Abstract
Mycoplasma pneumoniae (Mp), a common cause of pneumonia, is associated with asthma; however, the mechanisms underlying this association remain unclear. We investigated the cellular immune response to Mp in mice. Intranasal inoculation with Mp elicited infiltration of the lungs with neutrophils, monocytes and macrophages. Systemic depletion of macrophages, but not neutrophils, resulted in impaired clearance of Mp from the lungs. Accumulation and activation of macrophages were decreased in the lungs of MyD88−/− mice and clearance of Mp was impaired, indicating that MyD88 is a key signaling protein in the anti-Mp response. MyD88-dependent signaling was also required for the Mp-induced activation of NFκB, which was essential for macrophages to eliminate the microbe in vitro. Thus, MyD88-NFκB signaling in macrophages is essential for clearance of Mp from the lungs.
Introduction
Asthma is a chronic inflammatory disease of the airways, driven by Th2 lymphocytes and the type II cytokines IL-4, IL-5 and IL-13. These signals lead to the recruitment of neutrophils, monocytes, macrophages, lymphocytes, eosinophils and mast cells into the lung tissue and airway lumen. Infiltration of these cells into the lungs is associated with high-level production of airway mucus and the development of airway hyper-reactivity [1].
Typical of most complex diseases, asthma susceptibility appears to be multifactorial, including contributions by several genes and multiple environmental factors. Genetic susceptibility involves genes encoding functionally and structurally defined families of molecules that are thought to determine risk of both atopy and asthma. At least three groups of related genes have shown linkage to asthma susceptibility: genes that govern innate immune responses to environmental threats (CD14, TLR2, TLR4, TLR6, NOD1 and NOD2); genes involved in differentiation and activation of Th2 cells (IL-4, IL-13, IL-4R and GATA3); and genes that activate a broad range of inflammatory functions including recruitment of leukocytes to epithelial and endothelial surfaces (TNF, LTα, CCL5, CCL24 and CCL26) [2].
In addition to genetic susceptibility, environmental factors, including microbes, allergens and drugs, appear to contribute either to the induction or to increased severity of existing asthma [3]. One of the asthma-associated pathogens is Mycoplasma pneumoniae (Mp) [4], [5], [6]. In 1998, Kraft and colleagues showed, using PCR analysis of bronchoalveolar lavage (BAL) fluid, that more than 40% of patients with chronic stable asthma, compared to less than 10% of healthy control subjects, were positive for Mp in their airways [7]. Antibiotic treatment reduced the severity of asthma symptoms and improved pulmonary function in the subset of asthmatic patients whose BAL fluids or endobronchial biopsies were positive for Mp, whereas asthmatic patients who were Mp negative showed no improvement with antibiotic treatment [8]. Although not all studies have detected a higher rate of recovery of Mp from the airways of asthmatic subjects compared to controls [9], these combined observations suggest that the presence of microbes such as Mp in the airways may contribute to exacerbation of asthma symptoms in many populations.
Mycoplasmas are the smallest known self-replicating microorganisms. They are primarily mucosal pathogens, residing extracellularly in close association with epithelial surfaces. Mp is a human pathogen that typically infects ciliated epithelial cells in the respiratory tract, producing upper and lower airway infection [10]. Because Mp lacks all of the genes involved in amino acid synthesis, it is dependent on an exogenous supply of amino acids from its environment. Thus, Mp depends on a close association with host cells for its survival. The importance of this close association is underscored by the essential role of the Mp attachment organelle for microbial virulence. This specialized structure, located at the leading end of the bacterium, mediates close physical interactions between Mp and host epithelial cells. The attachment organelle of Mp is also essential for cell division and gliding motility [11]. The P1 and P30 adhesins, two critical components of this attachment organelle, play fundamental roles in the interactions of Mp with host airway epithelial cells and are required for virulence [10], [12], [13], [14].
Pattern recognition receptors (PRR) are essential for innate immunity to invading pathogens. One of the best-characterized groups of PRR is the family of Toll-like receptors (TLR). Signal transduction in the TLR pathways is mediated by four activating adaptors, including myeloid differentiation factor 88 (MyD88), TIR domain-containing adaptor protein (TIRAP, also called MyD88 adaptor-like (MAL)), TIR domain-containing adaptor inducing interferon-β (TRIF, also known as toll-IL-1 receptor adaptor molecule 1 (TICAM-1)), and TRIF-related adaptor molecule (TRAM, also named toll-like receptor adaptor molecule 2 (TICAM-2)) [15], [16]. MyD88 serves as the major adaptor molecule for all TLRs, except TLR3. TLR signals transduced through MyD88 ultimately activate NFκB and several of the interferon regulatory factors, all of which are transcription factors that regulate the expression of downstream genes required for TLR-induced cell activation. These downstream genes encode MHC molecules, T cell costimulatory molecules, cytokines, chemokines and their receptors, and many other activation associated genes [15], [17], which are critical for immune cells to respond to and eliminate invading pathogens.
Recent studies using experimental infection of mice have shown that IL-12 modulates the host response to Mp [18] and have established that signaling by TLR2 is required for the normal increased production of mucus by Mp-challenged airway epithelial cells [18], [19]. Nevertheless, the cellular mechanisms used by the host to control Mp in vivo remain largely undefined.
For this study, we developed a sensitive, specific and rapid method to detect Mp quantitatively in mouse lungs by using real-time PCR and verified that this assay detected similar numbers of Mp in the lungs of experimentally infected mice compared to quantitation by colony count of Mp recovered from minced lung tissue. Using this method, we focused on the cellular and molecular mechanisms used by the host to clear Mp from the lungs in an experimental mouse model. We show that macrophages, but not neutrophils, play a critical role in clearance of Mp from the lungs of mice. Additionally, we demonstrate an essential role for MyD88-NFκB signaling in the macrophage response to Mp in vivo and in vitro. This signaling pathway is required both for activation of macrophages in the lungs and for elimination of Mp both from the airways and from cultures of bone marrow-derived macrophages.
Results
Quantitative detection of Mp in the lungs of mice
Mp can be cultured on SP4 agar, but replicates slowly. Because of its lack of a cell wall and limited capacity to synthesize many important biomolecules, Mp is very sensitive to changes in its microenvironment. In addition, recent studies suggest that Mp may be a facultative intracellular pathogen, from which compartment it may be difficult to culture or affect with certain classes of antibiotics [20]. To quantify Mp rapidly from infected tissues, we developed a real-time PCR-based method for measuring the numbers of Mp in extracts of mouse lung.
For the real-time PCR assay we targeted the Mp 16S rRNA, because portions of this RNA are unique for each of the known mycoplasma strains and because the RNA is present in high copy number in the microbe. The assay is focused on the variable 5′ end region of the Mp 16S rRNA. This portion of the Mp 16S rRNA gene has been used as a target for a DNA-based semi-quantitative PCR assay in the past [21], but not using the RNA-based real-time PCR technology. We used the murine housekeeping gene, GAPDH, as an internal control for normalization of the amount of lung tissue represented in each RNA preparation.
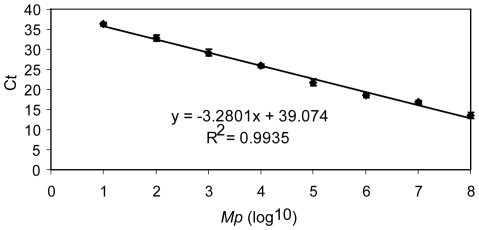
In order to generate a standard curve for numbers of Mp, total RNA was purified from the lungs of one non-infected mouse, and RNA from the indicated numbers (1 to 108) of Mp was added. 100 ng of total RNA was used for each real-time PCR reaction. Over a range of <102 to 108 mycoplasmas per lung, this assay showed a linear relationship between the numbers of Mp (x-axis, log10) and the threshold number of cycles (y-axis, Ct) (Figure 1). Thus, this assay provides a detection sensitivity of <100 Mp in the lungs of an individual mouse. In order to assess the degree to which real-time PCR and bacterial culture with colony count yield comparable data, we analyzed Mp numbers in the lungs of mice using both techniques in the same experiment. Our data showed that real-time PCR and conventional culture and colony count provided very similar numbers of Mp in the lungs of infected mice at d1, d3, and d5 after inoculation with Mp (Figure S1). Because the real-time PCR assay is more rapid and equally sensitive compared to bacterial culture, we have used it for the remainder of our studies.
Figure 1. Quantitative detection of Mp in mouse lung by real-time PCR.
A portion of the 16S rRNA (from nucleotides 201 to 265) that is specific for Mp was chosen as the molecular target for detection. Total RNA was purified from the lungs of one mouse, and RNA from the indicated number of Mp was added. 100 ng of total RNA was used for each real-time PCR reaction. Ct, cycle threshold. The sensitivity and linearity of the assay were confirmed in one additional experiment. Data shown are means ± SEM.
Time course for the clearance of Mp from the lungs of C57BL6 and BALB/c mice
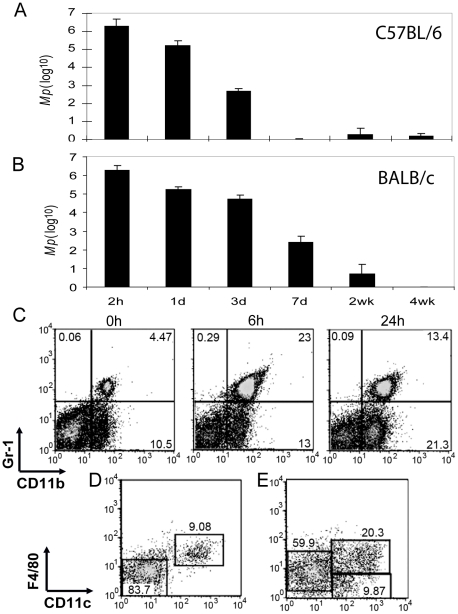
Mice were inoculated i.n. with 4×106 Mp on d0, and were sacrificed 2 h, 1 d, 3 d, 7 d, 2 wk, and 4 wk later. Based on an analysis of the numbers of Mp using the real-time PCR assay, Mp were cleared from the lungs of C57BL/6 mice within 7 days post-inoculation (Figure 2A); in BALB/c mice, the clearance of Mp from the lungs was reproducibly slower, reaching numbers below the level of detection of the assay by 4 weeks indicating that there are genetic background effects on Mp clearance (Figure 2B). To eliminate this type of variability, for the remainder of our study we used C57BL/6 as the wild type (WT) mouse strain. Importantly, in both strains of mice, 90% or more of the Mp were eliminated from the lungs over the first day (Figures 2A and 2B). This rapid, early clearance of Mp suggested that innate host responses play a major role in the elimination of Mp from the lungs of mice. We tested the impact of varying the inoculating dose of bacteria over a range from 4×105 to 4×107 cfu/animal. At days 2 and 3 after inoculation, the numbers of Mp recovered from the lungs were in proportion to the infectious dose, indicating that the ability of these innate responses to handle the bacterial load were not saturated at this level of challenge (data not shown).
