Abstract
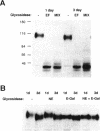
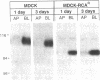
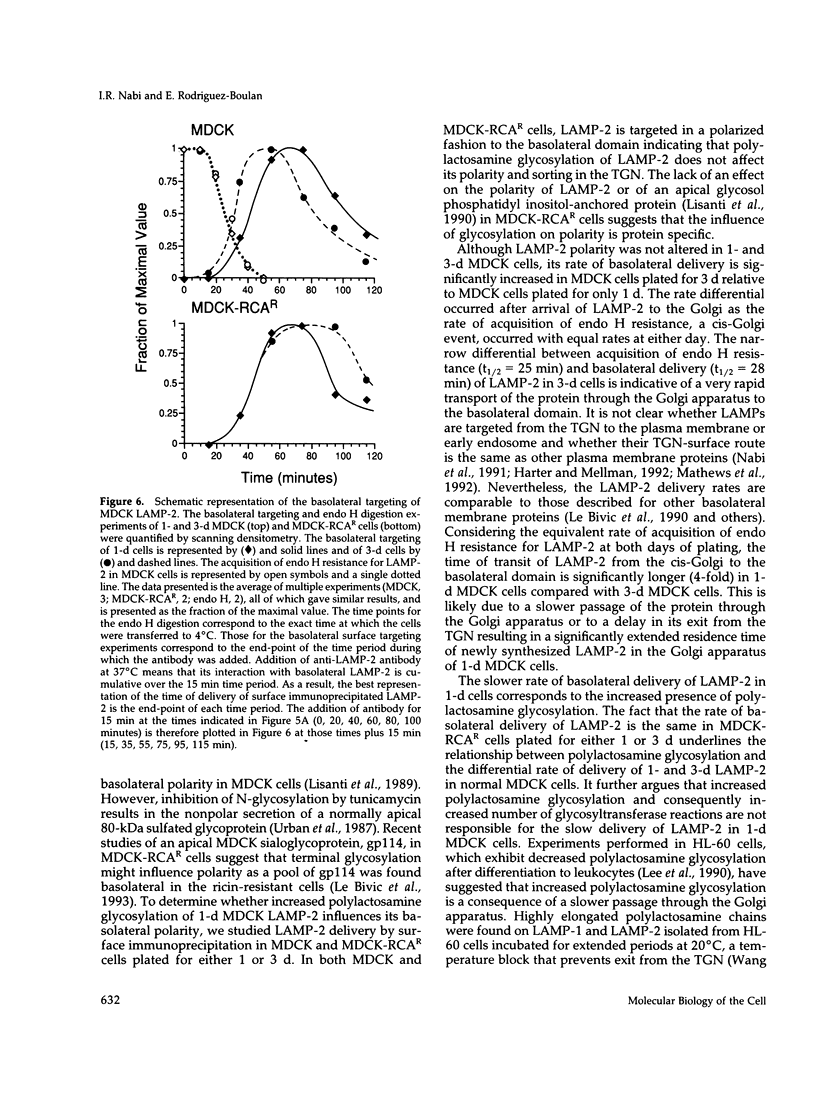
An endogenous Madin-Darby canine kidney (MDCK) lysosomal membrane glycoprotein that exhibits a basolateral targeting pathway to the lysosome is shown here to exhibit significant N-terminal amino acid sequence identity to lysosomal associated membrane proteins (LAMP-2) of other species. During establishment of the MDCK monolayer after only 1 d of culture, this canine LAMP-2 has a larger molecular size (110 kDa) than following formation of a confluent monolayer after 3 d of culture (100 kDa) due to the increased presence of N-linked polylactosamine oligosaccharide chains. Neither polylactosamine glycosylation of LAMP-2 in MDCK cells nor truncation of N-linked oligosaccharide chains of LAMP-2 in a ricin-resistant MDCK-RCAR cell line influenced the basolateral polarity of its targeting. However, the rate of basolateral delivery of LAMP-2 in MDCK cells plated for 3 d was significantly faster (t1/2 = 28 min) than in 1-d cells (t1/2 = 40 min); in MDCK-RCAR cells the rate of basolateral delivery at both 1 and 3 d of plating was similar (t1/2 = 40 min). The rate differential in MDCK cells occurred after arrival of LAMP-2 to the Golgi apparatus because the rate of acquisition of endoglycosidase H resistance was the same (t1/2 = 25 min) at both days of plating. The rate of transit of LAMP-2 through the Golgi apparatus to the basolateral domain was therefore far more rapid (approximately 4-fold) in 3 d compared with 1-d MDCK cultures. The increased polylactosamine glycosylation of MDCK LAMP-2 at early times of plating during the establishment of a confluent epithelial monolayer may thus be related to its longer residence time in the Golgi apparatus.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amos B., Lotan R. Modulation of lysosomal-associated membrane glycoproteins during retinoic acid-induced embryonal carcinoma cell differentiation. J Biol Chem. 1990 Nov 5;265(31):19192–19198. [PubMed] [Google Scholar]
- Bacallao R., Antony C., Dotti C., Karsenti E., Stelzer E. H., Simons K. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J Cell Biol. 1989 Dec;109(6 Pt 1):2817–2832. doi: 10.1083/jcb.109.6.2817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barriocanal J. G., Bonifacino J. S., Yuan L., Sandoval I. V. Biosynthesis, glycosylation, movement through the Golgi system, and transport to lysosomes by an N-linked carbohydrate-independent mechanism of three lysosomal integral membrane proteins. J Biol Chem. 1986 Dec 15;261(35):16755–16763. [PubMed] [Google Scholar]
- Brändli A. W., Hansson G. C., Rodriguez-Boulan E., Simons K. A polarized epithelial cell mutant deficient in translocation of UDP-galactose into the Golgi complex. J Biol Chem. 1988 Nov 5;263(31):16283–16290. [PubMed] [Google Scholar]
- Carlsson S. R., Fukuda M. The polylactosaminoglycans of human lysosomal membrane glycoproteins lamp-1 and lamp-2. Localization on the peptide backbones. J Biol Chem. 1990 Nov 25;265(33):20488–20495. [PubMed] [Google Scholar]
