Abstract
Incorporation of cross-linked quaternary ammonium polyethylenimine (QPEI) nanoparticles in dental resin composite has a long-lasting and wide antimicrobial effect with no measured impact on biocompatibility in vitro. We hypothesized that QPEI nanoparticles incorporated into a resin composite have a potent antibacterial effect in vivo and that this stress condition triggers a suicide module in the bacterial biofilm. Ten volunteers wore a removable acrylic appliance, in which two control resin composite specimens and two resin composite specimens incorporating 1% wt/wt QPEI nanoparticles were inserted to allow the buildup of intraoral biofilms. After 4 h, the specimens were removed and tested for bacterial vitality and biofilm thickness, using confocal laser scanning microscopy. The vitality rate in specimens incorporating QPEI was reduced by > 50% (p < 0.00001), whereas biofilm thickness was increased (p < 0.05). The ability of the biofilm supernatant to restore bacterial death was tested in vitro. The in vitro tests showed a 70% decrease in viable bacteria (p < 0.05). Biofilm morphological differences were also observed in the scanning electron microscope micrographs of the resin composite versus the resin composite incorporating QPEI. These results strongly suggest that QPEI nanoparticles incorporated at a low concentration in resin composite exert a significant in vivo antibiofilm activity and exhibit a potent broad spectrum antibacterial activity against salivary bacteria.
Resin composite materials are commonly used in dental practice as hard tissue substitutes owing to their superior esthetic properties. However, in vivo dental biofilm accumulates on resin composites to a greater extent than on enamel and other restorative materials (1, 2). Although dentin adhesive systems used for resin composites may strongly bind to enamel and dentin, they do not have the ability to prevent the occurrence of microgaps between the tooth and the restoration (3). Consequently, the restoration margins can provide a potential pathway for leakage of cariogenic microorganisms present in the normal human flora, resulting in secondary caries (4–6). Therefore, resin composite restorations that possess antibacterial properties could be beneficial in eliminating the detrimental effect caused by bacterial microleakage.
Numerous articles have demonstrated the antimicrobial utility of cationic polymers with quaternary ammonium groups (7–9). In particular, it was reported that quaternary ammonium polyethyleneimine (QPEI) possesses excellent antibacterial activity (10, 11). Bearing this in mind and understanding the pathogenesis of secondary caries and the properties of resin composite restorations, we showed that resin composites can be chemically modified to acquire potent and long-lasting antibacterial surface properties in vitro (12). For this purpose and to overcome the disadvantages of materials that release antibacterial agents (13–15), we prepared more than 50 derivatives of polycationic particles, starting from different polyamines and alkyl halides that had 4 to 16 methylene groups, using different methods of synthesis. Although some of the tested nanoparticles were found to be effective in inhibiting bacterial growth, octyl alkylated QPEI nanoparticles incorporated into dental resin composite at 1% wt/wt showed a superior antibacterial effect (16, 17). These nanoparticles were mixed into prepolymerized commercially available dental resin composites, modifying the restoration material. We thought that antibacterial modification of the dental resin composite is an advantageous approach for preparing materials that are continuously challenged physically in a harsh environment for many years. The incorporation of these QPEI nanoparticles in resin composite rendered a long-lasing antimicrobial effect against a wide range of bacteria with no measured influence on biocompatibility and without leaching (17–19). Furthermore, we have found that QPEI nanoparticle quarternization with octyl groups yields optimal antibacterial properties for dental composite restorative material, i.e. 6-mo aging of composite incorporating 1% wt/wt QPEI nanoparticles, resulted in complete inhibition of cariogenic mutans streptococci biofilm in vitro (20).
Although the detailed mechanism of the antibacterial effect of polycations bearing quaternary ammonium moieties has not been fully determined, it was suggested that they cause lysis of the bacterial cells. Consequently, stressful conditions such as exposure to antibiotics or other states causing cell death and lysis may initiate death in bacterial cultures, mediated by an intracellular death program (21, 22). An emerging paradigm in this field suggests that, analogous to programmed cell death in eukaryotes, regulated cell death and lysis in bacteria play an important role in developmental processes, such as competence and biofilm development, and in the elimination of damaged cells, such as those irreversibly injured by environmental or antibiotic stress (23). We hypothesized that QPEI when incorporated in resin composite has a potent antibacterial effect on salivary microorganisms and thus prominently affects oral biofilm in vivo.
We now report a quantitative and qualitative experimental investigation of the in vivo antibacterial effect of QPEI nanoparticles incorporated in a resin composite on intraoral biofilm. QPEI nanoparticles were incorporated at 1% wt/wt in commercially available composite, and their antibacterial effect on oral biofilm was assessed. The resultant resin composite showed a strong antibacterial and antibiofilm effect on biofilm at its outset. We also suggest that the QPEI nanoparticles may trigger a built-in death program in the oral biofilm in vivo.