Figure 2. Host responses to Mp.
Mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. Time course of Mp clearance from lungs of C57BL/6 mice (A) and BALB/c mice (B). For examining the cellular host response to respiratory Mp, whole lungs were digested into single cell suspensions and stained with Abs for FACS analysis. (C) At 6 h and 24 h after infection with Mp, CD11b+Gr-1hi and CD11b+Gr-1lo/− cell populations were recruited in the lungs. (D) Within the CD11b+Gr-1hi population of cells analyzed 24 h post-Mp infection, the major cell types were neutrophils (CD11c−F4/80−) and a small subset of pulmonary macrophages (CD11c+F4/80+). (E) The CD11b+Gr-1lo/− cell population includes pulmonary macrophages (CD11c+F4/80+), macrophage/monocytes (CD11c−F4/80lo/−) and DC (CD11c+F4/80− ). Each experiment was replicated at least two times. Data shown are means ± SEM (n = 6).
Consistent with this, there were no significant differences in the rate of clearance of Mp from the lungs of WT mice and Rag1−/− mice, which lack T and B lymphocyte-based adaptive immune responses [22] (Figure S2). These data support the central importance of the innate immune response in the elimination of Mp from the lungs of mice.
Rapid recruitment of neutrophils and macrophages/monocytes to the mouse lung following inoculation with Mp
To investigate the role of innate cells in the clearance of Mp, we first determined the nature of the host's cellular inflammatory response following airway inoculation with the microbe. Leukocytes that can be recovered from the lungs of microbe-challenged animals consist both of resident populations of innate cells and additional cells recruited from the periphery in response to the potential pathogen. The resident cell populations include macrophages, monocytes, dendritic cells (DC), NK cells, mast cells, neutrophils and other granulocytes, all cell types that can respond to invading pathogens.
Following i.n. inoculation with Mp, there was a rapid and substantial increase in the numbers of CD11b+ cells in the lungs (Figure 2C). Based on their staining with anti-Gr-1, these CD11b+ cells comprised two subpopulations, CD11b+Gr-1hi and CD11b+Gr-1lo/−. By 6 hr, the CD11b+Gr-1hi cells were dramatically increased from <5% of total lung leukocytes to >20% of total lung leukocytes. This population remained elevated at >10% of total lung cells at 24 h after inoculation with Mp (Figure 2C). The CD11b+Gr-1lo/− cells were increased from 10.5% of total lung cells in control animals to 13% of total cells 6 hr after inoculation with Mp, and to >20% by 24 h after microbial challenge (Figure 2C). The numbers of both of these two cell populations had returned to the baseline by 2–4 days after inoculation with Mp (data not shown).
Each of these CD11b+ cell populations could be further fractionated based on their surface expression of F4/80 (typically expressed on macrophages) and CD11c (typically expressed on DC and pulmonary macrophages). The largest cellular subpopulation, representing approximately 80–90% of the cells in the CD11b+Gr-1hi gate, did not stain with antibodies to CD11c or F4/80 (Figure 2D), and expressed Ly6G, but not Ly6C (data not shown). Based on this pattern of cell surface marker expression, this subset has phenotypic characteristics typical of neutrophils [23]. A minority subpopulation in the CD11b+Gr-1hi gate expressed CD11c and F4/80 (Figure 2D), typical of pulmonary macrophages [24].
Analysis of cells within the CD11b+Gr-1lo/− gate for expression of CD11c and F4/80 discriminated three subpopulations (Figure 2E). Cells that were CD11b+Gr-1lo/−CD11c−F4/80lo/− were monocytes/macrophages, whereas cells that were CD11b+Gr-1lo/−CD11c+F4/80+ were activated pulmonary macrophages. The minority subpopulation that was CD11b+Gr-1lo/− CD11c+F4/80− consisted primarily of myeloid-type DC [25], [26], [27].
Altogether, these data demonstrated that both neutrophils (CD11b+Gr-1hiCD11c−F4/80−) and monocytes/macrophages (CD11b+Gr-1lo/−CD11c+/−F4/80+/lo or CD11b+Gr-1hiCD11c+F4/80+) were recruited into the lungs within the first 24 h after i.n. instillation of Mp, during the period when 90% or more of the bacteria were cleared from the lungs (Figures 1, 2C, 2D, and 2E).
Clearance of Mp from the airways of mice is insensitive to depletion of neutrophils
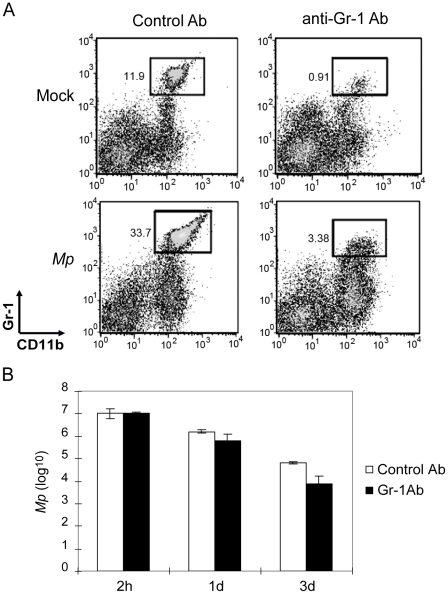
Because neutrophils were recruited rapidly and in high numbers into the lungs of mice following i.n. inoculation with Mp (Figures 2C and 2D), we tested whether this cell population contributed to the clearance of the organism from the lungs. We induced systemic depletion of neutrophils by treatment of adult WT mice with i.v. injection of 100 µg of an anti-Gr-1 mAb (clone RB6-8C5) prior to i.n. inoculation with Mp. Two days after injection with RB6-8C5, the depletion of CD11b+Gr-1hi cells in both the blood and the lungs was greater than 99% [28]. There was no significant depletion of Gr-1lo populations in either blood or lungs [28]. One day after inoculation with Mp, depletion of neutrophils remained at a high level in the anti-Gr-1 Ab-treated mice, with only 10% as many lung neutrophils as observed in microbe challenged mice that had received the control mAb (Figure 3A).
Figure 3. Neutrophils are not required for clearance of Mp from mouse lung.
(A) Greater than 90% of the CD11b+Gr-1hi cells (mainly neutrophils) in mouse lung were depleted by i.v. injection with anti-Gr-1 Ab (RB6-8C5) with or without Mp infection. Data shown were collected 3 d after injection of the anti-Gr-1 Ab, and 1 d after inoculation i.n. with Mp. (B) Neutrophil depleted mice (filled bars) showed no significant difference in clearance of Mp from the lungs at all indicated times post-Mp inoculation, compared to control mice (open bars). At least three similar experiments were performed with comparable results. Data shown are means ± SEM (n = 4 or 5). P>0.05, comparing control and Gr-1 Ab injected mice at each of the time points.
Neutrophil-depleted mice cleared Mp from their lungs at a rate equal to or modestly faster than mice treated with the control Ab (Figure 3B). In order to confirm that neutrophils are not essential for the clearance of Mp from the lungs, we tested CD11b−/− mice, which have morphologically abnormal neutrophils, and impaired neutrophil recruitment, activation, and apoptosis [29], [30]. As with mice treated with the RB6-8C5 mAb, we found no difference in the clearance of Mp from lungs of CD11b−/− mice compared to WT control animals (Figure S3). Together, these data indicate that neutrophils do not play an essential role for elimination of Mp from the lungs of naïve mice.
Systemic depletion of macrophages impairs the elimination of Mp from the lungs
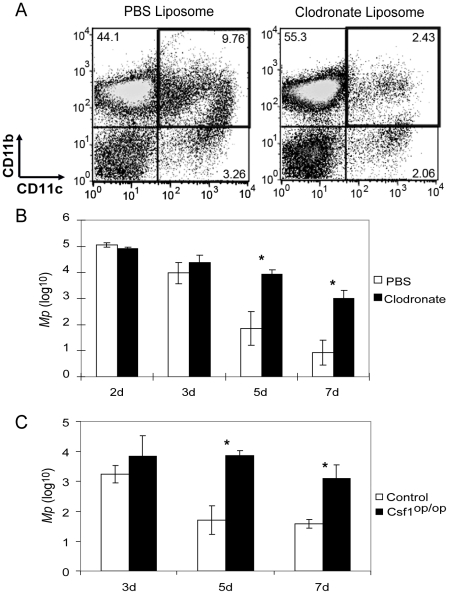
To test the role of macrophages in the host responses to Mp, we depleted macrophages systemically by injection of clodronate-containing liposomes both i.p. and i.n. Injection of clodronate liposomes by both the i.n. and the i.p. routes was required to obtain effective depletion of both resident pulmonary (CD11chiCD11blo) and recruited and interstitial (CD11cloCD11bhi) monocyte/macrophages following microbial challenge (Figure S4). One day after liposome injection, mice were inoculated i.n. with Mp. In animals that had been treated with the control liposomes, the percentage of CD11b+CD11c+ cells (mainly activated resident macrophages and recruited monocyte/macrophages, and a small subset of DC) was 9.76%. This cell population was substantially reduced (to <2.5%) in the lungs of mice that had been treated with clodronate liposomes prior to i.n. challenge with Mp (Figure 4A). This greater than 70% depletion in lung macrophages was associated with impaired clearance of Mp from the lungs. Numbers of Mp were not significantly different at days 2 and 3 after i.n. microbial challenge; however, at d 5 and d 7 after inoculation, there were several hundred-fold more Mp in the lungs of the clodronate liposome-treated mice compared to mice treated with the control liposomes (Figure 4B). These data show that, while macrophages are not essential for the initial elimination of Mp from lungs of mice observed in the first 3 days after microbial challenge of the airway, macrophages are essential for the final elimination of the microbe after d 3. Interestingly, varying the inoculating dose of Mp over a 100-fold range did not alter the ability of WT mice to clear the bacteria by d 5 (data not shown), suggesting that the ability of the respiratory tract macrophages to clear Mp remaining at these late time points is relatively independent of the initial bacterial load.
