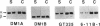Abstract
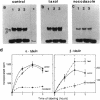
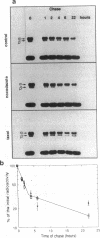
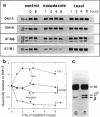
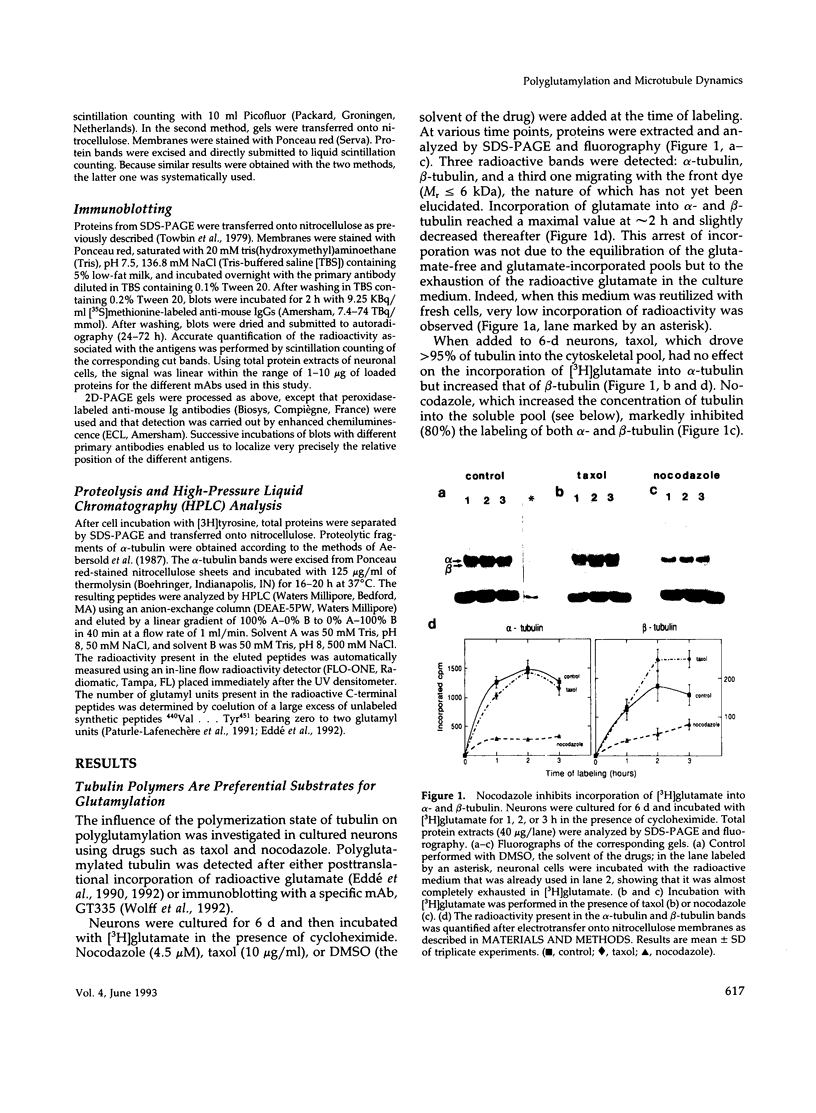
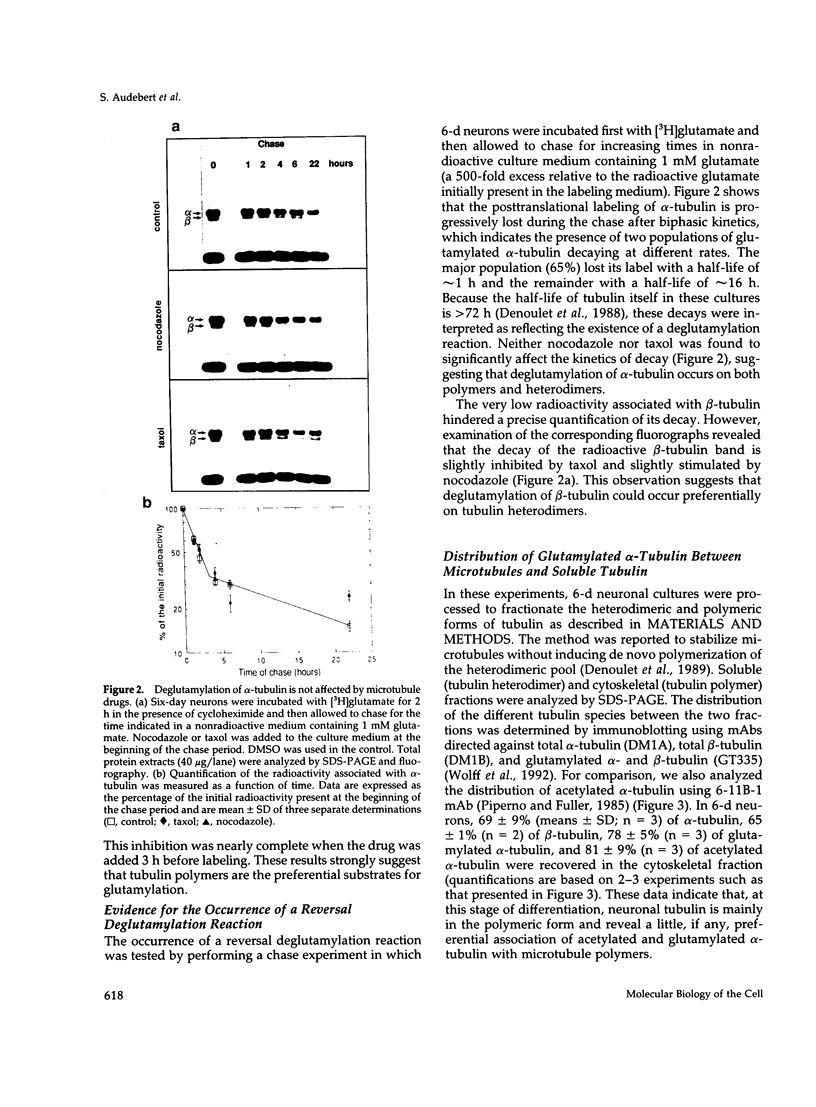
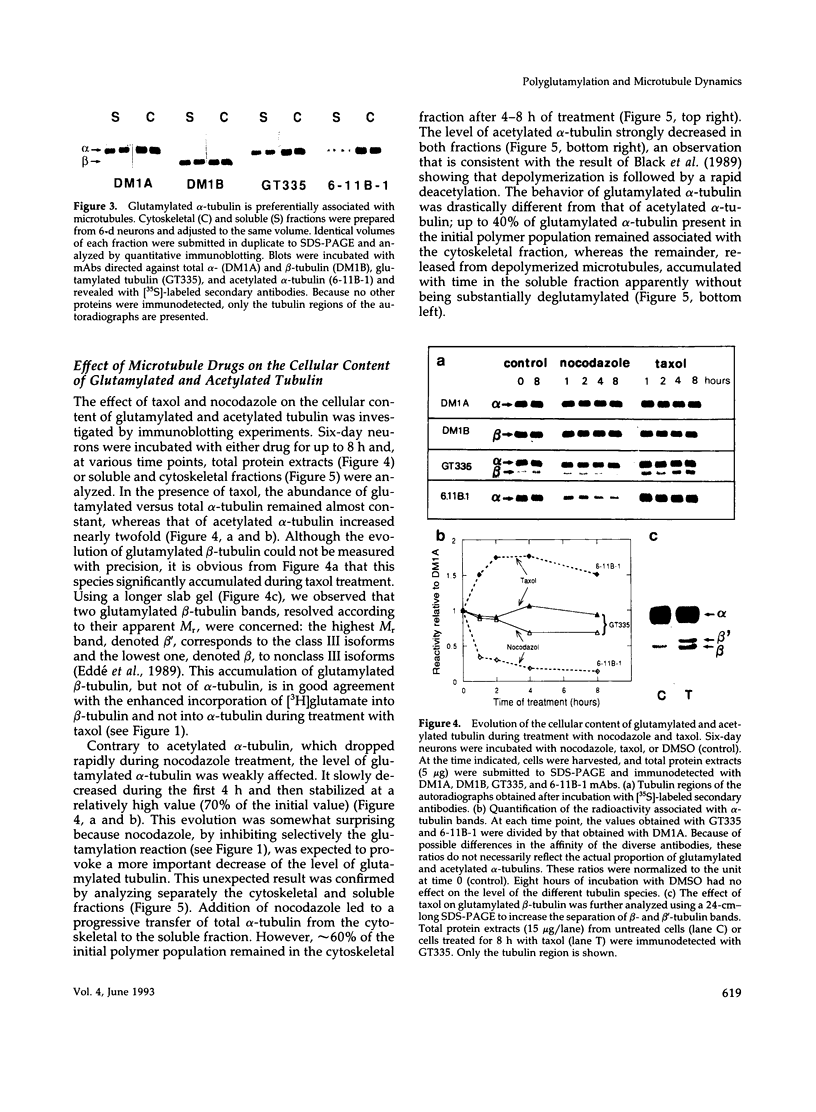
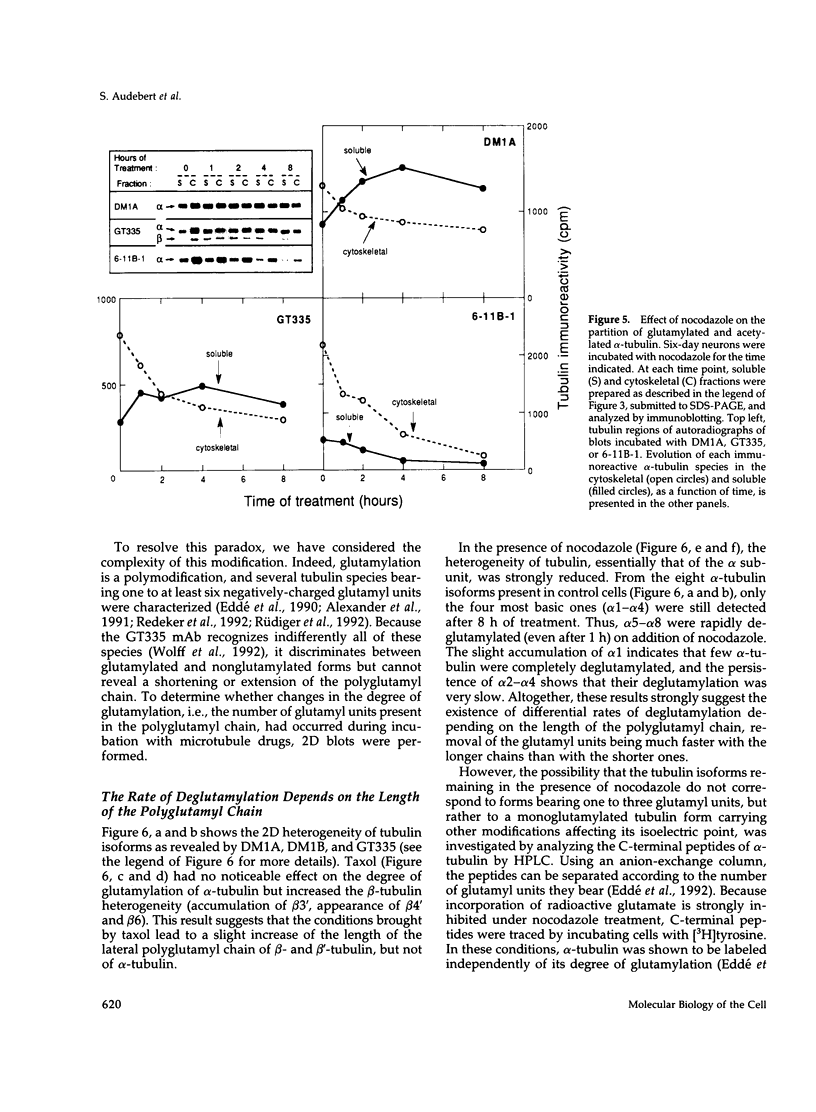
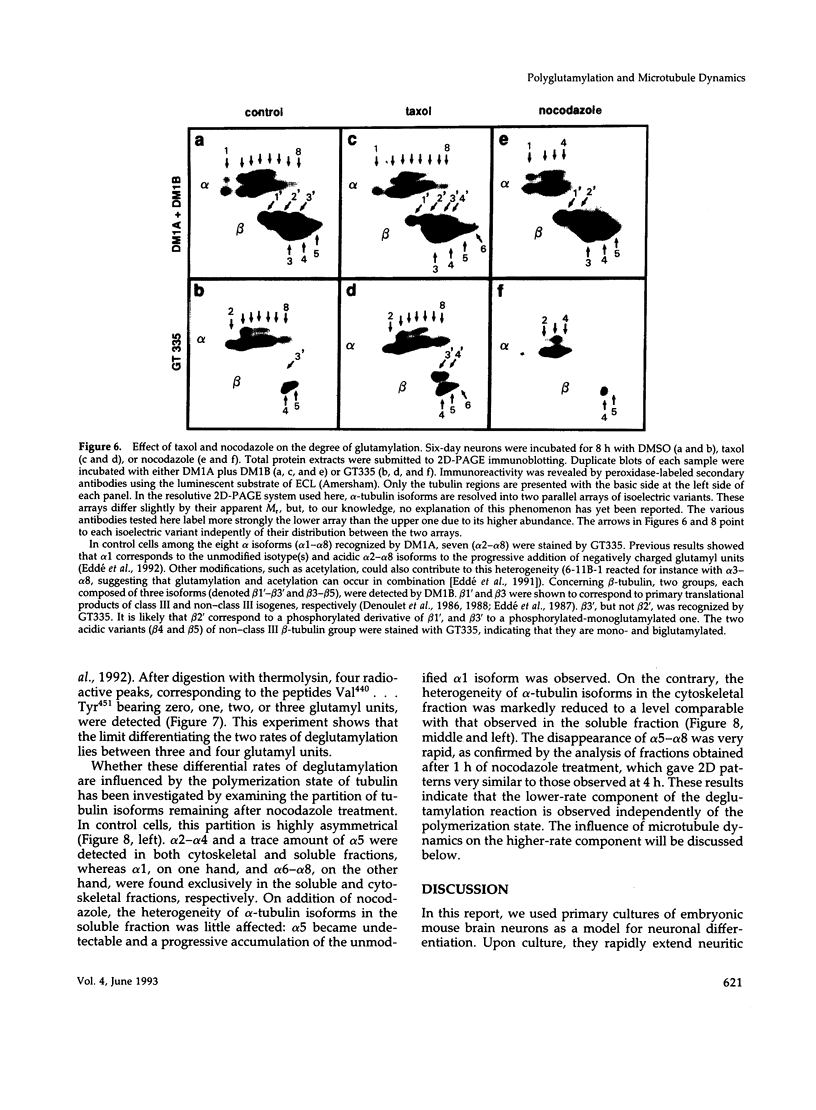
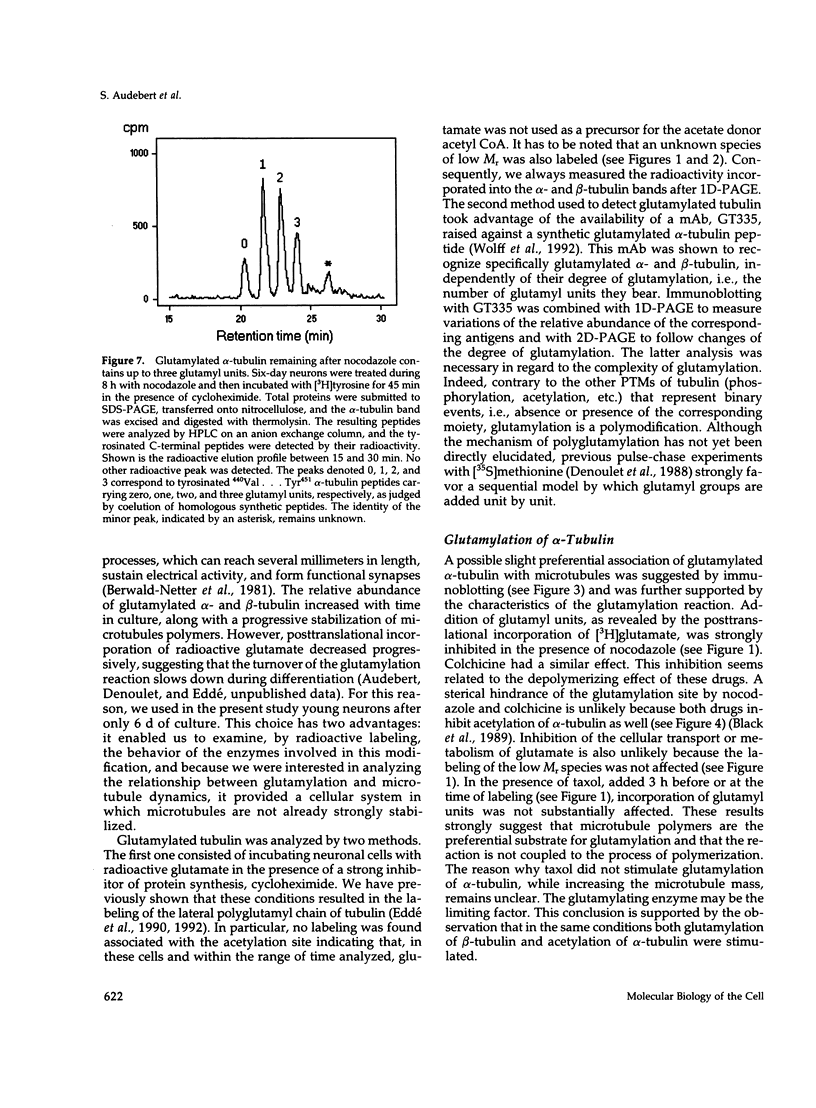
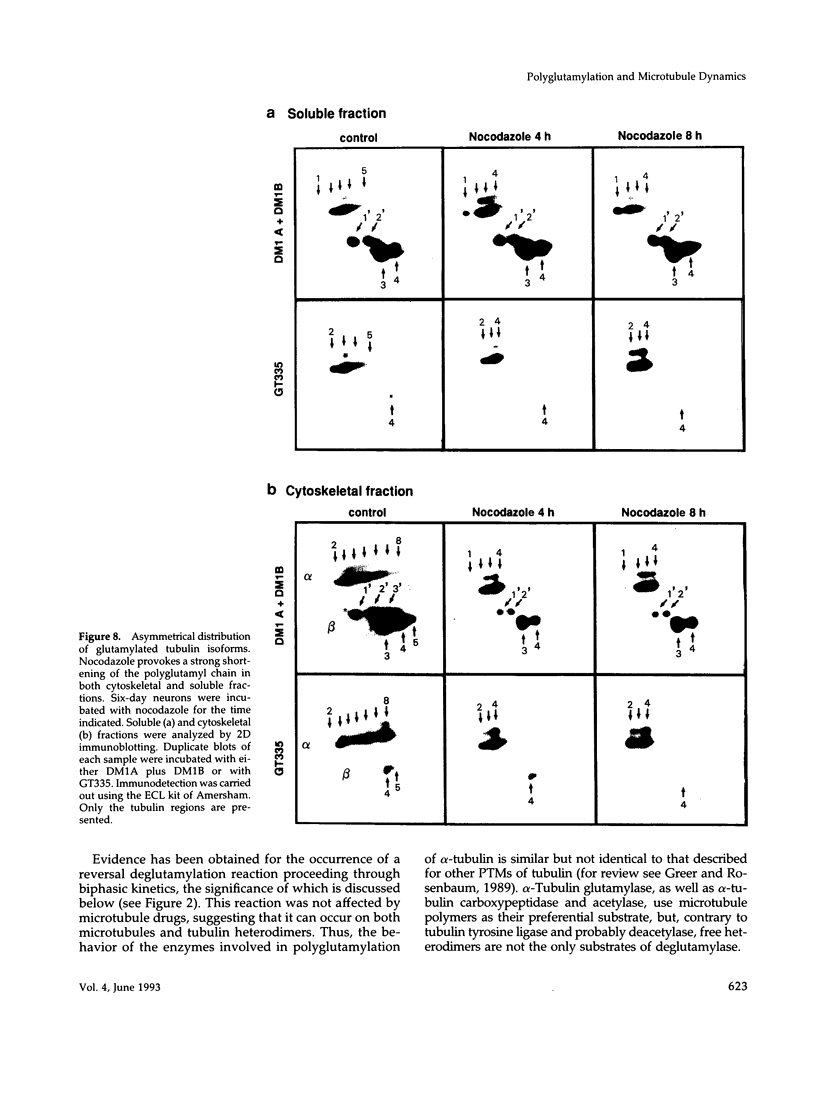
The relationship between microtubule dynamics and polyglutamylation of tubulin was investigated in young differentiating mouse brain neurons. Selective posttranslational labeling with [3H]glutamate and immunoblotting with a specific monoclonal antibody (GT335) enabled us to analyze polyglutamylation of both alpha and beta subunits. Nocodazole markedly inhibited incorporation of [3H]glutamate into alpha- and beta-tubulin, whereas taxol had no effect for alpha-tubulin and a stimulating effect for beta-tubulin. These results strongly suggest that microtubule polymers are the preferred substrate for polyglutamylation. Chase experiments revealed the existence of a reversal reaction that, in the case of alpha-tubulin, was not affected by microtubule drugs, suggesting that deglutamylation of this subunit can occur on both polymers and soluble tubulin. Evidence was