Results and Discussion
To achieve the long-term success of dental restorations, not only the professional carrying out the work, but also the different physical, chemical, and biological properties of the materials play an important role. These characteristics are also relevant factors affecting the attachment of organisms to the surface forming a biofilm. The lack of antibacterial properties of photopolymerized resin composites (24) means that there is no inhibitory effect against biofilm accumulation, and therefore cariogenic bacteria such as mutans streptococci can readily grow on composites and penetrate into the microgap at the tooth-restoration interface. Furthermore, dental resin composites confront a complex oral microbiological environment composed of hundreds of different strains of bacteria, which is unattainable in vitro. To better understand the metabolic process or the clinical effects of antibacterial substances such as QPEI nanoparticles against biofilm, it is necessary to choose an examination method in which the biofilm grows directly in the oral cavity and its three-dimensional structure is not manipulated.
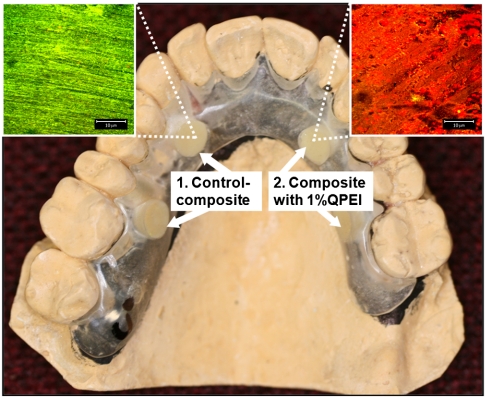
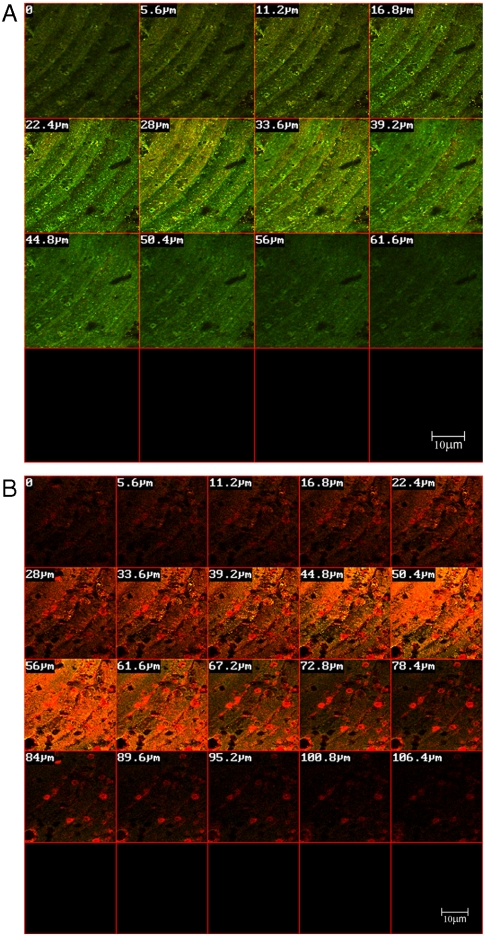
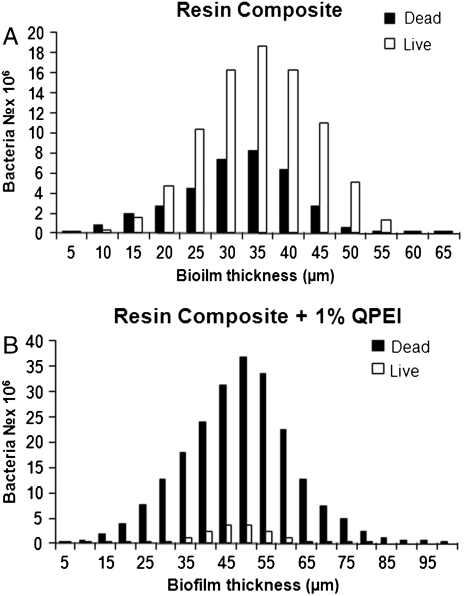
Accordingly, in the present study a custom-made removable acrylic appliance was placed intraorally in 10 volunteers (Fig. 1). Four disc-shaped specimens, two of resin composite and two of resin composite incorporating 1% wt/wt QPEI nanoparticles, were inserted in each appliance and the buildup of intraoral biofilms was allowed for 4 h. The percentage of QPEI nanoparticles to be added was decided after testing the antibacterial effect of various low concentrations (for details, see SI Text). The oral mucosa was examined carefully immediately after the appliance was removed and showed no signs of redness, warmth, tenderness, or swelling. This indicated that no inflammation or allergic reaction had developed. These findings coincide with our previous in vitro and in vivo histological finding that the incorporation of QPEI nanoparticles into resin composite does not compromise its safety for use (18, 19). Furthermore, the volunteers did not complain of pain or discomfort during the time they wore the appliance or afterward. The discs were recovered and the builtup biofilms were analyzed qualitatively and quantitatively. For this purpose, we utilized confocal scanning laser microscopy (CSLM), which serves as a powerful tool for studying bacteria in dental biofilm. First, using specific dyes, the discs were subjected to standard ex vivo staining of the bacteria, reflecting bacterial viability and biofilm thickness (for details see SI Text). The biofilm formed on the surface of the resin composite discs was stained mainly green (as seen in Fig. 1, Left), indicating that the majority of the bacterial cells were viable, whereas the red stain was evident on the resin composites incorporating QPEI nanoparticles, indicating that most of the cells were nonviable (as seen in Fig. 1, Right). The biofilm on each disc was further analyzed quantitatively, in four randomly captured images; i.e., the number of dead and live bacteria as well as the biofilm thickness in each image was determined and averaged for the assessed effect per sample as shown for one of the volunteers in Figs. 2 and 3. Analysis of the samples collected from all 10 volunteers showed a significant (p < 0.00001) reduction of the viable bacteria in the biofilm formed on the surface of the resin composites with incorporated QPEI nanoparticles. Interestingly, incorporation of the nanoparticles did not reduce biofilm thickness, but rather increased it (p < 0.05) as summarized in Table 1.