- Carlsson S. R., Roth J., Piller F., Fukuda M. Isolation and characterization of human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Major sialoglycoproteins carrying polylactosaminoglycan. J Biol Chem. 1988 Dec 15;263(35):18911–18919. [PubMed] [Google Scholar]
- Cha Y., Holland S. M., August J. T. The cDNA sequence of mouse LAMP-2. Evidence for two classes of lysosomal membrane glycoproteins. J Biol Chem. 1990 Mar 25;265(9):5008–5013. [PubMed] [Google Scholar]
- Cooper D. N., Massa S. M., Barondes S. H. Endogenous muscle lectin inhibits myoblast adhesion to laminin. J Cell Biol. 1991 Dec;115(5):1437–1448. doi: 10.1083/jcb.115.5.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. Characterization of the structural determinants required for the high affinity interaction of asparagine-linked oligosaccharides with immobilized Phaseolus vulgaris leukoagglutinating and erythroagglutinating lectins. J Biol Chem. 1982 Oct 10;257(19):11230–11234. [PubMed] [Google Scholar]
- Cummings R. D., Kornfeld S. The distribution of repeating [Gal beta 1,4GlcNAc beta 1,3] sequences in asparagine-linked oligosaccharides of the mouse lymphoma cell lines BW5147 and PHAR 2.1. J Biol Chem. 1984 May 25;259(10):6253–6260. [PubMed] [Google Scholar]
- Dennis J. W., Laferté S., Waghorne C., Breitman M. L., Kerbel R. S. Beta 1-6 branching of Asn-linked oligosaccharides is directly associated with metastasis. Science. 1987 May 1;236(4801):582–585. doi: 10.1126/science.2953071. [DOI] [PubMed] [Google Scholar]
- Do K. Y., Smith D. F., Cummings R. D. LAMP-1 in CHO cells is a primary carrier of poly-N-acetyllactosamine chains and is bound preferentially by a mammalian S-type lectin. Biochem Biophys Res Commun. 1990 Dec 31;173(3):1123–1128. doi: 10.1016/s0006-291x(05)80902-7. [DOI] [PubMed] [Google Scholar]
- Febbraio M., Silverstein R. L. Identification and characterization of LAMP-1 as an activation-dependent platelet surface glycoprotein. J Biol Chem. 1990 Oct 25;265(30):18531–18537. [PubMed] [Google Scholar]
- Feizi T. Carbohydrate differentiation antigens: probable ligands for cell adhesion molecules. Trends Biochem Sci. 1991 Mar;16(3):84–86. doi: 10.1016/0968-0004(91)90038-w. [DOI] [PubMed] [Google Scholar]
- Fukuda M. N., Watanabe K., Hakomori S. I. Release of oligosaccharides from various glycosphingolipids by endo-beta-galactosidase. J Biol Chem. 1978 Oct 10;253(19):6814–6819. [PubMed] [Google Scholar]
- Fukuda M. Cell surface glycoconjugates as onco-differentiation markers in hematopoietic cells. Biochim Biophys Acta. 1985;780(2):119–150. doi: 10.1016/0304-419x(84)90002-7. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Lysosomal membrane glycoproteins. Structure, biosynthesis, and intracellular trafficking. J Biol Chem. 1991 Nov 15;266(32):21327–21330. [PubMed] [Google Scholar]
- Fukuda M., Viitala J., Matteson J., Carlsson S. R. Cloning of cDNAs encoding human lysosomal membrane glycoproteins, h-lamp-1 and h-lamp-2. Comparison of their deduced amino acid sequences. J Biol Chem. 1988 Dec 15;263(35):18920–18928. [PubMed] [Google Scholar]
- Granger B. L., Green S. A., Gabel C. A., Howe C. L., Mellman I., Helenius A. Characterization and cloning of lgp110, a lysosomal membrane glycoprotein from mouse and rat cells. J Biol Chem. 1990 Jul 15;265(20):12036–12043. [PubMed] [Google Scholar]