obtained that deglutamylation of alpha-tubulin operates following two distinct rates depending on the length of the polyglutamyl chain, the distal units (4th-6th) being removed rapidly whereas the proximal ones (1st-3rd) appearing much more resistant to deglutamylation. Partition of glutamylated alpha-tubulin isoforms was also correlated with the length of the polyglutamyl chain. Forms bearing four to six units were recovered specifically in the polymeric fraction, whereas those bearing one to three units were distributed evenly between polymeric and soluble fractions. It thus appears that the slow rate component of the deglutamylation reaction offers to neurons the possibility to maintain a basal level of glutamylated alpha-tubulin in the soluble pool independently of microtubule dynamics. Finally, some differences observed in the glutamylation of alpha- and beta-tubulin suggest that distinct enzymes are involved.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander J. E., Hunt D. F., Lee M. K., Shabanowitz J., Michel H., Berlin S. C., MacDonald T. L., Sundberg R. J., Rebhun L. I., Frankfurter A. Characterization of posttranslational modifications in neuron-specific class III beta-tubulin by mass spectrometry. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4685–4689. doi: 10.1073/pnas.88.11.4685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baas P. W., Black M. M. Individual microtubules in the axon consist of domains that differ in both composition and stability. J Cell Biol. 1990 Aug;111(2):495–509. doi: 10.1083/jcb.111.2.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berwald-Netter Y., Martin-Moutot N., Koulakoff A., Couraud F. Na+-channel-associated scorpion toxin receptor sites as probes for neuronal evolution in vivo and in vitro. Proc Natl Acad Sci U S A. 1981 Feb;78(2):1245–1249. doi: 10.1073/pnas.78.2.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M., Baas P. W., Humphries S. Dynamics of alpha-tubulin deacetylation in intact neurons. J Neurosci. 1989 Jan;9(1):358–368. doi: 10.1523/JNEUROSCI.09-01-00358.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black M. M., Keyser P. Acetylation of alpha-tubulin in cultured neurons and the induction of alpha-tubulin acetylation in PC12 cells by treatment with nerve growth factor. J Neurosci. 1987 Jun;7(6):1833–1842. doi: 10.1523/JNEUROSCI.07-06-01833.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denoulet P., Edde B., Jeantet C., Gros F. Evolution of tubulin heterogeneity during mouse brain development. Biochimie. 1982 Mar;64(3):165–172. doi: 10.1016/s0300-9084(82)80466-5. