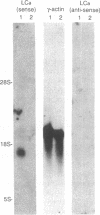
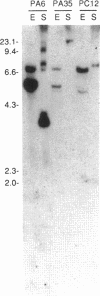
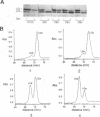
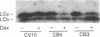Abstract
The light chain subunits of clathrin, LCa and LCb, have been implicated in the regulation of coated vesicle disassembly and other aspects of clathrin cycling within the cell. The potential for functional specialization of each light chain is suggested by tissue-specific variation in the relative amounts of the two light chains and by conservation of differences between LCa and LCb sequences during evolution. To investigate whether there might be exclusive roles for LCa and LCb in clathrin function, the expression of LCa was manipulated in C1R lymphoid cells and PC12 pheochromocytoma cells by transfection with light chain cDNA. These two cell lines differ in their ratios of LCa to LCb, expressing 86 and 25% LCa, respectively. After transfection with exogenous human LCa cDNA, a PC12 cell derivative was produced that completely lost the ability to manufacture LCa. Loss of LCa expression was found to be because of gene disruption and consequent lack of mRNA transcription. In C1R cells, the normally high level of LCa expression was reduced to 25% by overexpression of transfected LCb cDNA under the control of an inducible promoter. The C1R transfectants with reduced levels of LCa and the LCa-negative PC12 transfectant grow normally and show no change in clathrin distribution, clathrin assembly level, or impairment of endocytosis or secretion compared with wild-type cells and cells transfected with vectors lacking light chain cDNA. However, subtle alterations in the hsc70-mediated clathrin uncoating process were observed for vesicles derived from the LCa-negative cells, reflecting the preferential activity of LCa in stimulating the in vitro uncoating reaction.
Full text
PDF





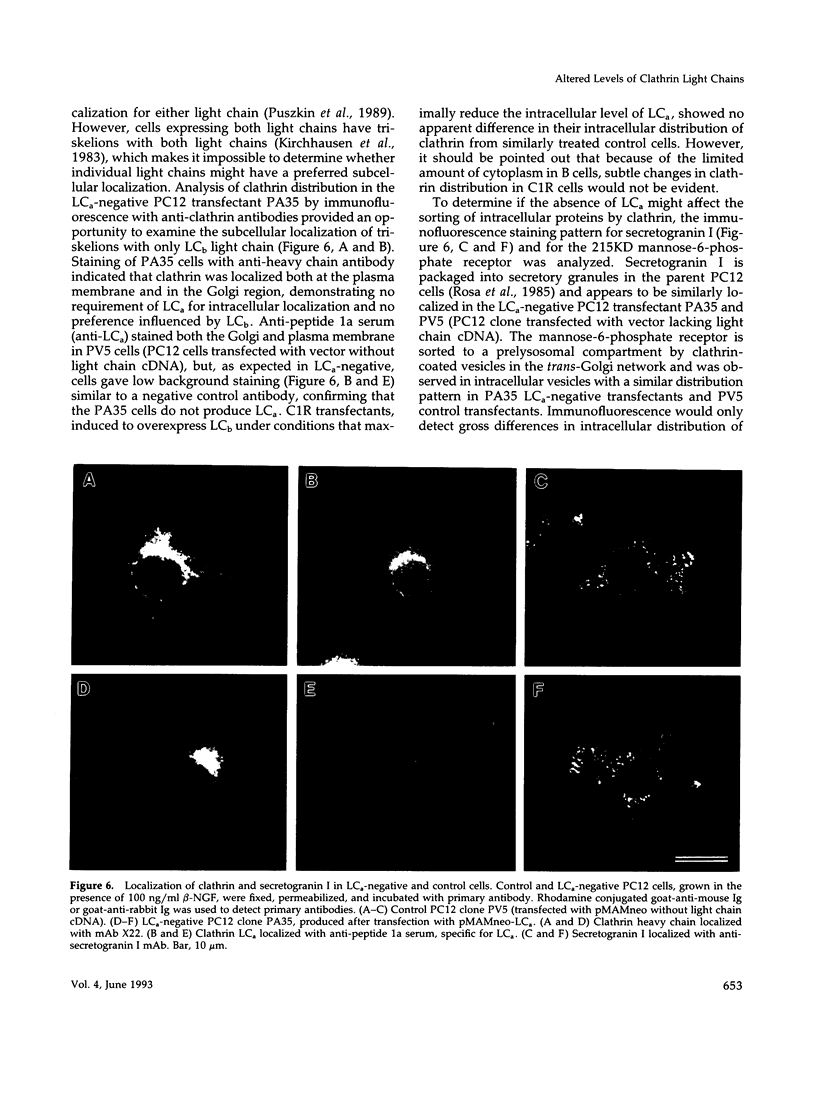







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acton S. L., Brodsky F. M. Predominance of clathrin light chain LCb correlates with the presence of a regulated secretory pathway. J Cell Biol. 1990 Oct;111(4):1419–1426. doi: 10.1083/jcb.111.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baeuerle P. A., Huttner W. B. Tyrosine sulfation is a trans-Golgi-specific protein modification. J Cell Biol. 1987 Dec;105(6 Pt 1):2655–2664. doi: 10.1083/jcb.105.6.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Zvi D., Mosley S. T., Branton D. In vivo phosphorylation of clathrin-coated vesicle proteins from rat reticulocytes. J Biol Chem. 1988 Mar 25;263(9):4408–4415. [PubMed] [Google Scholar]
- Blank G. S., Brodsky F. M. Site-specific disruption of clathrin assembly produces novel structures. EMBO J. 1986 Sep;5(9):2087–2095. doi: 10.1002/j.1460-2075.1986.tb04470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braell W. A., Schlossman D. M., Schmid S. L., Rothman J. E. Dissociation of clathrin coats coupled to the hydrolysis of ATP: role of an uncoating ATPase. J Cell Biol. 1984 Aug;99(2):734–741. doi: 10.1083/jcb.99.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresnahan P. A., Leduc R., Thomas L., Thorner J., Gibson H. L., Brake A. J., Barr P. J., Thomas G. Human fur gene encodes a yeast KEX2-like endoprotease that cleaves pro-beta-NGF in vivo. J Cell Biol. 1990 Dec;111(6 Pt 2):2851–2859. doi: 10.1083/jcb.111.6.2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M. Clathrin structure characterized with monoclonal antibodies. I. Analysis of multiple antigenic sites. J Cell Biol. 1985 Dec;101(6):2047–2054. doi: 10.1083/jcb.101.6.2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M. Clathrin structure characterized with monoclonal antibodies. II. Identification of in vivo forms of clathrin. J Cell Biol. 1985 Dec;101(6):2055–2062. doi: 10.1083/jcb.101.6.