Fig. 1.
Removable acrylic appliance withholding two resin composite discs and two discs of resin composite incorporating QPEI. Confocal laser microscope surface images of the attached biofilm formed on (1) resin composite mainly show dead cells stained red, whereas biofilm formed on (2) resin composite incorporating QPEI nanoparticles mainly shows live cells stained green.
Fig. 2.
Biofilms formed on resin composite incorporating QPEI nanoparticles and nonmodified resin composite. Confocal laser scanning microscope cross-section images of biofilms formed on resin composite (A) and resin composite with incorporated QPEI nanoparticles (B).
Fig. 3.
Bacterial vitality and biofilm thickness of biofilms formed on resin composite incorporating QPEI nanoparticles and nonmodified resin composite. Biofilms were stained using the BacLight LIVE/DEAD viability stain and scanned using a confocal laser scanning microscope. Average cross-section enumeration of viable and nonviable bacteria, as measured by Image ProPlus softwear of biofilms formed on resin composite (A) and resin composite with incorporated QPEI nanoparticles (B).
Table 1.
Total values of vital bacteria (% viable cells) as measured by Image ProPlus software, and biofilm thickness (μm)
| % Viable cells ± SD, n = 4* | Biofilm thickness, μm, ± SD, n = 4* | |||||||
| Resin composite | Resin composite +PEI | Resin composite | Resin composite +PEI | |||||
| Sample no. | I | II | I | II | I | II | I | II |
| Volunteer no. | ||||||||
| 1 | 90.1 ± 0.8 | 92.1 ± 1.4 | 33.7 ± 9.6 | 24.4 ± 5.0 | 52.5 ± 2.8 | 57.5 ± 2.8 | 50 ± 0 | 55 ± 10 |
| 2 | 79.9 ± 1.0 | 75.1 ± 1.8 | 13.9 ± 12.7 | 11.6 ± 6.2 | 60 ± 0 | 62.5 ± 5 | 173.7 ± 17.5 | 161.2 ± 31.7 |
| 3 | 88.5 ± 9.1 | 93.9 ± 4.7 | 9.6 ± 13.9 | 10.9 ± 2.5 | 87.5 ± 27.5 | 96.2 ± 56.4 | 80 ± 0 | 80 ± 0 |
| 4 | 70.6 ± 6.5 | 61.2 ± 3.9 | 21.5 ± 18.4 | 42.4 ± 27.7 | 105 ± 12.2 | 111.2 ± 19.3 | 111.2 ± 49.5 | 125 ± 62.7 |
| 5 | 56.1 ± 1.2 | 56.2 ± 2.3 | 27.5 ± 15.6 | 38.9 ± 2.6 | 73.7 ± 4.7 | 82.5 ± 9.5 | 117.5 ± 12.5 | 110 ± 11.5 |
| 6 | 60.5 ± 20.3 | 74.0 ± 2.4 | 6.7 ± 5.3 | 4.4 ± 1.6 | 57.5 ± 9.5 | 60 ± 0 | 92.5 ± 35.9 | 82.5 ± 8.6 |
| 7 | 55.1 ± 2.9 | 49.5 ± 4.9 | 12.0 ± 2.5 | 15.7 ± 1.9 | 46.2 ± 6.2 | 45 ± 7.0 | 106.2 ± 57.9 | 108.7 ± 62.2 |
| 8 | 57.2 ± 1.8 | 57.3 ± 4.0 | 15.0 ± 9.0 | 9.7 ± 8.5 | 78.7 ± 17.5 | 81.2 ± 18.8 | 103.7 ± 40.0 | 112.5 ± 50.0 |
| 9 | 84.3 ± 7.0 | 81.3 ± 2.8 | 22.2 ± 5.7 | 19.9 ± 6.4 | 53.7 ± 2.5 | 55 ± 0 | 137.5 ± 14.4 | 135 ± 17.3 |
| 10 | 66.9 ± 5.7 | 69.2 ± 4.3 | 16.7 ± 13.5 | 7.3 ± 5.0 | 57.5 ± 5 | 58.7 ± 2.5 | 108.7 ± 17.5 | 96.2 ± 11.0 |
| Mean | 70.9 ± 13.1 | 19.2 ± 11.2 | 69.1 ± 20.2 | 107.3 ± 26.0 | ||||
| p < 0.00001 | p < 0.05 | |||||||
*Four images were randomly taken from each disc sample; the number of dead and live bacteria, as well as the biofilm thickness in each image were determined and averaged.
