Abstract
Climate change has caused advances in spring phases of many plant species. Theoretically, however, strong warming in winter could slow the fulfillment of chilling requirements, which may delay spring phenology. This phenomenon should be particularly pronounced in regions that are experiencing rapid temperature increases and are characterized by highly temperature-responsive vegetation. To test this hypothesis, we used the Normalized Difference Vegetation Index ratio method to determine the beginning, end, and length of the growing season of meadow and steppe vegetation of the Tibetan Plateau in Western China between 1982 and 2006. We then correlated observed phenological dates with monthly temperatures for the entire period on record. For both vegetation types, spring phenology initially advanced, but started retreating in the mid-1990s in spite of continued warming. Together with an advancing end of the growing season for steppe vegetation, this led to a shortening of the growing period. Partial least-squares regression indicated that temperatures in both winter and spring had strong effects on spring phenology. Although warm springs led to an advance of the growing season, warm conditions in winter caused a delay of the spring phases. This delay appeared to be related to later fulfillment of chilling requirements. Because most plants from temperate and cold climates experience a period of dormancy in winter, it seems likely that similar effects occur in other environments. Continued warming may strengthen this effect and attenuate or even reverse the advancing trend in spring phenology that has dominated climate-change responses of plants thus far.
Climate change has influenced the timing of developmental stages of most plants of the temperate and cold regions (1–9). Many studies have provided evidence that increasing temperatures have led to progressive advances in spring phases. In a study that compared more than 125,000 observational time series of plant and animal phenology between 1971 and 2000, Menzel et al. found that 78% of species showed advanced phenology (significant advances for 22% of all time series) (3). An analysis of observations of the first flowering date of 385 plant species in England by Fitter and Fitter, showed three quarters of species flowering earlier in the decade between 1991 and 2000 than in the previous four decades (10). A recent comprehensive meta-analysis of several studies on phenology trends (5) supports the impression that most species show advanced spring phenology. However, all these studies contain a sizeable proportion of species that do not conform to this pattern. Twenty-two percent of species in Menzel et al.’s study (3) and 24% of species in Fitter and Fitter's article (10) showed trends toward later spring phenology. In both studies, this delay was significant for 3% of species. Because temperatures clearly increased over time in both studies, the spectrum of spring phenology trends suggests that plant responses to warming are not linear and differ among species.
The timing of spring phases in most temperate plants results from the interplay of winter cold and spring heat (11, 12). Plants that evolved in winter-cold climates fall dormant in fall to avoid frost damage in winter and will only resume growth in spring after their chilling requirements have been fulfilled. Temperature increases in spring then lead to advancing spring phenology, but warming in winter may delay the fulfillment of chilling requirements and thus lead to later onset of spring phases. The large proportion of plant species that have shown advancing phenology in recent decades suggests that effects of rising spring temperatures so far outweigh the possibly delaying impact of winter warming in most cases. However, plant species that are particularly sensitive to temperature cues, such as those at high latitudes or altitudes (13), may respond differently from those in more temperate climates, especially in regions that are experiencing particularly strong temperature increases. A recent remote sensing analysis of growing season changes at high Northern latitudes found a delaying trend in the onset of spring greening between 1993 and 2004, following an advancing trend between 1982 and 1991 (14).
Another region that has experienced extraordinary warming is the Tibetan Plateau in Western China (15) (Fig. 1). The climate of this region is characterized by a long period of frost and a relatively short growing season, and accurate determination of the ideal time to break dormancy and resume growth is crucial for the success of a plant species. Chilling and heat requirements of spring developmental phases are thus likely well adjusted to typical temperature regimes of the past; however, they may be less well adapted to the warmer conditions experienced in recent years. We hypothesize that strong warming of the Tibetan Plateau has led to changes in the effective growing season of meadow and steppe vegetation, with warming in winter leading to delayed fulfillment of chilling requirements, and thus later onset of the growing season.