- Griffiths G., Hoflack B., Simons K., Mellman I., Kornfeld S. The mannose 6-phosphate receptor and the biogenesis of lysosomes. Cell. 1988 Feb 12;52(3):329–341. doi: 10.1016/s0092-8674(88)80026-6. [DOI] [PubMed] [Google Scholar]
- Harter C., Mellman I. Transport of the lysosomal membrane glycoprotein lgp120 (lgp-A) to lysosomes does not require appearance on the plasma membrane. J Cell Biol. 1992 Apr;117(2):311–325. doi: 10.1083/jcb.117.2.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan M., Yousefi S., Dennis J. W. Molecular characterization of P2B/LAMP-1, a major protein target of a metastasis-associated oligosaccharide structure. Cancer Res. 1989 Nov 1;49(21):6077–6084. [PubMed] [Google Scholar]
- Kaplan H. A., Welply J. K., Lennarz W. J. Oligosaccharyl transferase: the central enzyme in the pathway of glycoprotein assembly. Biochim Biophys Acta. 1987 Jun 24;906(2):161–173. doi: 10.1016/0304-4157(87)90010-4. [DOI] [PubMed] [Google Scholar]
- Kornfeld S., Mellman I. The biogenesis of lysosomes. Annu Rev Cell Biol. 1989;5:483–525. doi: 10.1146/annurev.cb.05.110189.002411. [DOI] [PubMed] [Google Scholar]
- Le Bivic A., Garcia M., Rodriguez-Boulan E. Ricin-resistant Madin-Darby canine kidney cells missort a major endogenous apical sialoglycoprotein. J Biol Chem. 1993 Apr 5;268(10):6909–6916. [PubMed] [Google Scholar]
- Le Bivic A., Sambuy Y., Mostov K., Rodriguez-Boulan E. Vectorial targeting of an endogenous apical membrane sialoglycoprotein and uvomorulin in MDCK cells. J Cell Biol. 1990 May;110(5):1533–1539. doi: 10.1083/jcb.110.5.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee N., Wang W. C., Fukuda M. Granulocytic differentiation of HL-60 cells is associated with increase of poly-N-acetyllactosamine in Asn-linked oligosaccharides attached to human lysosomal membrane glycoproteins. J Biol Chem. 1990 Nov 25;265(33):20476–20487. [PubMed] [Google Scholar]
- Leffler H., Barondes S. H. Specificity of binding of three soluble rat lung lectins to substituted and unsubstituted mammalian beta-galactosides. J Biol Chem. 1986 Aug 5;261(22):10119–10126. [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Cycling of the integral membrane glycoprotein, LEP100, between plasma membrane and lysosomes: kinetic and morphological analysis. Cell. 1987 Jun 5;49(5):669–677. doi: 10.1016/0092-8674(87)90543-5. [DOI] [PubMed] [Google Scholar]
- Lippincott-Schwartz J., Fambrough D. M. Lysosomal membrane dynamics: structure and interorganellar movement of a major lysosomal membrane glycoprotein. J Cell Biol. 1986 May;102(5):1593–1605. doi: 10.1083/jcb.102.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Le Bivic A., Saltiel A. R., Rodriguez-Boulan E. Preferred apical distribution of glycosyl-phosphatidylinositol (GPI) anchored proteins: a highly conserved feature of the polarized epithelial cell phenotype. J Membr Biol. 1990 Feb;113(2):155–167. doi: 10.1007/BF01872889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisanti M. P., Le Bivic A., Sargiacomo M., Rodriguez-Boulan E. Steady-state distribution and biogenesis of endogenous Madin-Darby canine kidney glycoproteins: evidence for intracellular sorting and polarized cell surface delivery. J Cell Biol. 1989 Nov;109(5):2117–2127. doi: 10.1083/jcb.109.5.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotan R., Lotan D., Carralero D. M. Modulation of galactoside-binding lectins in tumor cells by differentiation-inducing agents. Cancer Lett. 1989 Nov 30;48(2):115–122. doi: 10.1016/0304-3835(89)90046-3. [DOI] [PubMed] [Google Scholar]