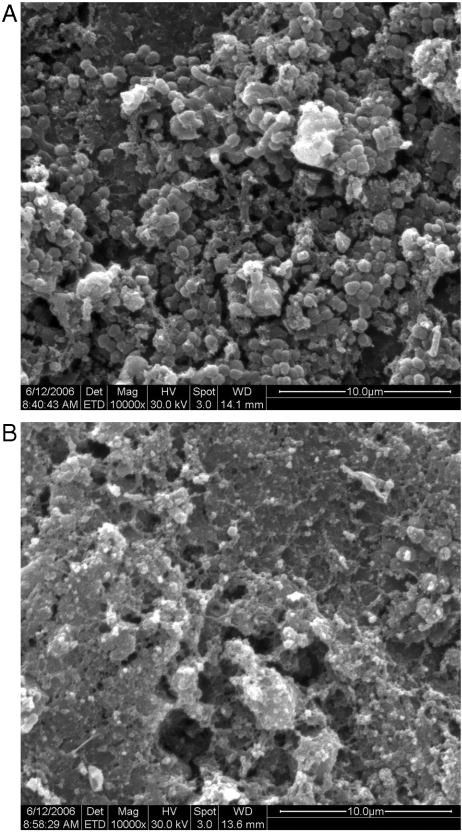
Second, a qualitative assessment of the same discs was performed using a scanning electron microscope (SEM) (SI Text). SEM images revealed differences in the biofilm structures formed on each of the tested groups. The resin composite SEM micrographs (Fig. 4A) depicted an established biofilm with numerous evident bacterial cells, whereas the biofilm morphology on the resin composite incorporating QPEI nanoparticles revealed only a few bacteria with clear membranes (Fig. 4B). Although in the present study SEM imaging of the specimens without biofilm cells did not reveal any difference in the surface views of resin composite versus resin composite incorporating 1% wt/wt QPEI nanoparticles (see SI Text), the presence and distribution of the active antibacterial groups on the surface was observed using CLSM (see SI Text), in concurrence with our previous findings (19, 20).
Fig. 4.
Biofilms formed on resin composite incorporating QPEI nanoparticles and on nonmodified resin composite. Scanning electron microscope micrographs (10,000×) of biofilms formed on resin composite (A) and resin composite with incorporated QPEI nanoparticles (B).
All nonshedding surfaces in the oral cavity, biological or artificial, are potential substrates for the development and growth of dental biofilm. Subsequently, following the formation of an acquired salivary pellicle, the first colonizers of these surfaces are streptococci, commonly Streptococcus sanguinis, later followed mainly by other streptococci and Gram-positive rods, such as actinomyces (25–27). Caries-associated mutans streptococci can be found in the early forming biofilm (27). In our study early biofilms after emerging naturally on the tested surfaces with and without incorporated antibacterial nanoparticles in vivo were examined ex vivo using standard dye reactivity.
Although bactericidal-immobilized materials usually show an inactivating effect only against bacteria that come into contact with the antibacterial molecules, surprisingly, in our study it was evident that > 50% of the bacteria in the biofilm formed on the surface of the modified resin composites incorporating QPEI nanoparticles were dead, even in the outer, more remote parts of the biofilm.
In previous studies we showed that incorporation of 1% wt/wt QPEI nanoparticles into resin composites is sufficient to exert a very potent and long-lasting antibacterial effect. The antibacterial compound is stable and does not leach out from the material into the surrounding environment. Is it likely that this small amount of QPEI nanoparticles is sufficient to cause stress not only to the bacteria with which they come into contact but to the other outer cell layers of the primary formed biofilm? Further investigation of this phenomenon is necessary to clarify whether this condition equilibrium distortion in the biofilm structure causes large-scale bacterial death.
