard C. S., Lochner L. R. Formation of cell-to-substrate contacts during fibroblast motility: an interference-reflexion study. J Cell Sci. 1980 Apr;42:81–116. doi: 10.1242/jcs.42.1.81. [DOI] [PubMed] [Google Scholar]
- Izzard C. S., Radinsky R., Culp L. A. Substratum contacts and cytoskeletal reorganization of BALB/c 3T3 cells on a cell-binding fragment and heparin-binding fragments of plasma fibronectin. Exp Cell Res. 1986 Aug;165(2):320–336. doi: 10.1016/0014-4827(86)90586-0. [DOI] [PubMed] [Google Scholar]
- Jalkanen S., Jalkanen M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J Cell Biol. 1992 Feb;116(3):817–825. doi: 10.1083/jcb.116.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kjellén L., Lindahl U. Proteoglycans: structures and interactions. Annu Rev Biochem. 1991;60:443–475. doi: 10.1146/annurev.bi.60.070191.002303. [DOI] [PubMed] [Google Scholar]
- LeBaron R. G., Esko J. D., Woods A., Johansson S., Hök M. Adhesion of glycosaminoglycan-deficient chinese hamster ovary cell mutants to fibronectin substrata. J Cell Biol. 1988 Mar;106(3):945–952. doi: 10.1083/jcb.106.3.945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leahy D. J., Hendrickson W. A., Aukhil I., Erickson H. P. Structure of a fibronectin type III domain from tenascin phased by MAD analysis of the selenomethionyl protein. Science. 1992 Nov 6;258(5084):987–991. doi: 10.1126/science.1279805. [DOI] [PubMed] [Google Scholar]
- Lories V., Cassiman J. J., Van den Berghe H., David G. Differential expression of cell surface heparan sulfate proteoglycans in human mammary epithelial cells and lung fibroblasts. J Biol Chem. 1992 Jan 15;267(2):1116–1122. [PubMed] [Google Scholar]
- Massia S. P., Hubbell J. A. Immobilized amines and basic amino acids as mimetic heparin-binding domains for cell surface proteoglycan-mediated adhesion. J Biol Chem. 1992 May 15;267(14):10133–10141. [PubMed] [Google Scholar]
- McCarthy J. B., Chelberg M. K., Mickelson D. J., Furcht L. T. Localization and chemical synthesis of fibronectin peptides with melanoma adhesion and heparin binding activities. Biochemistry. 1988 Feb 23;27(4):1380–1388. doi: 10.1021/bi00404a044. [DOI] [PubMed] [Google Scholar]
- McCarthy J. B., Skubitz A. P., Qi Z., Yi X. Y., Mickelson D. J., Klein D. J., Furcht L. T. RGD-independent cell adhesion to the carboxy-terminal heparin-binding fragment of fibronectin involves heparin-dependent and -independent activities. J Cell Biol. 1990 Mar;110(3):777–787. doi: 10.1083/jcb.110.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooradian D. L., McCarthy J. B., Skubitz A. P., Cameron J. D., Furcht L. T. Characterization of FN-C/H-V, a novel synthetic peptide from fibronectin that promotes rabbit corneal epithelial cell adhesion, spreading, and motility. Invest Ophthalmol Vis Sci. 1993 Jan;34(1):153–164. [PubMed] [Google Scholar]
- Nagai T., Yamakawa N., Aota S., Yamada S. S., Akiyama S. K., Olden K., Yamada K. M. Monoclonal antibody characterization of two distant sites required for function of the central cell-binding domain of fibronectin in cell adhesion, cell migration, and matrix assembly. J Cell Biol. 1991 Sep;114(6):1295–1305. doi: 10.1083/jcb.114.6.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obara M., Kang M. S., Yamada K. M. Site-directed mutagenesis of the cell-binding domain of human fibronectin: separable, synergistic sites mediate adhesive function. Cell. 1988 May 20;53(4):649–657. doi: 10.1016/0092-8674(88)90580-6. [DOI] [PubMed] [Google Scholar]
- Rapraeger A., Jalkanen M., Bernfield M. Cell surface proteoglycan associates with the cytoskeleton at the basolateral cell surface of mouse mammary epithelial cells. J Cell Biol. 1986 Dec;103(6 Pt 2):2683–2696. doi: 10.1083/jcb.103.6.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E. Fibronectin and its receptors. Annu Rev Biochem. 1988;57:375–413. doi: 10.1146/annurev.bi.57.070188.002111. [DOI] [PubMed] [Google Scholar]
- Streeter H. B., Rees D. A. Fibroblast adhesion to RGDS shows novel features compared with fibronectin. J Cell Biol. 1987 Jul;105(1):507–515. doi: 10.1083/jcb.105.1.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turnbull J. E., Fernig D. G., Ke Y., Wilkinson M. C., Gallagher J. T. Identification of the basic fibroblast growth factor binding sequence in fibroblast heparan sulfate. J Biol Chem. 1992 May 25;267(15):10337–10341. [PubMed] [Google Scholar]
- Wayner E. A., Garcia-Pardo A., Humphries M. J., McDonald J. A., Carter W. G. Identification and characterization of the T lymphocyte adhesion receptor for an alternative cell attachment domain (CS-1) in plasma fibronectin. J Cell Biol. 1989 Sep;109(3):1321–1330. doi: 10.1083/jcb.109.3.1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Focal adhesions and cell-matrix interactions. Coll Relat Res. 1988 Mar;8(2):155–182. doi: 10.1016/s0174-173x(88)80027-x. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Heparan sulfate proteoglycans and signalling in cell adhesion. Adv Exp Med Biol. 1992;313:87–96. doi: 10.1007/978-1-4899-2444-5_9. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Hök M. Heparan sulfate proteoglycans of rat embryo fibroblasts. A hydrophobic form may link cytoskeleton and matrix components. J Biol Chem. 1985 Sep 5;260(19):10872–10879. [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Johansson S., Hök M. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 1986 Apr;5(4):665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods A., Couchman J. R. Protein kinase C involvement in focal adhesion formation. J Cell Sci. 1992 Feb;101(Pt 2):277–290. doi: 10.1242/jcs.101.2.277. [DOI] [PubMed] [Google Scholar]
- Woods A., Hök M., Kjellén L., Smith C. G., Rees D. A. Relationship of heparan sulfate proteoglycans to the cytoskeleton and extracellular matrix of cultured fibroblasts. J Cell Biol. 1984 Nov;99(5):1743–1753. doi: 10.1083/jcb.99.5.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K. M. Fibronectins: structure, functions and receptors. Curr Opin Cell Biol. 1989 Oct;1(5):956–963. doi: 10.1016/0955-0674(89)90065-3. [DOI] [PubMed] [Google Scholar]