- Mane S. M., Marzella L., Bainton D. F., Holt V. K., Cha Y., Hildreth J. E., August J. T. Purification and characterization of human lysosomal membrane glycoproteins. Arch Biochem Biophys. 1989 Jan;268(1):360–378. doi: 10.1016/0003-9861(89)90597-3. [DOI] [PubMed] [Google Scholar]
- Mathews P. M., Martinie J. B., Fambrough D. M. The pathway and targeting signal for delivery of the integral membrane glycoprotein LEP100 to lysosomes. J Cell Biol. 1992 Sep;118(5):1027–1040. doi: 10.1083/jcb.118.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meiss H. K., Green R. F., Rodriguez-Boulan E. J. Lectin-resistant mutants of polarized epithelial cells. Mol Cell Biol. 1982 Oct;2(10):1287–1294. doi: 10.1128/mcb.2.10.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., Simons K. The Golgi complex: in vitro veritas? Cell. 1992 Mar 6;68(5):829–840. doi: 10.1016/0092-8674(92)90027-A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Asparagine-linked oligosaccharides containing poly-N-acetyllactosamine chains are preferentially bound by immobilized calf heart agglutinin. J Biol Chem. 1988 Nov 5;263(31):16143–16149. [PubMed] [Google Scholar]
- Merkle R. K., Cummings R. D. Relationship of the terminal sequences to the length of poly-N-acetyllactosamine chains in asparagine-linked oligosaccharides from the mouse lymphoma cell line BW5147. Immobilized tomato lectin interacts with high affinity with glycopeptides containing long poly-N-acetyllactosamine chains. J Biol Chem. 1987 Jun 15;262(17):8179–8189. [PubMed] [Google Scholar]
- Mostov K., Apodaca G., Aroeti B., Okamoto C. Plasma membrane protein sorting in polarized epithelial cells. J Cell Biol. 1992 Feb;116(3):577–583. doi: 10.1083/jcb.116.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Le Bivic A., Fambrough D., Rodriguez-Boulan E. An endogenous MDCK lysosomal membrane glycoprotein is targeted basolaterally before delivery to lysosomes. J Cell Biol. 1991 Dec;115(6):1573–1584. doi: 10.1083/jcb.115.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabi I. R., Raz A. Cell shape modulation alters glycosylation of a metastatic melanoma cell-surface antigen. Int J Cancer. 1987 Sep 15;40(3):396–402. doi: 10.1002/ijc.2910400319. [DOI] [PubMed] [Google Scholar]
- Ochieng J., Gerold M., Raz A. Dichotomy in the laminin-binding properties of soluble and membrane-bound human galactoside-binding protein. Biochem Biophys Res Commun. 1992 Aug 14;186(3):1674–1680. doi: 10.1016/s0006-291x(05)81601-8. [DOI] [PubMed] [Google Scholar]
- Pfeffer S. R., Rothman J. E. Biosynthetic protein transport and sorting by the endoplasmic reticulum and Golgi. Annu Rev Biochem. 1987;56:829–852. doi: 10.1146/annurev.bi.56.070187.004145. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Boulan E., Nelson W. J. Morphogenesis of the polarized epithelial cell phenotype. Science. 1989 Aug 18;245(4919):718–725. doi: 10.1126/science.2672330. [DOI] [PubMed] [Google Scholar]