It was shown that bacterial lysis may function as a stressful condition triggering programmed cell death (PCD) in the surrounding bacteria (28–32). Traditionally, PCD is associated with eukaryotic multicellular organisms (21). However, PCD systems have also been observed in bacteria. When challenged, the bacterial population appears to act like a multicellular organism in which a subpopulation dies, thereby permitting the survival of the bacterial population as a whole. Our results initially led us to suspect that the primary biofilm bacteria cells when coming in contact with QPEI nanoparticles caused cell death on a larger scale. Bearing this in mind, we examined in vitro whether bacterial cell death could be induced only by the cell extract of bacteria that were exposed to QPEI nanoparticles. The mechanism of action of antibacterial quaternary ammonium compounds is believed to be a sequence of events beginning with cationic binding and electrostatic interaction between the QPEI and the microorganism’s cell wall components. This results in strong adsorption that later disrupts membrane function and leads to leakage of constituents such as K+ ions, DNA, and RNA and culminates in cell death (33). To distinguish between the antibacterial effect of QPEI nanoparticles and the effect of the biofilm cells on the neighboring cells, we used the extract of a biofilm grown in vitro on the surface of resin composite incorporating QPEI nanoparticles. Bacteria from fresh whole saliva collected from a volunteer were inoculated on the surface of resin composite discs incorporating QPEI nanoparticles, as described previously (34). The resin composite discs were then placed in liquid medium to allow biofilm growth for 24 h. Next, biofilm cells were detached from the discs into the medium, incubated for 3 h and centrifuged. To isolate the factors secreted from the biofilm cells, the supernatant was then filtered. The biofilm extract obtained was lyophilized, and the powder obtained was adsorbed to a resin composite surface that was then photopolymerized (SI Text).
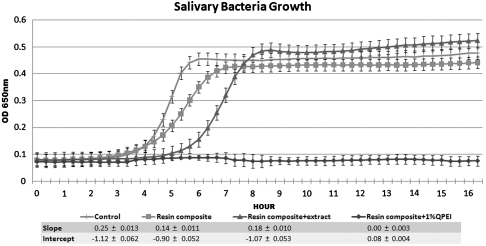
To gain more insight into the observed antibiofilm effect, we quantified the growth inhibition of salivary bacteria of the same volunteer using the direct contact test (24). Salivary bacteria were allowed to come in direct contact, under controlled conditions, with (i) resin composite with biofilm extract, (ii) resin composite incorporating 1% wt/wt QPEI nanoparticles; and for comparison (iii) resin composite, and (iv) microtiter plate surface. Medium was added and the growth of bacteria shed from the biofilm was estimated by recording the changes in optical density for a period of 16 h. The absorbance measurements were plotted, providing bacterial growth curves. As seen in Fig. 5, no bacterial growth was detected following direct contact with resin composite incorporating 1% wt/wt QPEI nanoparticles, indicating a potent antibacterial effect on total salivary bacteria ex vivo. Surprisingly, bacteria that came in contact with resin composites covered by the biofilm extract exhibited a partial bactericidal effect, as shown by the shift of the growth curve.
Fig. 5.
In vitro antibacterial effect of (i) resin composite with biofilm extract; (ii) resin composite incorporating 1% wt/wt of added QPEI nanoparticles, and (iii) nonmodified resin composite and (iv) microtiter plate surface on salivary bacteria. Kinetic measurements depicting growth of bacteria collected from saliva following direct contact with resin composite; resin composite incorporating 1% wt/wt of added QPEI nanoparticles; and with resin composite covered with an extract of bacteria previously grown on resin composite incorporating QPEI. Growth of the shed bacteria from the biofilm was measured every 20 min for 16 h. Each point on the curve is the average (± SD) optical density (650 nm) measured in 12 replica wells similarly prepared in the same microtiter plate. The linear portion of the logarithmic growth phase was analyzed and expressed according to two variables: the slope (a) and the constant (b) of the linear function ax + b = y. The slope (a) and the constant (b) correlate with growth rate and initial bacterial number, respectively.
The linear portion of the logarithmic growth curve, derived from the ascending part of the curve, was expressed by two variables: the slope (a) and the constant (b) of the linear function ax + b = y. These variables correlate with the growth rate (a = slope) and the initial number of viable bacteria (b = constant) as deducted from calibration growth curves that were obtained using serial salivary-bacteria dilutions in the same microtiter plate. A 70% decrease in viable bacteria at the onset of the experiment was extrapolated from the variables obtained from the linear portion of the logarithmic growth phase of bacteria grown on the resin composite covered with the biofilm extract and the variables obtained from the calibration curves. Thus, it is conceivable that < 30% of the bacterial population remained following contact with the extract that was adsorbed on the resin composite surface, compared with the control. The salivary bacteria brought in direct contact with the composite in the in vitro study simulated the effect of the modified resin composite on the very early events in the biofilm immediately after attachment, whereas the in vivo experiments depict the evolving effect on a 4-h biofilm. Consequently, the in vitro results showed that most of the bacteria that attached directly to the surface were killed, whereas in the in vivo test bacteria continued attaching thereafter not to the resin composite surface but to the killed bacteria. These findings coincide with recent studies that revealed the importance of regulated bacterial death for biofilm development (35). In the current study bacterial death supported, on the one hand, further biofilm adhesion increasing biofilm thickness, but on the other hand the stress effect caused by the QPEI nanoparticles was sufficient to cause significant in vivo antibiofilm activity.
