Sour taste can be agreeable in moderation. However, most people respond to drinking lemon juice with a violent contortion of their faces that clearly signals to everybody around that a mouth free of acid is greatly preferred. Such a strong aversive response must be based on a reliable acid-detection system on the tongue. Emily Liman's Laboratory of Sensory Neurobiology at the University of Southern California has discovered that specialized sour-taste cells on the tongue are equipped with proton channels. In PNAS (1), strong evidence is presented for the idea that H+ ions, resulting from the dissociation of food acids, enter sour-specific taste cells that inform the brain about the offensive material. The brain can then decide what to do—often to pull a “sour face.”
Chemically speaking, food is a highly complex mixture of compounds composed of many different organic and inorganic substances. When an animal eats, there is a brief but absolutely vital period during which it must reach a decision as to what to do with the food in its mouth: swallow it or spit it out. This decision indeed has an impact on the animal's chance for survival, because the world is full of harmful substances, both for the herbivore and for the carnivore. Although the animal has investigated the food beforehand using memorized visual and olfactory cues, it can never be sure what exactly it puts into its mouth. Fortunately, the sense of taste has evolved to stand sentinel over the digestive system. It provides last-moment information on the suitability of food, and it enables the animal to come to an almost binary decision: take it! or get rid of it!. To ensure swift and appropriate behavior, the taste system has direct access to key functions of the brain: it drives emotions, and it targets the brainstem, which controls many vital body functions. Consequently, the decision take it! will trigger pleasure, salivation, and swallowing, whereas the opposite decision will induce disgust and retching behavior. Clearly, this binary decision has little to do with the colloquial meaning of the term “taste.” Indeed, semantics differ profoundly between gustatory science and the vernacular. Tasting food in our daily life entails gustatory processes as well as olfactory, tactile, and probably also visual analysis. We describe this kind of multimodal sensation as flavor, aroma, or taste, experiences that we memorize and recall as we refine our knowledge of wholesome food. However, the connoisseurial appreciation of good food is only loosely related to the rapid, no-nonsense check for the good and the bad that is performed by the gustatory system on our tongue. The system probes the food for just five major qualities, three appetitive (salty, sweet, and umami) and two aversive (bitter and sour). Appetitive qualities represent the valuable food ingredients sodium chloride, carbohydrates, and protein. The aversive qualities signal harmful plant metabolites like strychnine, which often taste bitter, as well as acids that occur in unripe fruit or rotting material. Additional taste qualities like “fatty” or “metallic” may be added to this list in the future (2). Importantly, the response to these basic taste qualities are innate and do not have to be learned in a cooking school. Thus, newborn babies display the correct emotional responses to sweet, bitter, and sour stimuli through their facial expression (3).
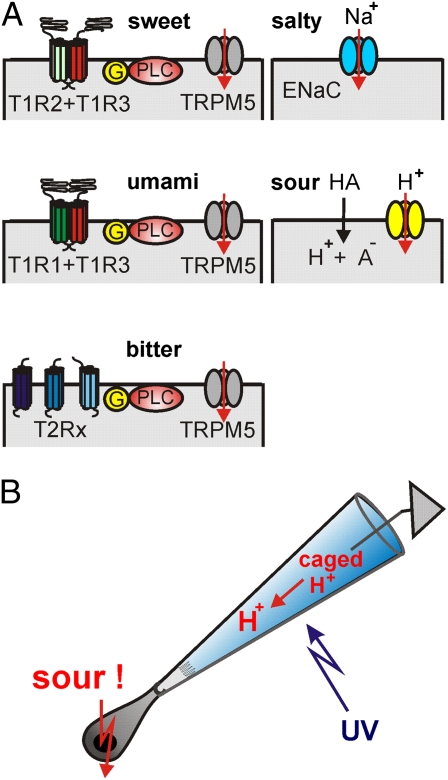
Gustatory physiologists have identified the signal transduction pathways that underlie the detection of four taste qualities in the taste cells (for a recent review see ref. 4). It turned out that the T1R family of receptors detects sweet and umami stimuli, whereas the T2R receptor family serves to detect bitter-tasting compounds (Fig. 1A). The T1R family consists of just three isoforms, which coassemble as dimeric receptors for sweet taste (T1R2/T1R3) and umami taste (T1R1/T1R3) (5, 6). The T2R bitter receptors form a larger family with 25 isoforms in the human taste system (7). T2R receptors do not form dimers, but several—possibly all—isoforms can be expressed in a single bitter-selective taste cell (8). Salt taste is mainly the gustatory perception of Na+ ions, which can enter salt-specific taste cells via an amiloride-sensitive epithelial Na+ channel of the ENaC family (9, 10). The fifth taste quality, the sour taste, has presented taste researchers with particularly intricate problems. The adequate stimulus of sour taste is an increase of the H+ concentration, a drop in pH on the surface of the tongue. If the food contains an acid, H+ ions interact with the chemosensory apical membrane of taste cells. However, H+ ions can also reach the basolateral membrane through the paracellular pathway of the lingual epithelium and act on proteins there. Moreover, most food acids (e.g., citric acid, malic acid, or acetic acid) have only weak acidity (pKa = 3–5). This means that a sizable fraction of food acids is still protonated when strong acids—like hydrochloric acid (pKa = 8)—are fully dissociated. In their protonated, electrically neutral form, the weak acids can cross the plasma membrane and enter taste cells, where they acidify the cytosol. Indeed, such induced cytosolic acidification is believed to contribute directly to the sour taste of weak acids (11, 12).