Figure 4. Macrophages play an essential role in the elimination of Mp from the lungs of mice.
(A) Greater than 70% of the pool of CD11c+CD11b+ cells (mainly macrophages) in the lungs of Mp-challenged mice was depleted by injection (i.p. + i.n.) with clodronate-containing liposomes (3 d after injection with liposomes, 1 d after inoculation with Mp). (B) and (C) Macrophage-depleted mice (filled bars) (B) and CSF1op/op mice (filled bars) (C) showed impaired clearance of Mp from the lungs at the indicated times post-Mp inoculation, compared to control mice (open bars). For clodronate depletion of macrophages, at least 5 experiments were performed with similar results. For CSF1op/op mice, the experiment was repeated once with similar results. Data shown are means ± SEM (n = 3 or 5). * p<0.05.
To confirm the importance of macrophages in the clearance of Mp, we also tested clearance from the lungs of Csf1op/op mice, which manifest reduced numbers of macrophages and monocytes [31] because of a mutation in CSF1 (also designated M-CSF). The numbers of Mp recovered from the lungs of Csf1op/op mice at 3 d after i.n. inoculation were not statistically different from the numbers recovered from control mice (either Csf1op/+ or Csf1+/+ mice) (Figure 4C); however, at d 5 and d 7 post-inoculation, there were 10- to 100-fold greater numbers of Mp in the lungs of Csf1op/op mice compared to control animals (Figure 4C). Thus, when the numbers of host macrophages are reduced either due to a congenital deficiency in the production of these cells (CSF1op/op mice) or by depletion using clodronate liposomes, then the terminal phase of elimination of Mp is impaired.
Phagocytosis of Mp in vitro by macrophages
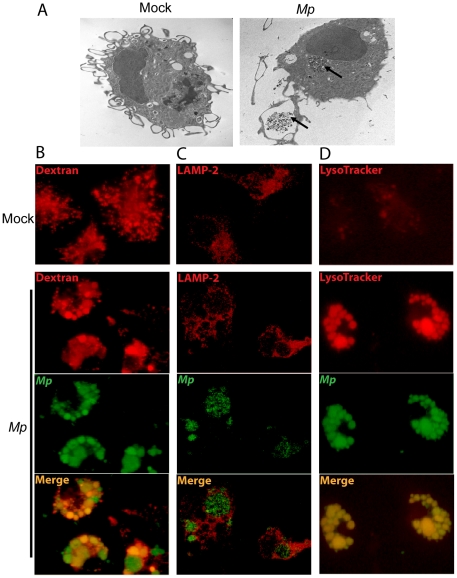
Having established the importance of macrophages in the elimination of Mp from the lungs and airways, we next investigated how macrophages respond when cultured with the microbe in vitro. In order to visualize the encounter of macrophages with the microbe, we co-cultured Mp with adherent bone marrow-derived macrophages (BMM) from WT mice at a ratio of 100∶1 for 1 h, washed the adherent cells with PBS, and analyzed the cells by transmission electron microscopy. Cells cultured with Mp showed large clusters of the microbe in phagocytic vacuoles (Figure 5A, upper arrow) as well as clusters of bacteria apparently in the early phases of cellular uptake (Figure 5A, lower arrow). To analyze the role of phagocytosis in greater detail, we stained the cells with reagents specific for endosomes or lysosomes and with the LysoTracker dye, then analyzed the cells by fluorescence microscopy. To test for phagosome-endosome fusion, we preloaded the endosomes of BMM with fluorophore-conjugated dextran [32] and then incubated with Mp carrying a gene encoding enhanced yellow fluorescent protein (EYFP-Mp; see Materials and Methods) for 1 hr (Figure 5B). This experiment demonstrated co-localization of dextran with the majority of Mp-containing cell organelles, indicating that phagosome-endosome fusion had occurred.
Figure 5. Macrophage phagocytosis of Mp in vitro.
(A) BMM were infected with Mp for 1 h, and the cells were fixed with 2% paraformaldehyde and 2% glutaraldehyde prior to embedding for TEM. Internalized Mp are highlighted by the black arrows. BMM were pre-loaded with dextran to label endosomes (B), and stained with anti-LAMP-2 antibody to visualize lysosomes (C) or LysoTracker dye to visualize acidified compartments (D). (B-D) cultures were infected with Mp (MOI 100∶1) or mock infected with medium alone as indicated. All portions of these experiments were repeated at least two times, always with similar results.
To further examine the process of phagosome maturation, we stained the cells with the lysosome-specific marker LAMP-2 (Figure 5C). The addition of Mp to the cultured cells resulted in enhanced localization of LAMP-2 in close approximation to vacuoles that contained abundant Mp, showing the localization of Mp with a phago-lysosomal compartment. To investigate whether the Mp-containing vesicles were acidified, we cultured BMM with EYFP-Mp and then stained the cells with LysoTracker Red, an indicator of phagolysosome acidification (Figure 5D). Addition of Mp to the cultured BMM resulted in dramatically enhanced LysoTracker-dependent staining of phagolysosomal vacuoles, with co-localization of the intracellular Mp and the acid indicator dye. Thus, as early as 1 h after infection, Mp had been taken up by macrophages into a phagosomal compartment that fused with lysosomal elements to form an acidified phagolysosome.
Cellular adhesive interaction is required for persistence of Mp in the murine respiratory tract but not for macrophage-dependent Mp clearance
To understand further the molecular mechanisms by which macrophages recognize invading Mp, we tested the importance of adhesive interactions for clearance of the microbe. The mycoplasmal P1 adhesin is an essential component of the mycoplasma attachment organelle by which the microbe adheres to the surface of host epithelial cells in the respiratory tract [12]. P1-deficient Mp loses its ability to attach to the host airway epithelium and expresses greatly reduced virulence [33]. This is manifested by accelerated clearance of P1-deficient Mp from the respiratory tract of WT mice (Figure S5). To test whether macrophages are required for clearance of P1-deficient Mp, we inoculated macrophage-depleted mice with P1-deficient Mp and determined the numbers of Mp present in the lungs at various subsequent time points. Depletion of macrophages resulted in prolongation of the persistence of the P1-deficient Mp, but they were still cleared more rapidly than WT Mp (Figure S5). Thus, P1-mediated interactions between Mp and the responding macrophage are not required for macrophage-dependent clearance of the microbe from the lungs and airways. These data suggest that, while P1-dependent Mp-host cell interactions contribute importantly to the ability of the microbe to persist in the respiratory tract, non-P1-dependent mechanisms are central to the interactions of Mp with host macrophages. We hypothesized, consequently, that macrophage PRR might contribute importantly to this response.
Signaling through the adaptor protein MyD88 is essential for the macrophage response to Mp in the lungs
The TLR family is one of the best-characterized families of PRR. It has been shown to play a key role in the triggering of both the innate and the adaptive arms of the immune response [15], and serves as a bridge between innate and adaptive immunity [34], [35], [36]. MyD88 is the major adaptor molecule for signaling downstream of TLR [37]. Therefore, we tested the role of TLR-MyD88 signaling in the clearance of Mp from the lungs of mice.
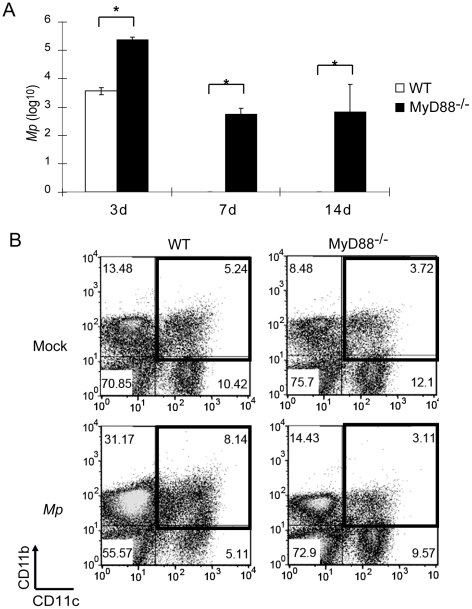
Three days after inoculation with 4×106 Mp, there were 105 Mp remaining in the lungs of MyD88−/− mice, compared to 103 Mp in lungs of WT mice (Figure 6A). Seven days and 14 days after inoculation with Mp, the microbe was undetectable in the lungs of WT mice; in contrast, 102-103 Mp were detected in the lungs of MyD88−/− mice (Figure 6A). This significant impairment in the elimination of Mp from the lungs of MyD88−/− mice indicates that the signaling adaptor MyD88 is essential for the elimination of Mp from the murine respiratory tract and suggests that TLR signaling plays an important role in this process.
Figure 6. The macrophage response to Mp in mouse lung is MyD88-dependent.
WT and MyD88−/− mice were inoculated with Mp, and (A) the survival of Mp in the lungs was determined at the indicated times. (*p<0.05) (B) Total leukocytes were recovered from collagenase-digested lungs, and cells were stained with anti-CD11c and anti-CD11b prior to analysis by flow cytometry. Three independent experiments showed similar results. Data shown are means ± SEM (n = 4 or 5). *p<0.05.
Because the TLR-MyD88 signaling pathway serves a major role in activation of macrophages, and because macrophages are critical for clearance of Mp, we tested whether MyD88 is required for recruitment into the lungs and activation of macrophages and monocyte/macrophages following inoculation with Mp. In naive WT mice, 5.2% of the total pulmonary cells were CD11c+CD11b+ macrophages (Figure 6B); one day after inoculation, the percentage of CD11c+CD11b+ cells was increased to 8.1% (Figure 6B), consisting of CD11ChiCD11blo and CD11cloCD11bhi subpopulations (Figure S4). The CD11chiCD11blo subpopulation appeared to represent activated cells generated from the CD11c+CD11b− subpopulation of resident lung macrophages (Figure 6B). The other population was CD11cloCD11bhi, and appeared to represent newly recruited monocyte/macrophages because they were absent from mice that had been treated with clodronate liposomes administered i.v., but not i.n. (Figure S4). In contrast to leukocytes detected in the lungs of WT mice, CD11c+CD11b+ macrophages were not increased in the lungs of Mp-treated MyD88−/− animals (Figure 6B). Rather, it appeared both that the CD11c+CD11b− lung resident macrophages of MyD88−/− mice were not activated following treatment with Mp, and that the recruitment of monocyte/macrophages from the periphery into the lungs was impaired in MyD88−/− mice (Figure 6B).