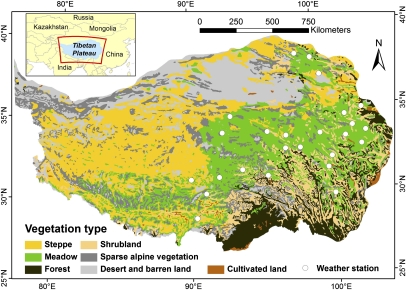
Fig. 1.
Vegetation types of the Tibetan Plateau according to the Atlas of the Tibetan Plateau (16) and locations of weather stations in the region. (Inset) The location of the Tibetan Plateau within Central Asia.
To test this hypothesis, we assessed changes in the average beginning, end, and length of the growing season of steppe and meadow vegetation of the Tibetan Plateau (16) between 1982 and 2006 by analyzing imagery created by the Global Inventory Modeling and Mapping Studies group (17) with the Normalized Difference Vegetation Index (NDVI) ratio method (18). We then used partial least-squares (PLS) regression (19, 20) to relate changes in the onset of spring greening to monthly gridded temperature data obtained from the China Meteorological Administration (21).
Results
Major phenological stages on the Tibetan Plateau could be detected by remote sensing, as indicated by comparison with phenological observations on the ground, which were available for 22 locations in Qinghai Province. The mean absolute error between remotely sensed and observed dates of the beginning and end of the growing season was 12.8 and 15.0 d, respectively. Root mean-square errors between remote sensing estimates and observed phenology were 10.6 d for the beginning and 13.5 d for the end of the growing season. In light of the temporal resolution of the NDVI dataset of 15 d, such errors were expected. Because onset and end dates of the growing season were averaged over the entire Tibetan Plateau, the temporal resolution of the data were deemed sufficiently high for detailed analysis.
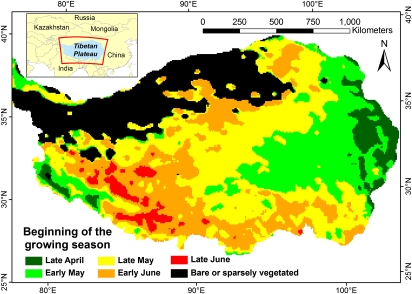
The onset of the growing season varied strongly across the Tibetan Plateau, with some areas in the Southwest greening up to 2 mo later than the Eastern region (Fig. 2). To analyze temporal trends in the data, we averaged these dates over all image pixels covered by steppe and meadow vegetation (16), respectively, for each year of the study period. The plot of these dates over time (Fig. 3 A and B) reveals strong changes in the beginning of the growing season but without one consistent trend over the entire time span. Instead of a single linear trend, both steppe and meadow vegetation show a slight advance in spring phenology from the early 1980s until the mid-1990s, followed by a fairly rapid delaying trend that persisted until the end of the study period in 2006.
Fig. 2.
Average timing of the beginning of the growing season (BGS) on the Tibetan Plateau between 1982 and 2006. BGS dates varied by up to 2 mo between different parts of the Plateau.
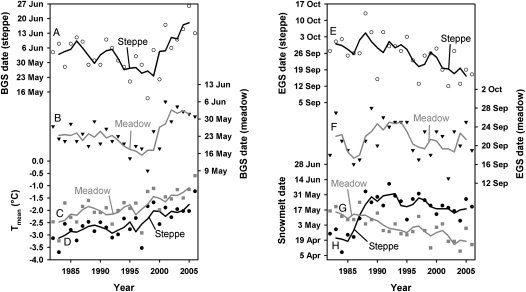
Fig. 3.