In conclusion, QPEI nanoparticles incorporated at a low concentration in resin composite exhibit significant in vivo antibiofilm activity as well as potent broad spectrum antibacterial activity against salivary bacteria. The hypothesis that QPEI nanoparticles incorporated into commercial resin composite may function as a trigger for a built-in death program in oral biofilm is now under investigation.
Because the occurrence of a microgap between the tooth and the restoration’s margins provides a pathway for cariogenic microorganisms, resulting in secondary caries, antibacterial restorative dental materials such as the one described in our study have the potential to prolong the service life of dental restorations. This may be a step toward devising better materials for longer-lasting dental restorations and reducing the need for repeated restorative procedures.
Materials and Methods
The protocol of the study was approved by the Helsinki Committee for Human Clinical Trials (http://clinicaltrials.gov/ Identifier: NCT00299598). Ten adults aged 25–55 yr volunteered to participate in the study. All participants were healthy and each signed an informed consent form. (for details, see SI Text).
PEI Nanoparticle Preparation.
The synthesis of quaternary ammonium PEI nanoparticle was previously described by Beyth et al. (12); for details, see SI Text. The average yield was 70% (mol/mol). FTIR (QPEI nanoparticles, KBr): 3,440 cm-1 (N-H), 2,956, 2,926, and 2,853 cm-1 (C-H), 1,617 cm-1 (N-H, small band), 1,465 cm-1 (C-H), 967 cm-1 quaternary nitrogen. 1H-NMR (DMSO): 0.845 ppm (t, 3H, CH3, octane hydrogens), 1.24 ppm (m, 10H, ─CH2─, octyl hydrogens) 1.65 ppm (m, 2H, CH, octyl hydrogens), 3.2–3.6 ppm (m, CH3 of quaternary amine, 4H, ─CH2─, PEI hydrogens, and 2H, ─CH2─, octyl hydrogens.
Preparation of Test Samples.
The test specimens were prepared by adding the synthesized polymer to a commercial resin composite FILTEK FLOW (47% zirconia/silica average particle size 0.01–6.0 μ; BIS-GMA, TEGDMA), 3M ESPE Dental. A 1% wt/wt polymer powder was added to 100 ± 20 mg of the commercial composite resin and homogeneously mixed in a dark room for 20 s with a spatula before polymerization in disc form. A detailed description of the removable acrylic appliance prepared for the volunteers and of specimen preparation appears in SI Text. Each participant wore the appliance (see Fig 1) on the upper jaw for 4 h.
Confocal Laser Scanning Microscopy.
CLSM allowed us to explore the vitality of bacteria in the different layers of the biofilm following treatment. Biofilm was allowed to form on discs. The removed discs (n = 40) were tested for biofilm formation and viability using a confocal laser microscope (36). After exposure, the samples were dyed using a live/dead kit (Live/Dead BacLight viability kit, Molecular Probes) described in SI Text. The stained bacteria were examined using a confocal microscope; the fluorescence emission of the discs was detected using a Zeiss LSM 410 confocal laser microscope (Carl Zeiss Microscopy). Biofilm was quantified by measuring the area occupied by the microorganisms in each individual layer in relation to the tested area. The bacterial index was determined with the aid of Image Pro 4.5 software (Media Cybernetics). Statistical analysis of the biofilm viability and biofilm thickness in the CLSM experiments was performed using the T-paired test and the Wilcoxon nonparametric paired rank test.
Scanning Electron Microscopy.
Following examination of the 40 discs using the CLSM—one test disc and one control disc from each volunteer were fixed in 2% glutaraldehyde, washed in cacodylate buffer (0.1 M, pH 7.2), postfixed in 2% osmium tetra oxide for 1 h, and examined with the aid of a Philips 505 SEM at accelerating voltage (for details, see SI Text). To compare the surface views of resin composite and resin composite discs incorporating 1% wt/wt of added QPEI nanoparticles, an additional set of control discs without the biofilm was examined (see SI Text).
Distribution Analysis.
The distribution of QPEI nanoparticles incorporated in resin composite was further examined in order to determine whether the nanoparticles are present on the surface of the modified material and can be used to achieve antibacterial surface properties. Surface distribution analysis of QPEI incorporated at 0, 0.25, 0.5, or 1% wt/wt labeled with dansyl chloride was performed using CLSM (see SI Text).
In Vitro Antibacterial Tests.
To distinguish between the antibacterial effect of QPEI nanoparticles and the effect of the biofilm cells on their neighboring cells, we used the extract of a biofilm grown on the surface of a resin composite incorporating QPEI (described in detail in SI Text).