Activation of macrophage NFκB by Mp depends on MyD88
In order to examine whether MyD88 has a direct role in the macrophage response to Mp, we analyzed the activation of NFκB, one of the major downstream transcription factors activated by TLR-MyD88 signaling, in WT or MyD88−/− BMM treated in vitro with EYFP-Mp. Before infection with Mp or following mock infection, the majority of NFκB was distributed diffusely in the cytoplasm in both WT and MyD88−/− BMM (Figure 7A). One hour after infection of WT BMM with Mp, NFκB had largely translocated into the nuclear compartment (Figure 7A), whereas it remained in the cytoplasm in BMM from MyD88−/− mice (Figure 7A). In order to assess the activation of NFκB quantitatively in Mp infected BMM from WT and MyD88−/− mice, we used an ELISA that only detected the activated form of this transcription factor by virtue of its ability to recognize its specific DNA target sequence. Before infection with Mp or mock infection, BMM from WT or MyD88−/− mice have similar low levels of activated NFκB in the total cell extracts (Figure S6A). One hour after Mp infection, there was a significantly increased level of NFκB activation in WT BMM, but not in MyD88−/− BMM (Figure S6A).
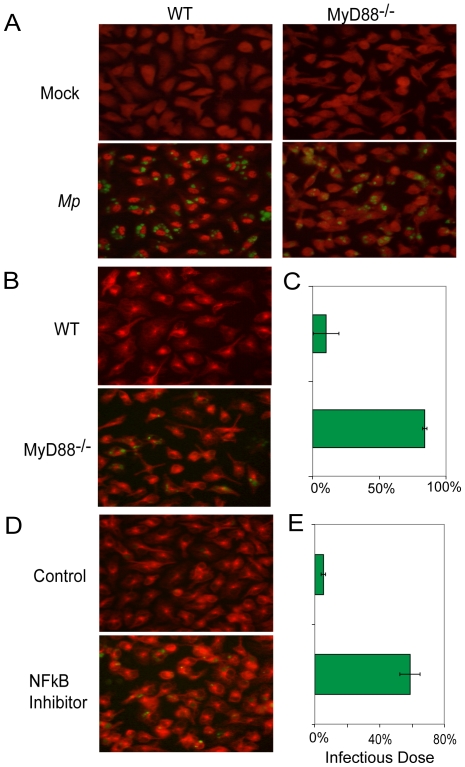
Figure 7. MyD88-NFκB signaling is essential for macrophages to eliminate Mp.
(A), (B) and (C), WT and MyD88−/− BMM were infected with EYFP-Mp or mock infected. (A) One hour after infection with EYFP-Mp (green), BMM were stained with anti-NFκB (p65) (red) to determine the subcellular location of this transcription factor. (B) BMM were stained with anti-α-tubulin (red) to highlight cell morphology. The survival of EYFP-Mp (green) was estimated by microscopy, and (C) the survival of EYFP-Mp was analyzed quantitatively by real-time PCR. (D) and (E), WT BMM were pretreated with the inhibitor of NFκB activation or with the diluent control (DMSO) for 1 h prior Mp infection. Eight hours after infection, BMM were stained with anti-α–tubulin (red) to show cell morphology and surviving EYFP-Mp (green) (D). The survival of EYFP-Mp in the cultures shown in (D) was determined by real-time PCR (E). Data shown are representative of two replicate experiments and are means ± SEM (n = 3).
We then tested in BMM and in F4/80+ macrophages purified from lungs of WT and MyD88−/− mice the impact of MyD88-deficiency on the mRNA expression of pro-inflammatory cytokines and chemokines that depend on NFκB for their induction. The mRNA expression of IL-1β, IL-6, TNF, and MIP-2 were up-regulated in Mp-infected WT BMM and purified macrophages at all time points tested; in contrast, there was only very low level expression of these pro-inflammatory genes in bone marrow-derived and lung macrophages of MyD88−/− mice following Mp infection (Figure S6B and data not shown). These data indicated that the MyD88-NFκB pathway responds rapidly to Mp, and suggested that this pathway may contribute importantly to the elimination of Mp by macrophages. They showed, further, that bone marrow-derived macrophages respond similarly to primary lung macrophages following contact with Mp.
Functional defect in elimination of Mp by MyD88-NFκB deficient macrophages
To test the hypothesis that MyD88-NFκB signaling contributes to the ability of macrophages to clear Mp, we analyzed the survival of EYFP-Mp in cultures of WT and MyD88−/− BMM using both semi-quantitative fluorescence microscopy and real-time PCR. One hour after addition of EYFP-Mp to BMM cultures, clusters of internalized bacteria were visible in both the WT and the MyD88−/− cells (Figure 7A). By 8 h after infection of WT BMM cultures with EYFP-Mp, there were almost no visible bacteria remaining (Figure 7B). In contrast, significant numbers of EYFP-Mp were easily detected at 8 h in cultures of MyD88−/− BMM (Figure 7B). Analysis of the numbers of cell-associated Mp by real-time PCR at 8 h after infection showed that approximately 10% of the original inoculum remained in the WT BMM culture (Figure 7C), compared to approximately 80% of the original inoculum in the MyD88−/− BMM culture (Figure 7C).
To test whether activation of NFκB, a major transcription factor downstream of MyD88, is required for the elimination of Mp by BMM, we treated BMM cultures with an inhibitor of NFκB activation for 1 hour prior to infection with EYFP-Mp. Eight hours after infection, there were only a few small clusters of cell-associated EYFP-Mp visible in the BMM cultured with the vehicle control, whereas a substantial amount of clustered EYFP-Mp were seen in the BMM cultures that had been treated with the inhibitor of NFκB activation (Figure 7D). Analysis by real-time PCR demonstrated that less than 10% of the original inoculum of Mp remained in the control BMM cultures, compared to approximately 60% of the original inoculum in the cultures of BMM that had been treated with the NFκB inhibitor (Figure 7E). These data underscore the critical role that the MyD88-NFκB pathway plays in the macrophage-mediated elimination of Mp.
Discussion
Mycoplasma pneumoniae (Mp) has been isolated and cultivated since the early 1960s and remains a common cause of community-acquired pneumonia. Over the past 17 years, several groups have observed that asthmatic subjects are at increased risk for colonization or productive infection with Mp compared to non-asthmatic controls [5], [38], [39], [40]; however, there has been only limited progress in understanding the pathogenic mechanisms that underlie Mp infections and the immunological mechanisms of their association with asthma. Our study has focused on identifying the cellular and molecular mechanisms that govern protective host responses to Mp in an experimental rodent model in order to permit development of therapies that interrupt the synergy between Mp and atopy in the expression of the asthmatic phenotype.
The finding of Mp in the airways of substantial numbers of individuals with asthma suggests either that the presence of Mp in the airways contributes to exacerbations of asthma symptoms in these asthmatic patients or that asthmatic inflammation alters the clearance of Mp from the airways, or both. Supporting a role for Mp in asthma exacerbations, one study showed that treatment with clarithromycin, an antibiotic that suppresses the growth of Mp resulted in significantly reduced asthma symptoms and improved lung function in Mp-positive asthmatic subjects, but not in Mp-negative subjects [8]. A recent follow-up study was not able to replicate this finding because the number of asthmatics PCR positive for Mp was too low (only 13%) [41]. The authors noted, however, that the study design may have impaired the detection of Mp since it included a run-in period with inhaled corticosteroids, a class of drugs that has been shown in a murine infection model to impair the growth of Mp in lung tissue [42]. In this group's previous studies [7] the chances that an asthmatic with a positive PCR test for Mp was not taking corticosteroids nearly reached statistical significance. This result underscores the importance of addressing the ways in which Mp may contribute to or modulate asthmatic inflammation and the mechanisms by which the host normally eliminates the microbe from the respiratory tract.
Mycoplasma pulmonis (M. pulmonis), a natural pathogen for rodents, has been widely utilized in mice and rats as a model system to mimic M. pneumoniae-induced pneumonia in humans. These studies have established several key features of the host response to M. pulmonis; however, much remains to be learned, and differences in the way the host handles the different mycoplasma species can be anticipated. Both alveolar macrophages and mast cells have been linked with the clearance of M. pulmonis in mice. Depletion of alveolar macrophages has been shown to cause exacerbation of respiratory mycoplasmosis in the M. pulmonis-resistant C57BL/6 mouse strain, although interestingly not in the M. pulmonis-susceptible strain C3H [43], [44]. These findings emphasize the role of macrophage lineage cells in effective host responsiveness to mycoplasma. These investigators have gone on to demonstrate that two of the mechanisms that alveolar macrophage utilize to eliminate M. pulmonis depend on normal levels of Surfactant protein A [45] and nitric oxide [46].
Additionally, mice deficient in mast cells due to their carriage of the Kitwsh/wsh mutation showed more severe weight loss and higher-grade pneumonia compared to WT mice infected with M. pulmonis. This phenotype was associated with a greater burden of mycoplasma in the lungs at early time points after experimental infection [47]. Although these investigators did not assess the effect of restoring mast cells in the Kit mutant mice, their findings suggested a protective role of mast cells in the host response to M. pulmonis.
Although Mp is not a natural pathogen in rodents, prior studies have shown that i.n. inoculation with Mp induces airway inflammation in mice [48]. Consistent with this, our data demonstrate that the microbe elicits a reproducible cellular inflammatory response in the lungs and airways of this experimental host. The inflammatory response is observed within several hours after inoculation with the microbe, and is dominated by the entry into the lungs of a variety of myeloid lineage cells, including neutrophils, macrophages, and dendritic cells (Figure 2). In spite of the rapid mobilization of these inflammatory cells, the microbe is able to survive for an extended period of time after inoculation, with complete clearance from the airway requiring 1 to 4 weeks, depending on the mouse strain being studied (Figure 1). Although full clearance requires a week or more and is associated with the development of circulating anti-Mp antibodies [49], the participation of Rag1-dependent adaptive immunity is not required (Figure S2).
These observations indicate that the primary mechanisms protecting the naïve murine host from Mp are components of the innate immune system. Prior studies in both humans and mice have focused on the roles of pro-inflammatory cytokines and chemokines, and have demonstrated increased production of IL-1β, IL-6, IL-8, IL-12, and KC in the respiratory tract [10], [48].