- Saitoh O., Wang W. C., Lotan R., Fukuda M. Differential glycosylation and cell surface expression of lysosomal membrane glycoproteins in sublines of a human colon cancer exhibiting distinct metastatic potentials. J Biol Chem. 1992 Mar 15;267(8):5700–5711. [PubMed] [Google Scholar]
- Sasaki H., Bothner B., Dell A., Fukuda M. Carbohydrate structure of erythropoietin expressed in Chinese hamster ovary cells by a human erythropoietin cDNA. J Biol Chem. 1987 Sep 5;262(25):12059–12076. [PubMed] [Google Scholar]
- Sato S., Hughes R. C. Binding specificity of a baby hamster kidney lectin for H type I and II chains, polylactosamine glycans, and appropriately glycosylated forms of laminin and fibronectin. J Biol Chem. 1992 Apr 5;267(10):6983–6990. [PubMed] [Google Scholar]
- Stults N. L., Asta L. M., Lee Y. C. Immobilization of proteins on oxidized crosslinked Sepharose preparations by reductive amination. Anal Biochem. 1989 Jul;180(1):114–119. doi: 10.1016/0003-2697(89)90097-3. [DOI] [PubMed] [Google Scholar]
- Stults N. L., Fechheimer M., Cummings R. D. Relationship between Golgi architecture and glycoprotein biosynthesis and transport in Chinese hamster ovary cells. J Biol Chem. 1989 Nov 25;264(33):19956–19966. [PubMed] [Google Scholar]
- Urban J., Parczyk K., Leutz A., Kayne M., Kondor-Koch C. Constitutive apical secretion of an 80-kD sulfated glycoprotein complex in the polarized epithelial Madin-Darby canine kidney cell line. J Cell Biol. 1987 Dec;105(6 Pt 1):2735–2743. doi: 10.1083/jcb.105.6.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W. C., Lee N., Aoki D., Fukuda M. N., Fukuda M. The poly-N-acetyllactosamines attached to lysosomal membrane glycoproteins are increased by the prolonged association with the Golgi complex. J Biol Chem. 1991 Dec 5;266(34):23185–23190. [PubMed] [Google Scholar]
- Woo H. J., Shaw L. M., Messier J. M., Mercurio A. M. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem. 1990 May 5;265(13):7097–7099. [PubMed] [Google Scholar]
- Youakim A., Romero P. A., Yee K., Carlsson S. R., Fukuda M., Herscovics A. Decrease in polylactosaminoglycans associated with lysosomal membrane glycoproteins during differentiation of CaCo-2 human colonic adenocarcinoma cells. Cancer Res. 1989 Dec 15;49(24 Pt 1):6889–6895. [PubMed] [Google Scholar]
- Yousefi S., Higgins E., Daoling Z., Pollex-Krüger A., Hindsgaul O., Dennis J. W. Increased UDP-GlcNAc:Gal beta 1-3GaLNAc-R (GlcNAc to GaLNAc) beta-1, 6-N-acetylglucosaminyltransferase activity in metastatic murine tumor cell lines. Control of polylactosamine synthesis. J Biol Chem. 1991 Jan 25;266(3):1772–1782. [PubMed] [Google Scholar]
- Zhou Q., Cummings R. D. The S-type lectin from calf heart tissue binds selectively to the carbohydrate chains of laminin. Arch Biochem Biophys. 1990 Aug 15;281(1):27–35. doi: 10.1016/0003-9861(90)90408-q. [DOI] [PubMed] [Google Scholar]
- van den Eijnden D. H., Koenderman A. H., Schiphorst W. E. Biosynthesis of blood group i-active polylactosaminoglycans. Partial purification and properties of an UDP-GlcNAc:N-acetyllactosaminide beta 1----3-N-acetylglucosaminyltransferase from Novikoff tumor cell ascites fluid. J Biol Chem. 1988 Sep 5;263(25):12461–12471. [PubMed] [Google Scholar]