Beginning (BGS; A and B) and end (EGS; E and F) of the growing season for steppe (A and E) and meadow (B and F) vegetation on the Tibetan Plateau between 1982 and 2006, derived from 15-d NDVI composites obtained from the Advanced Very High Resolution Radiometer (AVHRR) sensor. BGS dates advanced markedly between 1982 and the mid 1990s, before retreating significantly after that. EGS dates in the steppe region advanced progressively between 1982 and the mid 1990s, whereas variation in the meadow region was less consistent. Consistent increases in temperature (C and D) and gradual and steady change in snow melt dates (G and H; following a delay between 1985 and the early 1990s in the steppe environment) indicate that observed changes are not linear responses to temperature or snow cover changes. Lines in the graph represent 3-y running means.
The plot of annual mean temperatures calculated from all available weather data against time (Fig. 3 C and D) shows that the Tibetan Plateau has warmed consistently between 1982 and 2006. Similar warming trends were found for temperatures during all individual months (Tables S1 and S2), indicating that changes to the beginning of the growing season do not result from a monotonic response of vegetation to temperatures in any given time interval. The PLS regression analysis included both winter and spring temperatures as important variables into an explanatory model for the beginning of the growing season for both steppe (Fig. 4C) and meadow vegetation (Fig. 4D), according to the commonly used variable importance threshold of 0.8 (19). Temperatures in May and June had negative model coefficients for both vegetation types (Fig. 4 A and B), indicating that high temperatures during these months advanced the onset of the growing season. Model coefficients for temperatures between October and March were positive, indicating that high temperatures during these months have a delaying effect on spring phenology.
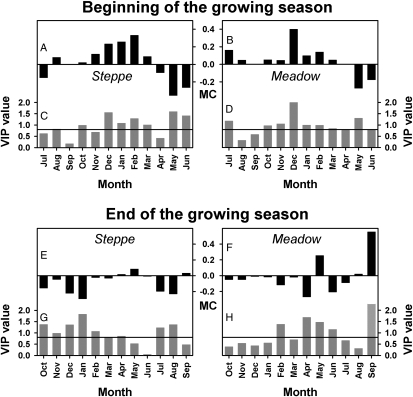
Fig. 4.
Response of the BGS (A–D) and EGS (E–H) in steppe and meadow vegetation of the Tibetan Plateau between 1982 and 2006 to monthly temperatures, according to PLS regression. For the BGS, the variable importance plots (VIP; C and D) indicate that temperatures in both spring (May and June) and winter (October through March) were important for explaining the response of BGS dates (VIP values above 0.8). Model coefficients (MC) of the centered and scaled data showed that warm winter temperatures delayed spring phenology (positive coefficients), whereas warm spring temperatures advanced the BGS (negative coefficients) for both steppe (A) and meadow (B). Including both effects into phenological models could substantially enhance our understanding of climate-change effects on vegetation at temperate and cold locations. For the EGS in the steppe region, the VIP (G) indicates that temperatures during most months contributed to explaining the variation in EGS dates. MC values (E) showed that warm temperatures in all of the important months contributed to an advance of the EGS date. This finding indicates that additional heat allows plants to complete their growth cycle earlier rather than extending the period, during which vegetation is active. EGS dates of meadow vegetation were mainly determined by temperatures in spring and September (H). Although temperature influence in spring is somewhat inconsistent (F), warm conditions in September delay the EGS date. In contrast to steppe, meadow vegetation can thus make use of the extended period of favorable conditions.
Across the Tibetan Plateau, the growing season ended between early September and early October (Fig. S1). The timing of this phenological phase showed no consistent change over time in the meadow region, but advanced progressively for steppe vegetation over the entire study period (Fig. 3 E and F). For the meadow region, the end of the growing season was mainly determined by temperatures in September, with warm conditions during this month extending the growing season (Fig. 4 E–H). For steppe vegetation, warm temperatures during the winter, as well as during July and August, advanced the end of the growing season (Fig. 4 E–H). The net effect of changes in the beginning and end of the growing season was a pronounced shortening of the growing season between 2000 and 2006 by about 1 mo for steppe and 3 wk for meadow vegetation (Fig. S2).