To gain some insight into the observed antibacterial effect, we quantified the growth inhibition of bacteria obtained from the saliva of the same volunteer using the direct contact test as described previously by Beyth et al. (24) (for details, see SI Text). Using the direct contact test we studied the kinetics of bacterial growth; the bacteria were allowed to come in direct contact, under controlled conditions, with the resin composite without added QPEI but covered with the previously obtained bacterial extract in a set of 8. Bacterial growth was also measured in additional wells after direct contact with the resin composite (n = 8) and with resin composite incorporating 1% wt/wt of added QPEI nanoparticles (n = 8), as shown in SI Text. Additionally, to choose the most effective concentration to be used in the in vivo experiment, various concentrations of added QPEI nanoparticles were tested in a different set of experiments (for details, see SI Text). The growth of bacteria shed from the biofilm was estimated by recording the changes in optical density during 16 h. The absorbance measurements were plotted, providing bacterial growth curves. The data were analyzed by one way ANOVA and the Tukey multiple comparison test. The level of significance was determined as p < 0.05.
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010341107/-/DCSupplemental.
References
- 1.Hahn R, Weiger R, Netuschil L, Bruch M. Microbial accumulation and vitality on different restorative materials. Dent Mater. 1993;9:312–316. doi: 10.1016/0109-5641(93)90049-v. [DOI] [PubMed] [Google Scholar]
- 2.Siegrist BE, Brecx MC, Gusberti FA, Joss A, Lang NP. In vivo early human dental plaque formation on different supporting substances. A scanning electron microscopic and bacteriological study. Clin Oral Implan Res. 1991;2:38–46. doi: 10.1034/j.1600-0501.1991.020105.x. [DOI] [PubMed] [Google Scholar]
- 3.Chigira H, et al. Efficacy of various commercial dentin bonding systems. Dent Mater. 1994;10:363–368. doi: 10.1016/0109-5641(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 4.Goracci G, Mori G, Bazzucchi M. Marginal seal and biocompatibility of a fourth-generation bonding agent. Dent Mater. 1995;11:343–347. doi: 10.1016/0109-5641(95)80032-8. [DOI] [PubMed] [Google Scholar]
- 5.Lobo MM, Goncalves RB, Ambrosano GM, Pimenta LA. Chemical or microbiological models of secondary caries development around different dental restorative materials. J Biomed Mater Res B. 2005;74:725–731. doi: 10.1002/jbm.b.30253. [DOI] [PubMed] [Google Scholar]
- 6.Seemann R, Bizhang M, Kluck I, Loth J, Roulet JF. A novel in vitro microbial-based model for studying caries formation—development and initial testing. Caries Res. 2005;39:185–190. doi: 10.1159/000084796. [DOI] [PubMed] [Google Scholar]
- 7.Imazato S, McCabe JF. Influence of incorporation of antibacterial monomer on curing behavior of a dental composite. J Dent Res. 1994;73:1641–1645. doi: 10.1177/00220345940730100901. [DOI] [PubMed] [Google Scholar]
- 8.Li G, Shen J, Zhu Y. A study of pyridinium-type functional polymers. III. Preparation and characterization of insoluble pyridinium-type polymers. J Appl Polym Sci. 2000;78:668–675. [Google Scholar]
- 9.Tiller JC, Liao CJ, Lewis K, Klibanov AM. Designing surfaces that kill bacteria on contact. Proc Natl Acad Sci USA. 2001;98:5981–5985. doi: 10.1073/pnas.111143098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gao B, Zhang X, Zhu Y. Studies on the preparation and antibacterial properties of quaternized polyethyleneimine. J Biomat Sci-Polym E. 2007;18:531–544. doi: 10.1163/156856207780852523. [DOI] [PubMed] [Google Scholar]
- 11.Lin J, Qiu S, Lewis K, Klibanov AM. Bactericidal properties of flat surfaces and nanoparticles derivatized with alkylated polyethylenimines. Biotechnol Prog. 2002;18:1082–1086. doi: 10.1021/bp025597w. [DOI] [PubMed] [Google Scholar]
- 12.Beyth N, Yudovin-Farber I, Bahir R, Domb AJ, Weiss EI. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials. 2006;27:3995–4002. doi: 10.1016/j.biomaterials.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 13.Kohnen W, Jansen B. Polymer materials for the prevention of catheter-related infections. Zbl Bakt. 1995;283:175–186. doi: 10.1016/s0934-8840(11)80199-4. [DOI] [PubMed] [Google Scholar]
- 14.Nohrb RS, Macdonald GJ. New biomaterials through surface segregation phenomenon: New quaternary ammonium compounds as antibacterial agents. J Biomat Sci-Polym E. 1994;5:607–619. doi: 10.1163/156856294x00239. [DOI] [PubMed] [Google Scholar]
- 15.Shearer AE, Paik JS, Hoover DG, Haynie SL, Kelley MJ. Potential of an antibacterial ultraviolet-irradiated nylon film. Biotechnol Bioeng. 2000;67:141–146. [PubMed] [Google Scholar]