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Eddé B., Gros F. Differential expression of several neurospecific beta-tubulin mRNAs in the mouse brain during development. Gene. 1986;50(1-3):289–297. doi: 10.1016/0378-1119(86)90333-1. [DOI] [PubMed] [Google Scholar]
- Denoulet P., Filliatreau G., de Néchaud B., Gros F., Di Giamberardino L. Differential axonal transport of isotubulins in the motor axons of the rat sciatic nerve. J Cell Biol. 1989 Mar;108(3):965–971. doi: 10.1083/jcb.108.3.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eddé B., Denoulet P., de Néchaud B., Koulakoff A., Berwald-Netter Y., Gros F. Posttranslational modifications of tubulin in cultured mouse brain neurons and astroglia. Biol Cell. 1989;65(2):109–117. doi: 10.1111/j.1768-322x.1989.tb00779.x. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Berwald-Netter Y., Koulakoff A., Gros F., Denoulet P. A combination of posttranslational modifications is responsible for the production of neuronal alpha-tubulin heterogeneity. J Cell Biochem. 1991 Jun;46(2):134–142. doi: 10.1002/jcb.240460207. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Desbruyères E., Gros F., Denoulet P. Posttranslational glutamylation of alpha-tubulin. Science. 1990 Jan 5;247(4938):83–85. doi: 10.1126/science.1967194. [DOI] [PubMed] [Google Scholar]