Discussion
Recent changes to the growing season on the Tibetan Plateau do not conform to the general trend of extended growing seasons reported elsewhere (1, 2, 22). In particular since about 2000, the growing season of both meadow and steppe vegetation has shortened substantially, driven primarily by delayed onset of spring phenological phases. Our findings are thus in line with results reported for vegetation at high northern latitudes, where a similar delay in spring phases has been reported (14). Delayed onset of spring greening of the Tibetan Plateau occurred despite strong recent warming and despite a phenology-advancing effect of temperature increases in spring. PLS regression assigned negative model coefficients to temperatures in May and June, confirming the phenology-advancing effect of warm springs that has been reported elsewhere (1, 3, 6). The delayed onset of the growing season in recent years appears to have been driven by increases in winter temperatures, between October and March, for which PLS regression assigned positive model coefficients. These positive coefficients mean that warm temperatures during these phases push the start of the growing season backward. The delaying effect may be a manifestation of reductions in winter chill leading to a later fulfillment of plants’ vernalization requirements. Like most plants of temperate and cold climates, the vegetation of the Tibetan Plateau overwinters in a state of dormancy, which protects sensitive growing tissue from frost damage. Overcoming dormancy and resuming growth in spring requires the fulfillment of a winter-chilling requirement, followed by the accumulation of spring heat (12, 23). Warmer temperatures during the winter may thus delay the fulfillment of the chilling requirement, slowing the dormancy-breaking process. Warm temperatures during the spring, however, accelerate the accumulation of heat and thus advance the resumption of active growth. Both effects appear to affect the timing of phenological phases.
Contrary to an extension of the growing season in fall, as has been reported for other regions, we found a shortening of the growing season, in particular for steppe vegetation. Despite warming in summer and fall, the growing season of the steppe region ended earlier in recent years than in the past. This finding indicates that plants of this environment cannot make use of the extended period of thermally favorable conditions, probably because of a determinate life cycle. Warm temperatures during July and August led to an advance rather than a delay of fall phenology. In contrast to this finding, vegetation of the meadow region responded to warm conditions in September with a delay in fall phenology, allowing it to exploit the longer period of thermally favorable conditions. Vegetation of the meadows thus seems to be more competitive under warmer conditions than plants of the steppe region, and may extend into the steppe region as warming continues.
On the Tibetan Plateau, the combination of the advancing effect of spring temperatures and the delaying effect of winter temperatures played out to result in an earlier onset of the growing season up to 1996 and a retreat afterward. This effect probably resulted from a combination of high chilling requirements, necessary for avoiding frost damage, and fairly low heat requirements, needed to exploit warm temperatures as early as possible. Because temperatures rose during all months of the winter, the phenology-advancing effect of spring warming was thus relatively low compared with the delaying effect of warming in winter. Because of the relatively cool temperatures that prevail in the steppe region even in summer, heat requirements of summer developmental stages are also low. Warming in summer thus rapidly accelerates plant development, leading to an earlier completion of the reproductive cycle of steppe species.
To exclude changes in snow cover or precipitation patterns as possible causes of the observed changes, we evaluated precipitation records from 25 weather stations in the region (China Meteorological Data Sharing Service System; shown in Fig. 1), as well as published information on snow cover and records of daily snow depth (24). Snow depth records were generated from passive shortwave sensing (Scanning Multichannel Microwave Radiometer) images and brightness temperature data (Special Sensor Microwave Imager) and processed by the American National Snow and Ice Center (24). We defined the last day during the first half of the year, at which snow depth dropped to zero, as the date of snow melt.