Consistent with the elevated expression of IL-1β, IL-6, IL-8/KC, and IL-12 after airway instillation of Mp, the microbe triggers rapid recruitment of a large number of neutrophils and macrophages into the lungs and airways (Figures 2C and 2D). Both of these cell types generally assist in the clearance of bacterial pathogens from the lungs of mice and humans [50], [51]. In the case of mycoplasma, however, prior data from Simberkoff and Elsbach has shown that in vitro co-incubation of mycoplasma with neutrophils had little impact on the viability of the microbe, suggesting that neutrophils are unlikely to participate in the killing and elimination of this microbe in vivo [52]. Thus, even though neutrophils are rapidly recruited in large numbers after airway inoculation with Mp, depletion of these cells by treatment with the anti-Gr-1 antibodies RB6-8C5 (Figure 3) or NIMP-R14 (data not shown) did not slow the clearance of the microbe from the lungs and airways. In fact, in some experiments, depletion of Gr-1+ cells resulted in a trend towards more rapid clearance of Mp from the airways (for example, Figure 3B), suggesting that neutrophils or other Gr-1+ cells may play an anti-inflammatory role in the host response to this microbe, modestly slowing its elimination. Regardless of the possible anti-inflammatory functions of neutrophils in the anti-mycoplasma response, other components of the innate immune system must be central in the elimination of the microbe.
Because NK cells, NK-T cells and mast cells produce mediators that can potently activate antibacterial host defenses [53], [54], [55], we tested whether these cells contribute importantly in the clearance of Mp by inoculating mice deficient in one or more of these lineages with the microbe. Mice that had been injected with anti-NK1.1 antibody (to deplete NK cells and NK-T cells), IL-15−/− mice (which are congenitally deficient in NK cells), and CD1d1−/− mice (deficient in NK-T cells) showed clearance of Mp from the airways that was indistinguishable from WT mice (data not shown), indicating no obligatory role for these cell lineages in elimination of the pathogen. Additionally, congenitally mast cell-deficient c-kitwsh/wsh mice showed no impairment of Mp clearance (Figure S7). These latter findings are of interest in the context of the findings of Xu et al. [47] who demonstrated that clearance of M. pulmonis is impaired in the c-kitwsh/wsh strain. The differences in the impact of this mutation on the ability of mice to clear Mp and M. pulmonis suggest that there may be microbial strain-specific aspects of the host response to this family of organisms.
Like neutrophils, macrophage lineage cells are also prominently recruited to the lungs within several hours after inoculation of Mp into the airways (Figures 2C, 2D and 2E). Experiments using pharmacologic depletion of macrophages (by treatment with clodronate liposomes; Figure 4B) or mice with congenital deficiency of macrophages (the CSF1op/op mouse strain; Figure 4C) showed that macrophages were indeed critical for the clearance of Mp. Interestingly, both of these approaches showed that the clearance of Mp occurred in at least two phases – an early macrophage-independent phase extending until approximately 3 d after i.n. inoculation with the microbe, and a late, macrophage-dependent phase, from day 3 to final eradication of the microbe. The initial macrophage-independent phase involves clearance of greater than 99% of the Mp inoculum. The nature of the innate mechanisms that govern this portion of the response remain undefined. As discussed above, neither the early nor the late phases of Mp clearance requires NK cell or neutrophil function. Using analysis of gene-targeted mice, we have also shown that neither the third component of complement (utilizing C3−/− mice) nor the free radical mediator nitric oxide produced by iNOS (using iNOS−/− mice) is essential for clearance of Mp (Figure S8). The reasons that iNOS is not required for clearance of Mp from the lungs of mice, but is required for the normal airway response to M. pulmonis [45] remain to be investigated.
In considering the potential molecular mechanisms by which macrophages govern the elimination of Mp from the lungs and airways, we noted that the rate of clearance of this microbe from the respiratory tract is substantially faster from C57BL/6 mice compared to BALB/c mice. This appeared to include differences in both the early phase of the response and the late phase. C57BL/6 and BALB/c mice are known to differ in their production of several key cytokines. Macrophages from C57BL/6 mice respond to stimulation by LPS with expression of higher quantities of IFN-γ and IL-12 compared to macrophages from BALB/c mice [56]. This and other studies suggest that C57BL/6 mice favor Th1 responses, whereas BALB/c mice favor Th2-type responses. Our data are consistent with host defense mechanisms associated with Th1-type responses participating importantly in the clearance of Mp from the airways. Interestingly, Salvatore et al. reported that mice deficient in IL-12 P35 showed accelerated clearance of Mp from the airways [18], suggesting that the prototypic Th1 response itself is not responsible for Mp clearance. Consistent with this is our finding using Rag1−/− mice that functional lymphocytes are not required for clearance (Figure S2).
Studies both in vivo, analyzing clearance from the lungs (Figure 6A), and in vitro, analyzing the handling of Mp by macrophages (Figure 7A), showed a pivotal role for the MyD88 adapter protein in clearance of Mp. MyD88 appears to affect both the early, macrophage-independent phase of microbial clearance and the late, macrophage-dependent clearance phase. This latter observation is consistent with the requirement for My88 for normal clearance of the microbe by Mp cultured in vitro. The MyD88-interacting receptor that governs this effect on clearance of Mp has not been defined. Using IL-1β−/− and type I IL-1 receptor−/− mice, we have excluded an essential role of the IL-1 axis in the clearance of the microbe (data not shown). Studies are underway to test the role of IL-18 and individual TLR in the clearance of Mp. Importantly, it has been identified that Mp-derived lipoproteins induce expression of the inflammatory cytokine TNF in macrophages through TLR2 paired with TLR1 or TLR6 [57]. Thus, TLR2 might be a candidate receptor that plays a role in the macrophage response to Mp.
In all settings that we have analyzed, BMM functioned similarly to macrophages purified from the lungs of mice (Figures S6B and S6C, and data not shown). Incubation of BMM with fluorescently labeled Mp followed by analysis both by transmission electron microscopy and by confocal fluorescence microscopy indicated that elimination of Mp is associated with their phagocytosis into LAMP-2-associated, acidified intracellular vacuoles. While we cannot rule out some degree of extracellular killing of the microbe, our data are consistent with the majority of the killing of Mp occurring in the phagolysosomal system. The central role of NFκB in disposal of the microbe suggests that pharmacological manipulations that altered the function of this transcription factor could have a profound effect on clearance of this organism.
Given that MyD88-dependent and NFκB-dependent pathways are involved in the activation of neutrophils [58], it is of considerable interest that neutrophils appear not to be engaged in the clearance of Mp from the lungs and airways. Additional studies will be required to determine the requirements for phagocytosis of this atypical microbe that lacks a cell wall. The fact that neutrophils are not required for the clearance of Mp from naïve mice does not exclude a potentially significant role for neutrophils in the clearance of opsonized Mp as might be encountered in mice that had established a robust anti-Mp antibody response.
The prominent roles of both macrophages and MyD88 in the anti-Mp response establish the key role of innate immune function in the elimination of this microbe. One might anticipate that deficiencies of MyD88 or TLR function could impair clearance of Mp and lead to persistence of asthma symptoms associated with chronic carriage of the organism as observed by Kraft et al. [40]. Such a potential relationship between TLR function and airway Mp may be particularly relevant in light of the findings by Kormann et al. that polymorphic variants of TLRs 1, 2, and 6 contribute to susceptibility and resistance to asthma in children [59].
Macrophages and TLRs should be studied further in asthmatic individuals who are colonized or infected by Mp. Additional studies should address the potential impact of asthmatic inflammation on the ability of pulmonary macrophages to respond to and eliminate this microbe. Furthermore, maneuvers that potentiate macrophage anti-mycoplasma function without up-regulating the proinflammatory activities of these cells might afford ways to improve, in an antibiotic-sparing fashion, asthma symptoms in individuals who are carrying this microbe.
Materials and Methods
Ethics statement
This study was carried out in strict accordance with the recommendations of the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health. The protocols for all experiments using vertebrate animals were approved by the Institutional Animal Care and Use Committee of the University of Alabama at Birmingham (Protocol number 091209016 under Institutional Animal Assurance Number A-3255-01).
Mice
C57BL/6, BALB/c, Rag1−/−, Csf1op/op, iNOS−/−, and c-kitwsh/wsh mice were obtained from The Jackson Laboratory (Bar Harbor, ME). C3−/− mice were from Dr. Harvey Colten [60] and were kindly provided by Dr. Scott Barnum (University of Alabama at Birmingham (UAB)). MyD88−/− mice [61] were from Dr. Shizuo Akira (Osaka University, Osaka, Japan) and were kindly provided by Dr. Suzanne Michalek (UAB). All mice were used between 6 to12 weeks of age and were kept in micro-isolator cages in the specific pathogen-free Animal Resources Program facility at UAB.
Reagents and antibodies
Anti-Ly6C-FITC (Abcam, Cambridge, MA), anti-Ly6G-PE, anti-CD11b-APC-Cy7, anti-CD11c-PE-Cy7 (BD Bioscience, San Jose, CA), anti-F4/80-PE-Alexa 647 (AbD Serotec, Raleigh, NC), anti-Gr-1- APC (Invitrogen, Carlsbad, CA), rabbit anti-NFκB p65 (Santa Cruz Biotechnology, Santa Cruz, CA), mouse anti-α-tubulin (Invitrogen), and goat anti-rabbit IgG-Rhodamine Red X (RRX) and goat anti-mouse IgG-RRX (Jackson ImmunoResearch Laboratory, West Grove, PA) were used for flow cytometry and immunofluorescence microscopy. The inhibitor of NFκB activation [62], [63] (Calbiochem, San Diego, CA) and DMSO (Sigma-Aldrich, St. Louis, MO) alone as control were used in vitro to assess the role of activated NFκB in the host response to Mp.
Culture of BMM
For preparation of bone marrow-derived macrophages (BMM), total bone marrow cells were recovered aseptically from the femurs of euthanized adult mice and filtered through a 40-µm cell strainer to remove undispersed cells and tissue debris. Approximately 2×106 bone marrow cells were cultured in a 10 cm Petri dish with 10 ml of RPMI 1640 medium plus 10% FBS, 2 mM L-glutamine, 1X Pen/Strep (complete RPMI medium) and 20% L929 cell conditioned medium. On d 5, an additional 5 ml of complete RPMI medium were added, and the following day the adherent cells were harvested by trypsinization and re-plated in complete RPMI medium at a density of 1.5×106 cells per well of a 6-well plate or 7.5×105 cells per well of a 12 well plate or per well of a 2 well chamber slide. BMM were rested for 1 day before infection with Mp.