- Eddé B., Rossier J., Le Caer J. P., Promé J. C., Desbruyères E., Gros F., Denoulet P. Polyglutamylated alpha-tubulin can enter the tyrosination/detyrosination cycle. Biochemistry. 1992 Jan 21;31(2):403–410. doi: 10.1021/bi00117a014. [DOI] [PubMed] [Google Scholar]
- Eddé B., de Nechaud B., Denoulet P., Gros F. Control of isotubulin expression during neuronal differentiation of mouse neuroblastoma and teratocarcinoma cell lines. Dev Biol. 1987 Oct;123(2):549–558. doi: 10.1016/0012-1606(87)90413-1. [DOI] [PubMed] [Google Scholar]
- Ferreira A., Caceres A. Expression of the class III beta-tubulin isotype in developing neurons in culture. J Neurosci Res. 1992 Aug;32(4):516–529. doi: 10.1002/jnr.490320407. [DOI] [PubMed] [Google Scholar]
- Goldsmith M., Connolly J. A., Kumar N., Wu J., Yarbrough L. R., van der Kooy D. Conserved beta-tubulin binding domain for the microtubule-associated motors underlying sperm motility and fast axonal transport. Cell Motil Cytoskeleton. 1991;20(3):249–262. doi: 10.1002/cm.970200308. [DOI] [PubMed] [Google Scholar]
- Joshi H. C., Cleveland D. W. Diversity among tubulin subunits: toward what functional end? Cell Motil Cytoskeleton. 1990;16(3):159–163. doi: 10.1002/cm.970160302. [DOI] [PubMed] [Google Scholar]