Averaged records of monthly precipitation over all stations showed no significant changes over time (Table S3). The snow-melt date, averaged over all pixels used in the PLS regression analysis, showed a delayed trend until the early 1990s, but displayed only slight changes for the rest of the study period (Fig. 3 G and H). In recent years, snow melt appears to have advanced slightly, probably in response to increasing spring temperatures. Moreover, a recent analysis of snow depth across China showed low snow depths at all weather stations on the Tibetan Plateau (25, 26), without significant change trends (25). Significant increases in snow depths, as reported for the late 1970s (27), did not occur during our study period. It therefore seems unlikely that observed changes in phenology are a result of changes in snow cover or precipitation regime.
High northern latitudes appear to be experiencing similar developments. The vegetation of both the northern latitudes and the highlands of the Tibetan Plateau are characterized by strong temperature dependence, and their environments have experienced abnormally strong temperature increases in recent years. It stands to reason, therefore, that as temperatures continue to increase, vegetation in different parts of the world may also experience a phenology-delaying effect caused by winter warming. Because plants of the temperate zones vary greatly in their temperature responses, with a wide range of chilling and heat requirements, it is likely that phenology-delaying effects of winter warming are already occurring. Many of the species reported to experience delayed onsets of spring phases in the studies by Menzel et al. (3) and Fitter and Fitter (10) may show this behavior as a result of winter warming. Further temperature increases may thus push more and more species toward delayed rather than advanced phenology. Initial advances in spring phenology, which have been reported in many places (1, 4–6, 22, 28), may then be halted or even reversed as temperature increases continue.
It is important to note, however, that scientific understanding of the intricacies of the chilling and dormancy-breaking process is relatively limited and convincing quantitative models are lacking at present. In particular, little is known about the range of temperatures that is effective for accumulating chilling in high-altitude vegetation. Trees are generally assumed to not accumulate winter chill during freezing conditions (23, 29), but annual plants are likely to respond primarily to soil temperature, which is moderated in winter by protective snow cover, raising it substantially above air temperature (30, 31). Much more research is needed to elucidate the interactions of plants with temperatures during different parts of the year to enhance our ability to predict the effects of temperature increases on plant phenology. Because of these limitations, accurate predictions of how plant phenology will respond to increasing temperatures are not possible at present. Nevertheless, under a range of assumptions, indicative projections can be made for species with known chilling and heat requirements. We use the example of the walnut cultivar Payne in California to illustrate that delayed phenology in response to warming is plausible. Long-term observations of spring phenology have indicated that this cultivar needs about 66.1 chill portions and 5,139 growing degree hours for leaf-out (12). Assuming that both requirements must be fulfilled in sequence, leaf-out dates for different climate regimes can be predicted. Using this process to predict leaf-out for projected temperatures for a range of future scenarios in Northern California (described in ref. 32) indicates a delay in this phenological stage from mid-March to mid-April by the end of the 21st century, because of later fulfillment of the chilling requirement. Moreover, such projection suggests that chilling requirements will no longer be fulfilled in about 50% of all years, with unclear consequences for tree development. Although these estimates are only approximations and rely on assumptions that strongly simplify the dormancy process, they support our hypothesis that warming may lead to phenology delays.
Although the existence of a delaying effect of winter warming on spring phenology has been suspected occasionally in the past (3, 9), it has not been included in analyses of observational time series, which have typically focused on identifying linear trends in plant phenology (1, 3, 9). This approach has recently been called into question (33, 34), however, and phenological analyses may benefit from differentiating between temperature effects in spring and in winter. Our results indicate that the combined effect of warming in both winter and spring on early season phenology may not be linear or monotonic, but rather consist of an advancing followed by a retreating trend. Recent studies have identified historic (35–37) and projected future (32, 36, 37) losses in winter chill in many parts of the world, leading us to hypothesize that phenology delays may be in store for many regions in the future. Future studies of climate-change effects on phenology may be well advised to take the additional effects of winter warming into consideration. This theory may substantially reduce the amount of unexplained variation in regressions of phenology with temperature and help produce much more accurate projections of future trends in phenology than are currently available.