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodsky F. M., Galloway C. J., Blank G. S., Jackson A. P., Seow H. F., Drickamer K., Parham P. Localization of clathrin light-chain sequences mediating heavy-chain binding and coated vesicle diversity. Nature. 1987 Mar 12;326(6109):203–205. doi: 10.1038/326203a0. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M., Hill B. L., Acton S. L., Näthke I., Wong D. H., Ponnambalam S., Parham P. Clathrin light chains: arrays of protein motifs that regulate coated-vesicle dynamics. Trends Biochem Sci. 1991 Jun;16(6):208–213. doi: 10.1016/0968-0004(91)90087-c. [DOI] [PubMed] [Google Scholar]
- Brodsky F. M. Living with clathrin: its role in intracellular membrane traffic. Science. 1988 Dec 9;242(4884):1396–1402. doi: 10.1126/science.2904698. [DOI] [PubMed] [Google Scholar]
- Burstein D. E., Greene L. A. Nerve growth factor has both mitogenic and antimitogenic activity. Dev Biol. 1982 Dec;94(2):477–482. doi: 10.1016/0012-1606(82)90364-5. [DOI] [PubMed] [Google Scholar]
- Chu G., Hayakawa H., Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987 Feb 11;15(3):1311–1326. doi: 10.1093/nar/15.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLuca-Flaherty C., McKay D. B., Parham P., Hill B. L. Uncoating protein (hsc70) binds a conformationally labile domain of clathrin light chain LCa to stimulate ATP hydrolysis. Cell. 1990 Sep 7;62(5):875–887. doi: 10.1016/0092-8674(90)90263-e. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Hill B. L., Drickamer K., Brodsky F. M., Parham P. Identification of the phosphorylation sites of clathrin light chain LCb. J Biol Chem. 1988 Apr 25;263(12):5499–5501. [PubMed] [Google Scholar]
- Hopkins C. R., Trowbridge I. S. Internalization and processing of transferrin and the transferrin receptor in human carcinoma A431 cells. J Cell Biol. 1983 Aug;97(2):508–521. doi: 10.1083/jcb.97.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson A. P., Parham P. Structure of human clathrin light chains. Conservation of light chain polymorphism in three mammalian species. J Biol Chem. 1988 Nov 15;263(32):16688–16695. [PubMed] [Google Scholar]
- Jackson A. P., Seow H. F., Holmes N., Drickamer K., Parham P. Clathrin light chains contain brain-specific insertion sequences and a region of homology with intermediate filaments. Nature. 1987 Mar 12;326(6109):154–159. doi: 10.1038/326154a0. [DOI] [PubMed] [Google Scholar]
- Karin M., Mintz B. Receptor-mediated endocytosis of transferrin in developmentally totipotent mouse teratocarcinoma stem cells. J Biol Chem. 1981 Apr 10;256(7):3245–3252. [PubMed] [Google Scholar]
- Kirchhausen T., Harrison S. C., Parham P., Brodsky F. M. Location and distribution of the light chains in clathrin trimers. Proc Natl Acad Sci U S A. 1983 May;80(9):2481–2485. doi: 10.1073/pnas.80.9.2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lisanti M. P., Shapiro L. S., Moskowitz N., Hua E. L., Puszkin S., Schook W. Isolation and preliminary characterization of clathrin-associated proteins. Eur J Biochem. 1982 Jul;125(2):463–470. doi: 10.1111/j.1432-1033.1982.tb06706.x. [DOI] [PubMed] [Google Scholar]
- Lowe A. W., Madeddu L., Kelly R. B. Endocrine secretory granules and neuronal synaptic vesicles have three integral membrane proteins in common. J Cell Biol. 1988 Jan;106(1):51–59. doi: 10.1083/jcb.106.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooibroek M. J., Michiel D. F., Wang J. H. Clathrin light chains are calcium-binding proteins. J Biol Chem. 1987 Jan 5;262(1):25–28. [PubMed] [Google Scholar]
- Näthke I. S., Heuser J., Lupas A., Stock J., Turck C. W., Brodsky F. M. Folding and trimerization of clathrin subunits at the triskelion hub. Cell. 1992 Mar 6;68(5):899–910. doi: 10.1016/0092-8674(92)90033-9. [DOI] [PubMed] [Google Scholar]