- Khawaja S., Gundersen G. G., Bulinski J. C. Enhanced stability of microtubules enriched in detyrosinated tubulin is not a direct function of detyrosination level. J Cell Biol. 1988 Jan;106(1):141–149. doi: 10.1083/jcb.106.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Littauer U. Z., Giveon D., Thierauf M., Ginzburg I., Ponstingl H. Common and distinct tubulin binding sites for microtubule-associated proteins. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7162–7166. doi: 10.1073/pnas.83.19.7162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maccioni R. B., Rivas C. I., Vera J. C. Differential interaction of synthetic peptides from the carboxyl-terminal regulatory domain of tubulin with microtubule-associated proteins. EMBO J. 1988 Jul;7(7):1957–1963. doi: 10.1002/j.1460-2075.1988.tb03033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Cytoskeletal dynamics and nerve growth. Neuron. 1988 Nov;1(9):761–772. doi: 10.1016/0896-6273(88)90124-9. [DOI] [PubMed] [Google Scholar]
- Murphy D. B. Functions of tubulin isoforms. Curr Opin Cell Biol. 1991 Feb;3(1):43–51. doi: 10.1016/0955-0674(91)90164-t. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- Paschal B. M., Obar R. A., Vallee R. B. Interaction of brain cytoplasmic dynein and MAP2 with a common sequence at the C terminus of tubulin. Nature. 1989 Nov 30;342(6249):569–572. doi: 10.1038/342569a0. [DOI] [PubMed] [Google Scholar]