Materials and Methods
We analyzed changes in the beginning (BGS), end (EGS), and length (LGS) of the growing season for steppe and meadow vegetation of the Tibetan Plateau between 1982 and 2006 based on the NDVI dataset created by the Global Inventory Modeling and Mapping Studies group (17). This dataset is derived from imagery obtained from the Advanced Very High Resolution Radiometer (AVHRR) and has a spatial and temporal resolution of 8 km and 15 d, respectively.
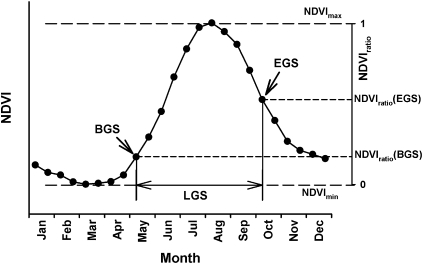
BGS, EGS, and LGS were modeled using the NDVI ratio method developed by White et al. (18) for specific plant communities (Fig. 5). The NDVI ratio is the difference between the NDVI value at a certain time [NDVI (t)] and the minimum NDVI value (NDVImin) for the time span of interest, normalized by the total range of NDVI values during this time span (NDVImax − NDVImin). Evaluation of the average NDVI curve over 24 y showed that the NDVI reached its minimum value in February and March. The average NDVI in these 2 mo was thus used as NDVImin, rather than the annual NDVI minimum, which might occur at the time of maximum snow cover. Ground observation data from 22 grassland-monitoring stations in Qinghai Province were then used to determine the appropriate threshold. The final thresholds were selected according to mean absolute error (MAE) and root mean-square error (RMSE), which were calculated between the ground observations and modeled growing season parameters. For the BGS, we selected an NDVI ratio threshold of 0.2 (MAE = 12.8 d and RMSE = 10.6 d); a drop of the NDVI ratio below 0.6 (MAE = 15.0 d and RMSE = 13.5 d) was interpreted to signify the EGS. Using these thresholds, the BGS, EGS, and LGS were estimated for each pixel of the study area for each year on record. Monthly temperatures for the entire observation period were obtained from the China Meteorological Administration (21). This dataset integrates various observational temperature time series in a 1°-by-1° grid.
Fig. 5.
Illustration of the NDVI ratio method used to model the BGS and the EGS. BGS is assumed to occur when the BGS-specific threshold [NDVIratio (BGS)] is exceeded and followed by three consecutive data points with increasing NDVI ratio. EGS occurs when the NDVI ratio falls below the NDVI ratio (EGS) threshold, followed by three consecutive points of falling NDVI.
We evaluated the response of spring phenology to monthly temperatures, relating both datasets by PLS regression (19, 20) (implemented in JMP 8, SAS Institute). PLS regression describes variation in both the independent and dependent variables by determining latent factors, which are linear-weighted combinations of the input variables. This strategy is useful for highly autocorrelated variables, such as the temperatures of the individual months used here, and allows detecting the influence of deviations from “normal” patterns on the response variable. This analysis provides an indication of which independent variables are useful for explaining the variation in the dependent variable (variable-importance-plot), and of the direction in which that influence is exerted (model coefficients of the centered and scaled data). We used two latent factors to relate the onset of the growing season dates with monthly temperatures for steppe and meadow vegetation. In preparation for this analysis, we spatially averaged data for all image pixels of the temperature grids that had more than 50% cover by the respective vegetation type.
Supplementary Material
Acknowledgments
Field research was funded by the Knowledge Innovation Program of the Chinese Academy of Sciences (Grant KSCX2-YW-Z-1019). The field phenological observation data were provided by the Climate Data Center of Qinghai Province and snow-depth data were provided by the Cold and Arid Regions Environmental and Engineering Research Institute of the Chinese Academy of Sciences.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012490107/-/DCSupplemental.