- Näthke I., Hill B. L., Parham P., Brodsky F. M. The calcium-binding site of clathrin light chains. J Biol Chem. 1990 Oct 25;265(30):18621–18627. [PubMed] [Google Scholar]
- Orci L., Halban P., Amherdt M., Ravazzola M., Vassalli J. D., Perrelet A. A clathrin-coated, Golgi-related compartment of the insulin secreting cell accumulates proinsulin in the presence of monensin. Cell. 1984 Nov;39(1):39–47. doi: 10.1016/0092-8674(84)90189-2. [DOI] [PubMed] [Google Scholar]
- Puszkin S., Kohtz J. D., Schook W. J., Kohtz D. S. Clathrin-coated vesicle subtypes in mammalian brain tissue: detection of polypeptide heterogeneity by immunoprecipitation with monoclonal antibodies. J Neurochem. 1989 Jul;53(1):51–63. doi: 10.1111/j.1471-4159.1989.tb07294.x. [DOI] [PubMed] [Google Scholar]
- Rajewsky K. A phenotype or not: targeting genes in the immune system. Science. 1992 Apr 24;256(5056):483–483. doi: 10.1126/science.1570513. [DOI] [PubMed] [Google Scholar]
- Rosa P., Hille A., Lee R. W., Zanini A., De Camilli P., Huttner W. B. Secretogranins I and II: two tyrosine-sulfated secretory proteins common to a variety of cells secreting peptides by the regulated pathway. J Cell Biol. 1985 Nov;101(5 Pt 1):1999–2011. doi: 10.1083/jcb.101.5.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salter R. D., Clayberger C., Lomen C. E., Krensky A. M., Parham P. In vitro mutagenesis at a single residue introduces B and T cell epitopes into a class I HLA molecule. J Exp Med. 1987 Jul 1;166(1):283–288. doi: 10.1084/jem.166.1.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid S. L., Braell W. A., Schlossman D. M., Rothman J. E. A role for clathrin light chains in the recognition of clathrin cages by 'uncoating ATPase'. Nature. 1984 Sep 20;311(5983):228–231. doi: 10.1038/311228a0. [DOI] [PubMed] [Google Scholar]
- Silveira L. A., Wong D. H., Masiarz F. R., Schekman R. Yeast clathrin has a distinctive light chain that is important for cell growth. J Cell Biol. 1990 Oct;111(4):1437–1449. doi: 10.1083/jcb.111.4.1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soriano P., Montgomery C., Geske R., Bradley A. Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell. 1991 Feb 22;64(4):693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- Südhof T. C. Synaptic vesicles. Curr Opin Cell Biol. 1989 Aug;1(4):655–659. doi: 10.1016/0955-0674(89)90030-6. [DOI] [PubMed] [Google Scholar]
- Tooze J., Tooze S. A. Clathrin-coated vesicular transport of secretory proteins during the formation of ACTH-containing secretory granules in AtT20 cells. J Cell Biol. 1986 Sep;103(3):839–850. doi: 10.1083/jcb.103.3.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tooze S. A., Huttner W. B. Cell-free protein sorting to the regulated and constitutive secretory pathways. Cell. 1990 Mar 9;60(5):837–847. doi: 10.1016/0092-8674(90)90097-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E. Biochemical and immunological studies on clathrin light chains and their binding sites on clathrin triskelions. EMBO J. 1983;2(8):1401–1408. doi: 10.1002/j.1460-2075.1983.tb01598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungewickell E., Ungewickell H. Bovine brain clathrin light chains impede heavy chain assembly in vitro. J Biol Chem. 1991 Jul 5;266(19):12710–12714. [PubMed] [Google Scholar]
- Winkler F. K., Stanley K. K. Clathrin heavy chain, light chain interactions. EMBO J. 1983;2(8):1393–1400. doi: 10.1002/j.1460-2075.1983.tb01597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D. H., Ignatius M. J., Parosky G., Parham P., Trojanowski J. Q., Brodsky F. M. Neuron-specific expression of high-molecular-weight clathrin light chain. J Neurosci. 1990 Sep;10(9):3025–3031. doi: 10.1523/JNEUROSCI.10-09-03025.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]