Infection with Mp
For analysis of the clearance of Mp in vivo, mice were anesthetized lightly with isoflurane, and then inoculated intranasally (i.n.) with 4×106 WT Mp strain M129 (ATCC 29342) in 40 µl of SP4 medium. Mock inoculations were with SP4 medium alone. At various times after inoculation, whole lungs were harvested for extraction of total RNA or preparation of cell suspensions. For in vitro studies, BMM were infected with WT Mp or with a strain of Mp that was deficient in the P30 adhesin and restored to WT by transformation with a recombinant transposon carrying a fusion gene encoding P30 and EYFP (EYFP-Mp) [64]. Infections of cultures of BMM in vitro were at a 100∶1 multiplicity of infection (MOI). Controls were mock infected with complete RPMI 1640 medium alone. For transmission electron microscopy and for analysis of translocation of NFκB, BMM were analyzed 1 hour after infection with Mp in vitro. For determining the Mp-induced expression of RNA encoding cytokines and chemokines, BMM were harvested 3 hours after infection. For investigating the elimination of Mp from BMM cultures, BMM were harvested 8 hours after infection.
Detection of Mp by bacterial culture
Numbers of viable M. pneumoniae in the lungs and airways of experimentally infected mice were determined by a modification of the procedure of Cartner et al. [43]. Whole lungs were harvested by dissection from the main stem bronchi and placed in a small volume of SP4 medium at room temperature. The lung tissue was finely minced in 2 ml SP4 broth and vortexed for 30 sec. Tenfold dilutions were prepared and 20 µl aliquots were plated on SP4 agar and incubated at 37°C in 5% CO2 for 7 to 14 days until colonies were visible. Colonies were counted under a dissecting microscope. In addition, the 10-fold dilutions were incubated at 37°C for 14 days, then observed for development of a color change indicating growth. All samples were processed and analyzed in a double blind fashion.
Detection of Mp by real-time PCR
Whole lungs were harvested by dissection from the main stem bronchi, stored in the RNA stabilizing solution RNAlater (Ambion, Austin, TX), and kept at −20°C prior to isolation of total cellular RNA. Cultured macrophages were directly dissolved in RLT lysis buffer (Qiagen, Valencia, CA). Then total RNA was extracted from whole lungs or from macrophage lysates using the RNeasy kit (Qiagen), and reverse transcribed into cDNA using the SuperScript III RTS First-Strand cDNA Synthesis Kit (Invitrogen). The numbers of Mp in lung samples were determined by Taqman real-time PCR using a set of primers and probe that were specific for the 16S rRNA of Mp. We used the 16S rRNA as the target for quantifying Mp because this RNA is present in >1,000 copies per bacterium, enhancing the sensitivity of the assay [65]. The primer sequences providing specificity for Mp were 5′-GGA CCT GCA AGG GTT CGT T-3′ (forward primer), 5′-AGT TGG TGG GGT AAC GGC C-3′ (reverse primer), and 5′-/56-FAM/-TTG ATG AGG GTG CGC CAT ATC AGC T-/3BHQ-1/-3′ (probe). We used the murine glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene as a control for normalizing the quantity of total lung RNA recovered in each experiment. The GAPDH primers were 5′-TCC ATG ACA ACT TTG GCA TTG-3′ (forward primer), 5′-CAG TCT TCT GGG TGG CAG TGA-3′ (reverse primer), and 5′-/5TexEd-XN/-AGG GCT CAT GAC CAC AGT CCA TGC C-/3BHQ-2/-3′ (probe) (all purchased from Integrated DNA Technologies (IDT), Coralville, IA).
Flow cytometry
Freshly harvested lungs were minced and digested with Collagenase B (2 mg/ml) and DNase I (0.02 mg/ml) for 30 minutes at 37°C. Single cell suspensions were filtered through a 40-µm cell strainer and then fixed with 2% paraformaldehyde prior to staining with fluorescently conjugated Abs, washing and analysis using an LSRII flow cytometer (BD Biosciences) using FlowJo software (Tree Star, Inc., Portland, OR).
Depletion of leukocyte populations in vivo
For depletion of neutrophils, mice were injected i.v. with 100 µg of the rat anti-murine Gr-1 mAb (RB6-8C5; kindly provided by Emil R. Unanue, Washington University, St. Louis, MO) or rat IgG (Sigma-Aldrich) in 100 µl of PBS. Two days after injection with the depleting antibody, mice were inoculated i.n. with Mp. Depletion of macrophages was performed using liposomes containing dichloromethylene bisphosphonate (clodronate; [66]). Clodronate was a gift of Roche (Mannheim, Germany). It was encapsulated in liposomes as previously described [67]. Phosphatidylcholine (LIPOID E PC) was obtained from Lipoid GmbH. Cholesterol was purchased from Sigma-Aldrich. Mice were injected i.n. with 50 µl and i.p. with 100 µl of clodronate- or PBS-containing liposomes 24 h prior to inoculation with Mp.
Analysis of NFκB localization and cell morphology by immunofluorescence microscopy
At various times after infection with EYFP-Mp, BMM cultured in chamber slides were washed twice with PBS, fixed with 2% paraformaldehyde for 20 min at RT, and permeabilized with CSK buffer (10 mM PIPES, pH 6.8, 50 mM NaCl, 300 mM sucrose, 3 mM MgCl2, 0.05% Triton X-100) for 5 min at RT. Translocation of NFκB from the cytoplasm to the nucleus was detected by staining with rabbit anti-murine NFκB Ab followed by RRX labeled goat anti-rabbit IgG. Activation of the BMM cytoskeleton was assessed by staining with mouse anti-murine α-tubulin mAb followed by RRX labeled goat anti-mouse IgG.
Statistical analyses
Statistical analyses were performed by two-tailed equal variant Student's t test. A p value <0.05 was considered statistically significant.
Supporting Information
Comparison of Mp quantification in lungs by real-time PCR and bacterial culture. WT mice were inoculated intranasally with 4×106 Mp at d0. At d1, d3 and d5 after inoculation, mice were sacrificed, and the whole lungs were harvested for RNA extraction (4 mice) or bacterial culture (4 different mice) for quantification of Mp. Data shown are means ± SEM (n = 4).
(0.48 MB EPS)
Adaptive immune responses are not required for the elimination of Mp from the lungs of naive mice. WT (open bars) and Rag1−/− (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. Data shown are means ± SEM (n = 4 or 5). This experiment was repeated with similar results.
(0.29 MB EPS)
Mice with dysfunctional neutrophils show no defect in the elimination of Mp from the lungs. WT (blue bars) and CD11b−/− (purple bars) mice were inoculated intranasally with 4×106 Mp. Five days after inoculation, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. This experiment was repeated with similar results. Data shown are means ± SEM (n = 5).
(0.47 MB EPS)
Two major populations of macrophages found in the lungs of mice following i.n. inoculation with Mp can be depleted differentially by treatment with clodronate liposomes. Mice were injected with clodronate liposomes or control (PBS) liposomes via the i.n. and i.p. routes one day prior to inoculation with Mp. On d1 after inoculation with Mp, lungs were harvested and digested into single cell suspensions, and then lung leukocytes were stained with anti-CD11c and anti-CD11b and analyzed by FACS. (A) Mice received i.n. and i.p. injection of control (PBS) liposomes. (B) Mice were pretreated with clodronate liposomes via the i.n. route only. (C) Mice were injected with clodronate liposomes by both the i.n. and i.p. routes. R1 (green box) represented CD11chiCD11blo macrophages (activated resident pulmonary macrophages), and R2 (orange box) showed CD11cloCD11bhi macrophages (recruited peripheral macrophages). All parts of this experiment were repeated at least twice with similar results. Data shown are means ± SEM (n = 5).
(0.69 MB EPS)
The clearance of P1-deficient Mp is macrophage-dependent. Mice were treated i.n. and i.p. with clodronate liposomes to deplete macrophages, or with control (PBS) liposomes at day -1, followed by i.n. inoculation with P1-deficient Mp at day 0. At various times after inoculation with Mp, lungs were harvested and total RNA was extracted to determine the numbers of Mp by real-time PCR. (* p<0.05). This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 5).
(0.33 MB EPS)
MyD88 signaling is essential for activation of NFκB and mRNA expression of pro-inflammatory genes in the macrophage response to Mp. BMM derived from wild type or MyD88−/− mice were infected with Mp (MOI 100∶1), or mock infected as control. (A) One hour after infection, BMM were harvested and lysed with lysis buffer containing a phosphatase inhibitor. Five µg of total cell lysate were used for TransAM transcription factor ELISA (Actif Motif) to detect the activated form of NFκB. (B) BMM and (C) lung macrophages, total RNA was harvested at 0 h, 2 h, 4 h, 6 h and 8 h after Mp infection, and the mRNA expression of the pro-inflammatory genes TNF, IL-6, and MIP-2 was analyzed by real-time PCR. The mRNA expression levels of pro-inflammatory genes were compared to the levels in macrophages from WT mice without Mp infection (0 h). This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 3 for BMM; n = 1 for primary lung macrophages).
(0.42 MB EPS)
The clearance of Mp from the airways of mice is independent of mast cells. WT (open bars) and c-kitwsh/wsh (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 5).
(0.43 MB EPS)
The clearnce of Mp from the airways of mice is independent of C3 or nitric oxide produced by iNOS. (A) WT (open bars) and C3−/− (filled bars) mice and (B) WT (open bars) and iNOS−/− (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. Data shown are means ± SEM (n = 5).