References
- 1.Chmielewski FM, Rötzer T. Response of tree phenology to climate change across Europe. Agric For Meteorol. 2001;108(2):101–112. [Google Scholar]
- 2.Schwartz MD. Green-wave phenology. Nature. 1998;394:839–840. [Google Scholar]
- 3.Menzel A, et al. European phenological response to climate change matches the warming pattern. Glob Change Biol. 2006;12:1969–1976. [Google Scholar]
- 4.Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR. Increased plant growth in the northern high latitudes from 1981 to 1991. Nature. 1997;386:698–702. [Google Scholar]
- 5.Parmesan C. Influences of species, latitudes and methodologies on estimates of phenological response to global warming. Glob Change Biol. 2007;13:1860–1872. [Google Scholar]
- 6.Shabanov NV, Zhou LM, Knyazikhin Y, Myneni RB, Tucker CJ. Analysis of interannual changes in northern vegetation activity observed in AVHRR data from 1981 to 1994. IEEE Trans Geosci Rem Sens. 2002;40(1):115–130. [Google Scholar]
- 7.Sparks TH, Menzel A. Observed changes in seasons: An overview. Int J Climatol. 2002;22:1715–1725. [Google Scholar]
- 8.Zhou LM, et al. Variations in northern vegetation activity inferred from satellite data of vegetation index during 1981 to 1999. J Geophys Res-Atmos. 2001;106(D17):20069–20083. [Google Scholar]
- 9.Luterbacher J, et al. Exceptional European warmth of autumn 2006 and winter 2007: Historical context, the underlying dynamics, and its phenological impacts. Geophys Res Lett. 2007;34 L12704:1–6. [Google Scholar]
- 10.Fitter AH, Fitter RSR. Rapid changes in flowering time in British plants. Science. 2002;296:1689–1691. doi: 10.1126/science.1071617. [DOI] [PubMed] [Google Scholar]
- 11.Linkosalo T, Lappalainen HK, Hari P. A comparison of phenological models of leaf bud burst and flowering of boreal trees using independent observations. Tree Physiol. 2008;28:1873–1882. doi: 10.1093/treephys/28.12.1873. [DOI] [PubMed] [Google Scholar]
- 12.Luedeling E, Zhang M, McGranahan G, Leslie C. Validation of winter chill models using historic records of walnut phenology. Agric For Meteorol. 2009;149(11):1854–1864. [Google Scholar]
- 13.Körner C. Mountain biodiversity, its causes and functions. Ambio Special Report. 2004;13:11–17. [PubMed] [Google Scholar]
- 14.Delbart N, Le Toan T, Kergoat L, Fedotova V. Remote sensing of spring phenology in boreal regions: A free of snow-effect method using NOAA-AVHRR and SPOT-VGT data (1982–2004) Remote Sens Environ. 2006;101(1):52–62. [Google Scholar]
- 15.Xu J, et al. The melting Himalayas: Cascading effects of climate change on water, biodiversity and livelihoods. Conserv Biol. 2009;23:520–530. doi: 10.1111/j.1523-1739.2009.01237.x. [DOI] [PubMed] [Google Scholar]
- 16.Institute of Geographic Sciences and Natural Resources Research CAS . Atlas of the Tibetan Plateau. Beijing, China: Science Press; 1990. [Google Scholar]
- 17.Tucker CJ, et al. An extended AVHRR 8-km NDVI dataset compatible with MODIS and SPOT vegetation NDVI data. Int J Remote Sens. 2005;26:4485–4498. [Google Scholar]
- 18.White MA, Thornton PE, Running SW. A continental phenology model for monitoring vegetation responses to interannual climatic variability. Global Biogeochem Cycles. 1997;11:217–234. [Google Scholar]
- 19.Wold S. PLS for multivariate linear modeling. In: van der Waterbeemd H, editor. Chemometric Methods in Molecular Design: Methods and Principles in Medicinal Chemistry. Vol. 2. Weinheim, Germany: Verlag-Chemie; 1995. pp. 195–218. [Google Scholar]