- Paturle-Lafanechère L., Eddé B., Denoulet P., Van Dorsselaer A., Mazarguil H., Le Caer J. P., Wehland J., Job D. Characterization of a major brain tubulin variant which cannot be tyrosinated. Biochemistry. 1991 Oct 29;30(43):10523–10528. doi: 10.1021/bi00107a022. [DOI] [PubMed] [Google Scholar]
- Piperno G., Fuller M. T. Monoclonal antibodies specific for an acetylated form of alpha-tubulin recognize the antigen in cilia and flagella from a variety of organisms. J Cell Biol. 1985 Dec;101(6):2085–2094. doi: 10.1083/jcb.101.6.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redeker V., Melki R., Promé D., Le Caer J. P., Rossier J. Structure of tubulin C-terminal domain obtained by subtilisin treatment. The major alpha and beta tubulin isotypes from pig brain are glutamylated. FEBS Lett. 1992 Nov 23;313(2):185–192. doi: 10.1016/0014-5793(92)81441-n. [DOI] [PubMed] [Google Scholar]
- Rüdiger M., Plessman U., Klöppel K. D., Wehland J., Weber K. Class II tubulin, the major brain beta tubulin isotype is polyglutamylated on glutamic acid residue 435. FEBS Lett. 1992 Aug 10;308(1):101–105. doi: 10.1016/0014-5793(92)81061-p. [DOI] [PubMed] [Google Scholar]