(0.49 MB EPS)
Acknowledgments
We thank Donna M. Crabb for help with culture of Mycoplasma pneumoniae. We also thank Melissa Chimento and the UAB High Resolution Imaging Facility for excellent help with transmission electron microscopy. We are grateful to Dr. Mary Ann Accaviti-Loper and the UAB Epitope Recognition and Immunoreagent Core Facility for large-scale preparation of depleting monoclonal antibodies. We thank Dr. John F. Kearney for providing L929 cells used for preparation of conditioned media for bone marrow-derived macrophage culture, Dr. Emil R. Unanue for providing the RB6-8C5 monoclonal Ab, Dr. Scott Barnum for providing the C3-deficient mouse strain, Dr. Sadis Matalon for providing the iNOS-deficient mouse strain, and Dr. Suzanne Michalek for providing the MyD88-deficient mouse strain, and for many helpful discussions. We are grateful to Dr. Eric Brown, Genentech Inc., for helpful suggestions regarding evaluation of endosomal and lysosomal compartments in the killing of Mycoplasma pneumoniae. We thank Ming-Chi Tsai and Dr. Jacques Wadiche for help with confocal microscopy. We especially thank Dr. Gail Cassell for helpful discussions. All authors state that they have no conflict of interest regarding the work reported in this manuscript.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: Portions of this research were supported by National Institutes of Health grants 5P01HL073907 (DDC), 5R21AI083873 (TPA and KBW) and 5R01AI23362 (DCK). The NIH had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454:445–454. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sleiman PM, Hakonarson H. Recent advances in the genetics and genomics of asthma and related traits. Curr Opin Pediatr. 2010;22:307–312. doi: 10.1097/MOP.0b013e328339553d. [DOI] [PubMed] [Google Scholar]
- 3.Vercelli D. Discovering susceptibility genes for asthma and allergy. Nat Rev Immunol. 2008;8:169–182. doi: 10.1038/nri2257. [DOI] [PubMed] [Google Scholar]
- 4.Martin RJ. Infections and asthma. Clin Chest Med. 2006;27:87–98. doi: 10.1016/j.ccm.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 5.Seggev JS, Lis I, Siman-Tov R, Gutman R, Abu-Samara H, et al. Mycoplasma pneumoniae is a frequent cause of exacerbation of bronchial asthma in adults. Ann Allergy. 1986;57:263–265. [PubMed] [Google Scholar]
- 6.Sutherland ER, Martin RJ. Asthma and atypical bacterial infection. Chest. 2007;132:1962–1966. doi: 10.1378/chest.06-2415. [DOI] [PubMed] [Google Scholar]
- 7.Martin RJ, Kraft M, Chu HW, Berns EA, Cassell GH. A link between chronic asthma and chronic infection. J Allergy Clin Immunol. 2001;107:595–601. doi: 10.1067/mai.2001.113563. [DOI] [PubMed] [Google Scholar]
- 8.Kraft M, Cassell GH, Pak J, Martin RJ. Mycoplasma pneumoniae and Chlamydia pneumoniae in asthma: effect of clarithromycin. Chest. 2002;121:1782–1788. doi: 10.1378/chest.121.6.1782. [DOI] [PubMed] [Google Scholar]
- 9.Fayon M, Just J, Thien HV, Chiba T, Pascual L, et al. Bacterial flora of the lower respiratory tract in children with bronchial asthma. Acta Paediatr. 1999;88:1216–1222. doi: 10.1080/080352599750030310. [DOI] [PubMed] [Google Scholar]
- 10.Waites KB, Talkington DF. Mycoplasma pneumoniae and its role as a human pathogen. Clin Microbiol Rev. 2004;17:697–728. doi: 10.1128/CMR.17.4.697-728.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hasselbring BM, Jordan JL, Krause RW, Krause DC. Terminal organelle development in the cell wall-less bacterium Mycoplasma pneumoniae. Proc Natl Acad Sci U S A. 2006;103:16478–16483. doi: 10.1073/pnas.0608051103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Baseman JB, Cole RM, Krause DC, Leith DK. Molecular basis for cytadsorption of Mycoplasma pneumoniae. J Bacteriol. 1982;151:1514–1522. doi: 10.1128/jb.151.3.1514-1522.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dallo SF, Lazzell AL, Chavoya A, Reddy SP, Baseman JB. Biofunctional domains of the Mycoplasma pneumoniae P30 adhesin. Infect Immun. 1996;64:2595–2601. doi: 10.1128/iai.64.7.2595-2601.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Waldo RH, 3rd, Krause DC. Synthesis, stability, and function of cytadhesin P1 and accessory protein B/C complex of Mycoplasma pneumoniae. J Bacteriol. 2006;188:569–575. doi: 10.1128/JB.188.2.569-575.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaisho T, Akira S. Toll-like receptor function and signaling. J Allergy Clin Immunol. 2006;117:979–987. doi: 10.1016/j.jaci.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 16.O'Neill LA, Bowie AG. The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat Rev Immunol. 2007;7:353–364. doi: 10.1038/nri2079. [DOI] [PubMed] [Google Scholar]
- 17.Kawai T, Akira S. TLR signaling. Semin Immunol. 2007;19:24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Salvatore CM, Fonseca-Aten M, Katz-Gaynor K, Gomez AM, Mejias A, et al. Respiratory tract infection with Mycoplasma pneumoniae in interleukin-12 knockout mice results in improved bacterial clearance and reduced pulmonary inflammation. Infect Immun. 2007;75:236–242. doi: 10.1128/IAI.01249-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chu HW, Jeyaseelan S, Rino JG, Voelker DR, Wexler RB, et al. TLR2 signaling is critical for Mycoplasma pneumoniae-induced airway mucin expression. J Immunol. 2005;174:5713–5719. doi: 10.4049/jimmunol.174.9.5713. [DOI] [PubMed] [Google Scholar]
- 20.Katz B, Waites K. Emerging intracellular bacterial infections. Clin Lab Med. 2004;24:627–649. doi: 10.1016/j.cll.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 21.Williamson J, Marmion BP, Worswick DA, Kok TW, Tannock G, et al. Laboratory diagnosis of Mycoplasma pneumoniae infection. 4. Antigen capture and PCR-gene amplification for detection of the Mycoplasma: problems of clinical correlation. Epidemiol Infect. 1992;109:519–537. doi: 10.1017/s0950268800050512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mombaerts P, Iacomini J, Johnson RS, Herrup K, Tonegawa S, et al. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992;68:869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- 23.Lagasse E, Weissman IL. Flow cytometric identification of murine neutrophils and monocytes. J Immunol Methods. 1996;197:139–150. doi: 10.1016/0022-1759(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 24.Gonzalez-Juarrero M, Shim TS, Kipnis A, Junqueira-Kipnis AP, Orme IM. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- 25.Hall JD, Woolard MD, Gunn BM, Craven RR, Taft-Benz S, et al. Infected-host-cell repertoire and cellular response in the lung following inhalation of Francisella tularensis Schu S4, LVS, or U112. Infect Immun. 2008;76:5843–5852. doi: 10.1128/IAI.01176-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Dea KP, Wilson MR, Dokpesi JO, Wakabayashi K, Tatton L, et al. Mobilization and margination of bone marrow Gr-1high monocytes during subclinical endotoxemia predisposes the lungs toward acute injury. J Immunol. 2009;182:1155–1166. doi: 10.4049/jimmunol.182.2.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.O'Dea KP, Young AJ, Yamamoto H, Robotham JL, Brennan FM, et al. Lung-marginated monocytes modulate pulmonary microvascular injury during early endotoxemia. Am J Respir Crit Care Med. 2005;172:1119–1127. doi: 10.1164/rccm.200504-605OC. [DOI] [PubMed] [Google Scholar]
- 28.Jung YW, Zindl CL, Lai JF, Weaver CT, Chaplin DD. MMP induced by Gr-1+ cells are crucial for recruitment of Th cells into the airways. Eur J Immunol. 2009;39:2281–2292. doi: 10.1002/eji.200838985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, et al. A novel role for the β2 integrin CD11b/CD18 in neutrophil apoptosis: a homeostatic mechanism in inflammation. Immunity. 1996;5:653–666. doi: 10.1016/s1074-7613(00)80278-2. [DOI] [PubMed] [Google Scholar]
- 30.Tang T, Rosenkranz A, Assmann KJ, Goodman MJ, Gutierrez-Ramos JC, et al. A role for Mac-1 (CDIIb/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcγ receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med. 1997;186:1853–1863. doi: 10.1084/jem.186.11.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wiktor-Jedrzejczak WW, Ahmed A, Szczylik C, Skelly RR. Hematological characterization of congenital osteopetrosis in op/op mouse. Possible mechanism for abnormal macrophage differentiation. J Exp Med. 1982;156:1516–1527. doi: 10.1084/jem.156.5.1516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galloway CJ, Dean GE, Marsh M, Rudnick G, Mellman I. Acidification of macrophage and fibroblast endocytic vesicles in vitro. Proc Natl Acad Sci U S A. 1983;80:3334–3338. doi: 10.1073/pnas.80.11.3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause DC, Leith DK, Wilson RM, Baseman JB. Identification of Mycoplasma pneumoniae proteins associated with hemadsorption and virulence. Infect Immun. 1982;35:809–817. doi: 10.1128/iai.35.3.809-817.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisenbarth SC, Piggott DA, Huleatt JW, Visintin I, Herrick CA, et al. Lipopolysaccharide-enhanced, toll-like receptor 4-dependent T helper cell type 2 responses to inhaled antigen. J Exp Med. 2002;196:1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 36.Schnare M, Barton GM, Holt AC, Takeda K, Akira S, et al. Toll-like receptors control activation of adaptive immune responses. Nat Immunol. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 37.Kawai T, Akira S. Signaling to NFκB by Toll-like receptors. Trends Mol Med. 2007;13:460–469. doi: 10.1016/j.molmed.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Biscardi S, Lorrot M, Marc E, Moulin F, Boutonnat-Faucher B, et al. Mycoplasma pneumoniae and asthma in children. Clin Infect Dis. 2004;38:1341–1346. doi: 10.1086/392498. [DOI] [PubMed] [Google Scholar]
- 39.Gil JC, Cedillo RL, Mayagoitia BG, Paz MD. Isolation of Mycoplasma pneumoniae from asthmatic patients. Ann Allergy. 1993;70:23–25. [PubMed] [Google Scholar]
- 40.Kraft M, Cassell GH, Henson JE, Watson H, Williamson J, et al. Detection of Mycoplasma pneumoniae in the airways of adults with chronic asthma. Am J Respir Crit Care Med. 1998;158:998–1001. doi: 10.1164/ajrccm.158.3.9711092. [DOI] [PubMed] [Google Scholar]
- 41.Sutherland ER, King TS, Icitovic N, Ameredes BT, Bleecker E, et al. A trial of clarithromycin for the treatment of suboptimally controlled asthma. J Allergy Clin Immunol. 2010;126:747–753. doi: 10.1016/j.jaci.2010.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chu HW, Campbell JA, Rino JG, Harbeck RJ, Martin RJ. Inhaled fluticasone propionate reduces concentration of Mycoplasma pneumoniae, inflammation, and bronchial hyperresponsiveness in lungs of mice. J Infect Dis. 2004;189:1119–1127. doi: 10.1086/382050. [DOI] [PubMed] [Google Scholar]