- 20.Wold S, Sjostrom M, Eriksson L. PLS-regression: A basic tool of chemometrics. Chemom Intell Lab Syst. 2001;58(2):109–130. [Google Scholar]
- 21.Zhang Q. China Land Surface Monthly Temperature Grid. Beijing, China: Climate Information Center of the China Meteorological Administration; 2007. [Google Scholar]
- 22.Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. [Google Scholar]
- 23.Richardson EA, Seeley SD, Walker DR. A model for estimating the completion of rest for Redhaven and Elberta peach trees. HortScience. 1974;9:331–332. [Google Scholar]
- 24.Che T. Long-Term Snow Depth Dataset of China [EB/OL]. Dataset provided by the Environmental & Ecological Science Data Center for West China NNSFoC (Cold and Arid Regions Environmental and Engineering Research Institute, Chinese Academy of Sciences, Lanzhou) 2006 [Google Scholar]
- 25.Peng S, Piao S, Ciais P, Fang J, Wang X. Change in winter snow depth and its impacts on vegetation in China. Glob Change Biol. 2010;16:3004–3013. [Google Scholar]
- 26.Qin DH, Liu SY, Li PJ. Snow cover distribution, variability, and response to climate change in western China. J Clim. 2006;19:1820–1833. [Google Scholar]
- 27.Xin XG, Zhou TJ, Yu RC. Increased Tibetan Plateau snow depth: An indicator of the connection between enhanced winter NAO and late-spring tropospheric cooling over East Asia. Adv Atmos Sci. 2010;27:788–794. [Google Scholar]
- 28.Tucker CJ, et al. Higher northern latitude normalized difference vegetation index and growing season trends from 1982 to 1999. Int J Biometeorol. 2001;45(4):184–190. doi: 10.1007/s00484-001-0109-8. [DOI] [PubMed] [Google Scholar]
- 29.Luedeling E, Zhang M, Luedeling V, Girvetz EH. Sensitivity of winter chill models for fruit and nut trees to climate change. Agric Ecosyst Environ. 2009;133(1–2):23–31. [Google Scholar]
- 30.Groffman PM, et al. Colder soils in a warmer world: A snow manipulation study in a northern hardwood forest ecosystem. Biogeochemistry. 2001;56(2):135–150. [Google Scholar]
- 31.Freppaz M, Celi L, Marchelli M, Zanini E. Snow removal and its influence on temperature and N dynamics in alpine soils (Vallee d'Aoste, northwest Italy) J Plant Nutr Soil Sc. 2008;171:672–680. [Google Scholar]
- 32.Luedeling E, Zhang M, Girvetz EH. Climatic changes lead to declining winter chill for fruit and nut trees in California during 1950–2099. PLoS ONE. 2009;4:e6166. doi: 10.1371/journal.pone.0006166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chuine I, Morin X, Bugmann H. Warming, photoperiods, and tree phenology. Science. 2010;329:277–278. doi: 10.1126/science.329.5989.277-e. author reply 278. [DOI] [PubMed] [Google Scholar]
- 34.Körner C, Basler D. Plant science. Phenology under global warming. Science. 2010;327:1461–1462. doi: 10.1126/science.1186473. [DOI] [PubMed] [Google Scholar]
- 35.Luedeling E, Blanke M, Gebauer J. Climate change effects on winter chill for fruit crops in Germany (Translated from German) Erwerbs-Obstbau. 2009;51(3):81–94. [Google Scholar]
- 36.Luedeling E, Gebauer J, Buerkert A. Climate change effects on winter chill for tree crops with chilling requirements on the Arabian Peninsula. Clim Change. 2009;96(1–2):219–237. [Google Scholar]
- 37.Baldocchi D, Wong S. Accumulated winter chill is decreasing in the fruit growing regions of California. Clim Change. 2008;87(Suppl 1):S153–S166. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.