- Schulze E., Asai D. J., Bulinski J. C., Kirschner M. Posttranslational modification and microtubule stability. J Cell Biol. 1987 Nov;105(5):2167–2177. doi: 10.1083/jcb.105.5.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Dynamic and stable populations of microtubules in cells. J Cell Biol. 1987 Feb;104(2):277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano L., Montejo de Garcini E., Hernández M. A., Avila J. Localization of the tubulin binding site for tau protein. Eur J Biochem. 1985 Dec 16;153(3):595–600. doi: 10.1111/j.1432-1033.1985.tb09342.x. [DOI] [PubMed] [Google Scholar]
- Sullivan K. F. Structure and utilization of tubulin isotypes. Annu Rev Cell Biol. 1988;4:687–716. doi: 10.1146/annurev.cb.04.110188.003351. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster D. R., Wehland J., Weber K., Borisy G. G. Detyrosination of alpha tubulin does not stabilize microtubules in vivo. J Cell Biol. 1990 Jul;111(1):113–122. doi: 10.1083/jcb.111.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff A., Denoulet P., Jeantet C. High level of tubulin microheterogeneity in the mouse brain. Neurosci Lett. 1982 Aug 31;31(3):323–328. doi: 10.1016/0304-3940(82)90041-6. [DOI] [PubMed] [Google Scholar]
- Wolff A., de Néchaud B., Chillet D., Mazarguil H., Desbruyères E., Audebert S., Eddé B., Gros F., Denoulet P. Distribution of glutamylated alpha and beta-tubulin in mouse tissues using a specific monoclonal antibody, GT335. Eur J Cell Biol. 1992 Dec;59(2):425–432. [PubMed] [Google Scholar]