- 43.Cartner SC, Simecka JW, Lindsey JR, Cassell GH, Davis JK. Chronic respiratory mycoplasmosis in C3H/HeN and C57BL/6N mice: lesion severity and antibody response. Infect Immun. 1995;63:4138–4142. doi: 10.1128/iai.63.10.4138-4142.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hickman-Davis JM, Michalek SM, Gibbs-Erwin J, Lindsey JR. Depletion of alveolar macrophages exacerbates respiratory mycoplasmosis in mycoplasma-resistant C57BL mice but not mycoplasma-susceptible C3H mice. Infect Immun. 1997;65:2278–2282. doi: 10.1128/iai.65.6.2278-2282.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hickman-Davis J, Gibbs-Erwin J, Lindsey JR, Matalon S. Surfactant protein A mediates mycoplasmacidal activity of alveolar macrophages by production of peroxynitrite. Proc Natl Acad Sci U S A. 1999;96:4953–4958. doi: 10.1073/pnas.96.9.4953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hickman-Davis JM, Wang Z, Fierro-Perez GA, Chess PR, Page GP, et al. Surfactant dysfunction in SP-A-/- and iNOS-/- mice with mycoplasma infection. Am J Respir Cell Mol Biol. 2007;36:103–113. doi: 10.1165/rcmb.2006-0049OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu X, Zhang D, Lyubynska N, Wolters PJ, Killeen NP, et al. Mast cells protect mice from Mycoplasma pneumonia. Am J Respir Crit Care Med. 2006;173:219–225. doi: 10.1164/rccm.200507-1034OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fonseca-Aten M, Rios AM, Mejias A, Chavez-Bueno S, Katz K, et al. Mycoplasma pneumoniae induces host-dependent pulmonary inflammation and airway obstruction in mice. Am J Respir Cell Mol Biol. 2005;32:201–210. doi: 10.1165/rcmb.2004-0197OC. [DOI] [PubMed] [Google Scholar]
- 49.Wubbel L, Jafri HS, Olsen K, Shelton S, Barton Rogers B, et al. Mycoplasma pneumoniae pneumonia in a mouse model. J Infect Dis. 1998;178:1526–1529. doi: 10.1086/314439. [DOI] [PubMed] [Google Scholar]
- 50.Craig A, Mai J, Cai S, Jeyaseelan S. Neutrophil recruitment to the lungs during bacterial pneumonia. Infect Immun. 2009;77:568–575. doi: 10.1128/IAI.00832-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Marriott HM, Dockrell DH. The role of the macrophage in lung disease mediated by bacteria. Exp Lung Res. 2007;33:493–505. doi: 10.1080/01902140701756562. [DOI] [PubMed] [Google Scholar]
- 52.Simberkoff MS, Elsbach P. The interaction in vitro between polymorphonuclear leukocytes and mycoplasma. J Exp Med. 1971;134:1417–1430. doi: 10.1084/jem.134.6.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hofmann AM, Abraham SN. New roles for mast cells in modulating allergic reactions and immunity against pathogens. Curr Opin Immunol. 2009;21:679–686. doi: 10.1016/j.coi.2009.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Strowig T, Brilot F, Munz C. Noncytotoxic functions of NK cells: direct pathogen restriction and assistance to adaptive immunity. J Immunol. 2008;180:7785–7791. doi: 10.4049/jimmunol.180.12.7785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tupin E, Kinjo Y, Kronenberg M. The unique role of natural killer T cells in the response to microorganisms. Nat Rev Microbiol. 2007;5:405–417. doi: 10.1038/nrmicro1657. [DOI] [PubMed] [Google Scholar]
- 56.Kuroda E, Kito T, Yamashita U. Reduced expression of STAT4 and IFN-γ in macrophages from BALB/c mice. J Immunol. 2002;168:5477–5482. doi: 10.4049/jimmunol.168.11.5477. [DOI] [PubMed] [Google Scholar]
- 57.Shimizu T, Kida Y, Kuwano K. Mycoplasma pneumoniae-derived lipopeptides induce acute inflammatory responses in the lungs of mice. Infect Immun. 2008;76:270–277. doi: 10.1128/IAI.00955-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yanagisawa S, Koarai A, Sugiura H, Ichikawa T, Kanda M, et al. Oxidative stress augments toll-like receptor 8 mediated neutrophilic responses in healthy subjects. Respir Res. 2009;10:50. doi: 10.1186/1465-9921-10-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kormann MS, Depner M, Hartl D, Klopp N, Illig T, et al. Toll-like receptor heterodimer variants protect from childhood asthma. J Allergy Clin Immunol. 2008;122:86–92. doi: 10.1016/j.jaci.2008.04.039. [DOI] [PubMed] [Google Scholar]
- 60.Circolo A, Garnier G, Fukuda W, Wang X, Hidvegi T, et al. Genetic disruption of the murine complement C3 promoter region generates deficient mice with extrahepatic expression of C3 mRNA. Immunopharmacology. 1999;42:135–149. doi: 10.1016/s0162-3109(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 61.Adachi O, Kawai T, Takeda K, Matsumoto M, Tsutsui H, et al. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity. 1998;9:143–150. doi: 10.1016/s1074-7613(00)80596-8. [DOI] [PubMed] [Google Scholar]
- 62.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, et al. Discovery of quinazolines as a novel structural class of potent inhibitors of NFκB activation. Bioorg Med Chem. 2003;11:383–391. doi: 10.1016/s0968-0896(02)00440-6. [DOI] [PubMed] [Google Scholar]
- 63.Tobe M, Isobe Y, Tomizawa H, Nagasaki T, Takahashi H, et al. A novel structural class of potent inhibitors of NFκB activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives. Bioorg Med Chem. 2003;11:3869–3878. doi: 10.1016/s0968-0896(03)00438-3. [DOI] [PubMed] [Google Scholar]
- 64.Romero-Arroyo CE, Jordan J, Peacock SJ, Willby MJ, Farmer MA, et al. Mycoplasma pneumoniae protein P30 is required for cytadherence and associated with proper cell development. J Bacteriol. 1999;181:1079–1087. doi: 10.1128/jb.181.4.1079-1087.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.van Kuppeveld FJ, van der Logt JT, Angulo AF, van Zoest MJ, Quint WG, et al. Genus- and species-specific identification of mycoplasmas by 16S rRNA amplification. Appl Environ Microbiol. 1992;58:2606–2615. doi: 10.1128/aem.58.8.2606-2615.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McGill J, Van Rooijen N, Legge KL. Protective influenza-specific CD8 T cell responses require interactions with dendritic cells in the lungs. J Exp Med. 2008;205:1635–1646. doi: 10.1084/jem.20080314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Comparison of Mp quantification in lungs by real-time PCR and bacterial culture. WT mice were inoculated intranasally with 4×106 Mp at d0. At d1, d3 and d5 after inoculation, mice were sacrificed, and the whole lungs were harvested for RNA extraction (4 mice) or bacterial culture (4 different mice) for quantification of Mp. Data shown are means ± SEM (n = 4).
(0.48 MB EPS)
Adaptive immune responses are not required for the elimination of Mp from the lungs of naive mice. WT (open bars) and Rag1−/− (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. Data shown are means ± SEM (n = 4 or 5). This experiment was repeated with similar results.
(0.29 MB EPS)
Mice with dysfunctional neutrophils show no defect in the elimination of Mp from the lungs. WT (blue bars) and CD11b−/− (purple bars) mice were inoculated intranasally with 4×106 Mp. Five days after inoculation, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. This experiment was repeated with similar results. Data shown are means ± SEM (n = 5).
(0.47 MB EPS)
Two major populations of macrophages found in the lungs of mice following i.n. inoculation with Mp can be depleted differentially by treatment with clodronate liposomes. Mice were injected with clodronate liposomes or control (PBS) liposomes via the i.n. and i.p. routes one day prior to inoculation with Mp. On d1 after inoculation with Mp, lungs were harvested and digested into single cell suspensions, and then lung leukocytes were stained with anti-CD11c and anti-CD11b and analyzed by FACS. (A) Mice received i.n. and i.p. injection of control (PBS) liposomes. (B) Mice were pretreated with clodronate liposomes via the i.n. route only. (C) Mice were injected with clodronate liposomes by both the i.n. and i.p. routes. R1 (green box) represented CD11chiCD11blo macrophages (activated resident pulmonary macrophages), and R2 (orange box) showed CD11cloCD11bhi macrophages (recruited peripheral macrophages). All parts of this experiment were repeated at least twice with similar results. Data shown are means ± SEM (n = 5).
(0.69 MB EPS)
The clearance of P1-deficient Mp is macrophage-dependent. Mice were treated i.n. and i.p. with clodronate liposomes to deplete macrophages, or with control (PBS) liposomes at day -1, followed by i.n. inoculation with P1-deficient Mp at day 0. At various times after inoculation with Mp, lungs were harvested and total RNA was extracted to determine the numbers of Mp by real-time PCR. (* p<0.05). This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 5).
(0.33 MB EPS)
MyD88 signaling is essential for activation of NFκB and mRNA expression of pro-inflammatory genes in the macrophage response to Mp. BMM derived from wild type or MyD88−/− mice were infected with Mp (MOI 100∶1), or mock infected as control. (A) One hour after infection, BMM were harvested and lysed with lysis buffer containing a phosphatase inhibitor. Five µg of total cell lysate were used for TransAM transcription factor ELISA (Actif Motif) to detect the activated form of NFκB. (B) BMM and (C) lung macrophages, total RNA was harvested at 0 h, 2 h, 4 h, 6 h and 8 h after Mp infection, and the mRNA expression of the pro-inflammatory genes TNF, IL-6, and MIP-2 was analyzed by real-time PCR. The mRNA expression levels of pro-inflammatory genes were compared to the levels in macrophages from WT mice without Mp infection (0 h). This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 3 for BMM; n = 1 for primary lung macrophages).
(0.42 MB EPS)
The clearance of Mp from the airways of mice is independent of mast cells. WT (open bars) and c-kitwsh/wsh (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. This experiment was repeated one time with similar results. Data shown are means ± SEM (n = 5).
(0.43 MB EPS)
The clearnce of Mp from the airways of mice is independent of C3 or nitric oxide produced by iNOS. (A) WT (open bars) and C3−/− (filled bars) mice and (B) WT (open bars) and iNOS−/− (filled bars) mice were inoculated intranasally with 4×106 Mp. At the indicated times, total lung RNA was harvested and the numbers of Mp were measured by real-time PCR. Data shown are means ± SEM (n = 5).
(0.49 MB EPS)