- 16.Yudovin-Farber I, Golenser J, Beyth N, Weiss EI, Domb AJ. Quaternary ammonium polyethyleneimine: Antibacterial activity. J Nanomater. 2010;2010:1–11. [Google Scholar]
- 17.Yudovin-Farber I, Beyth N, Weiss EI, Domb AJ. Antibacterial effect of composite resins containing quaternary ammonium polyethyleneimine nanoparticles. J Nanopart Res. 2009;12:591–603. [Google Scholar]
- 18.Beyth N, et al. Surface antimicrobial activity and biocompatibility of incorporated polyethylenimine nanoparticles. Biomaterials. 2008;29:4157–4163. doi: 10.1016/j.biomaterials.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 19.Yudovin-Farber I, et al. Surface characterization and biocompatibility of restorative resin containing nanoparticles. Biomacromolecules. 2008;9:3044–3050. doi: 10.1021/bm8004897. [DOI] [PubMed] [Google Scholar]
- 20.Beyth N, Yudovin-Farber I, Domb AJ, Weiss EI. Long-term antibacterial surface properties of resin composite incorporating polyethyleneimine nanoparticles. Quintessence Int J. 2010;41:827–835. [PubMed] [Google Scholar]
- 21.Engelberg-Kulka H, Hazan R, Amitai S. mazEF: A chromosomal toxin-antitoxin module that triggers programmed cell death in bacteria. J Cell Sci. 2005;118:4327–4332. doi: 10.1242/jcs.02619. [DOI] [PubMed] [Google Scholar]
- 22.Kolodkin-Gal I, Verdiger R, Shlosberg-Fedida A, Engelberg-Kulka H. A differential effect of E. coli toxin-antitoxin systems on cell death in liquid media and biofilm formation. PLoS One. 2009;4:e6785. doi: 10.1371/journal.pone.0006785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rice KC, Bayles KW. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev. 2008;72:85–109. doi: 10.1128/MMBR.00030-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beyth N, Domb AJ, Weiss EI. An in vitro quantitative antibacterial analysis of amalgam and composite resins. J Dent. 2007;35:201–206. doi: 10.1016/j.jdent.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 25.Nyvad B, Kilian M. Comparison of the initial streptococcal microflora on dental enamel in caries-active and in caries-inactive individuals. Caries Res. 1990;24:267–272. doi: 10.1159/000261281. [DOI] [PubMed] [Google Scholar]
- 26.Quirynen M, Bollen CM. The influence of surface roughness and surface-free energy on supra- and subgingival plaque formation in man. A review of the literature. J Clin Periodontol. 1995;22:1–14. doi: 10.1111/j.1600-051x.1995.tb01765.x. [DOI] [PubMed] [Google Scholar]
- 27.Theilade E, Theilade J, Mikkelsen L. Microbiological studies on early dento-gingival plaque on teeth and Mylar strips in humans. J Periodontal Res. 1982;17:12–25. doi: 10.1111/j.1600-0765.1982.tb01127.x. [DOI] [PubMed] [Google Scholar]
- 28.Engelberg-Kulka H, Amitai S, Kolodkin-Gal I, Hazan R. Bacterial programmed cell death and multicellular behavior in bacteria. PLoS Genet. 2006;2:e135. doi: 10.1371/journal.pgen.0020135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gerdes K, Christensen SK, Lobner-Olesen A. Prokaryotic toxin-antitoxin stress response loci. Nat Rev Microbiol. 2005;3:371–382. doi: 10.1038/nrmicro1147. [DOI] [PubMed] [Google Scholar]
- 30.Masuda Y, Ohtsubo E. Mapping and disruption of the chpB locus in Escherichia coli. J Bacteriol. 1994;176:5861–5863. doi: 10.1128/jb.176.18.5861-5863.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Christensen SK, Mikkelsen M, Pedersen K, Gerdes K. RelE, a global inhibitor of translation, is activated during nutritional stress. Proc Natl Acad Sci USA. 2001;98:14328–14333. doi: 10.1073/pnas.251327898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grady R, Hayes F. Axe-Txe, a broad-spectrum proteic toxin-antitoxin system specified by a multidrug-resistant, clinical isolate of Enterococcus faecium. Mol Microbiol. 2003;47:1419–1432. doi: 10.1046/j.1365-2958.2003.03387.x. [DOI] [PubMed] [Google Scholar]
- 33.Kenawy ER. Biologically active polymers. IV. Synthesis and antimicrobial activity of polymers containing 8-hydroxyquinoline moiety. J Appl Polym Sci. 2001;82:1364–1372. [Google Scholar]
- 34.Beyth N, Bahir R, Matalon S, Domb AJ, Weiss EI. Streptococcus mutans biofilm changes surface-topography of resin composites. Dent Mater. 2008;24:732–736. doi: 10.1016/j.dental.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 35.Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol. 2007;5:721–726. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- 36.Zaura-Arite E, van Marle J, ten Cate JM. Conofocal microscopy study of undisturbed and chlorhexidine-treated dental biofilm. J Dent Res. 2001;80:1436–1440. doi: 10.1177/00220345010800051001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.