Fig. 1.
Transduction of the five basic taste qualities. (A) The sensory membrane of taste cells comes into contact with food. The taste receptor family T1R detects the presence of sweet substances, such as sugars and artificial sweeteners, using the isoforms T1R2 and T1R3 to form a receptor dimer. The alternative dimer (T1R1 + T1R3) detects protein components, in particular monosodium glutamate, and hence mediates the taste quality umami (Japanese for “tasty” or “savory”). The bitter taste arises from cells expressing several members of the T2R family of taste receptors. All taste receptors transmit their signal to the target enzyme phospholipase β2 (PLC) through a GTP-binding signaling protein (G). At the end of this signal transduction cascade is the activation of the cation channel TRPM5 that causes a depolarization of the taste cell. An ion channel that allows Na+ ions to enter the cell generates salt taste (ENaC: epithelial sodium channel). Acids may enter the cell in their undissociated form (HA) and cause intracellular acidification. A previously uncharacterized proton channel provides a direct pathway for H+ ions into the cell. (B) The method used by Liman et al. (1) to test the effect of acid signals on the sensory membrane of isolated sour-taste cells. The apical pole of the cell is sucked into a glass micropipette so that the sensory apical membrane is exposed to the solution that fills the pipette. This solution contains a compound (“caged H+”) that releases H+ ions upon illumination with a UV flash. Through the previously uncharacterized proton channels in the sensory membrane, H+ ions enter the cell and elicit action potentials in the sour-taste cell.
A serious difficulty with these multiple H+ effects on proteins all over the taste cell is to establish whether the effects are specific for sour-sensitive cells or, alternatively, nonspecific effects without relevance for the sour taste. A decisive method for the identification of sour-selective taste cells was introduced with the finding that those taste cells that express the protein PKD2L1 are necessary for sour taste in mice (13). Genetically driven ablation of PKD2L1-expressing cells specifically removed the sour taste, whereas the other taste qualities persisted. Liman's group (1) used this observation to unambiguously mark the subset of sour-specific taste cells using YFP expressed under the PKD2L1 promotor. Isolated PKD2L1-YFP–positive cells responded to mild acid stimulation with an inward current that was not carried by Na+ ions but that caused intracellular acidification, indicating the influx of H+ ions. The current was insensitive to a set of channel blockers including amiloride but could be blocked by application of Zn2+. To exclude nonspecific acid effects on the basolateral membrane of the isolated taste cells, the acid stimulus was applied exclusively to the apical membrane. This was achieved by enclosing the apical cell pole with the chemosensory membrane inside a glass micropipette, where it was shielded from the solution bathing the basolateral
Dissociated acids on the tongue can be detected by sour-specific cells via a proton channel.
membrane (Fig. 1B). The apical acid stimulus was then generated by photoreleasing H+ with a UV flash only inside the micropipette. This elegant method ensured that the sour-taste stimulus hit the chemosensory membrane, the membrane area that is exposed to food in vivo. When stimulating PKD2L1-YFP–positive cells in this way, the Liman laboratory observed an excitatory, Na+-independent, and Zn2+-sensitive current entering the cell across the apical membrane. The current was specific for sour cells and was not seen in cells expressing TRPM5, an ion channel that operates in the transduction of sweet, bitter, and umami transduction. Taste cells, which are not neurons but epithelial cells, are nevertheless able to fire action potentials (14, 4), and the sour taste resides in one particular subpopulation of taste cells that can also form synapses with afferent nerves (15). Accordingly, the current triggered by the apical acid stimulus elicited bursts of action potentials and caused a transient increase of the cytosolic Ca2+ concentration, the signal that induces transmitter release in these cells. Thus, the data presented by Liman and colleagues provide a consistent functional concept for acid transduction. H+ ions of food acids flow through a proton channel into presynaptic, PKD2L1-expressing taste cells and cause electrical excitation and transmitter release.
With this work, taste research takes an important step forward. It becomes clear that the presence of dissociated acids on the tongue can be detected by sour-specific cells via a proton channel. The challenge is now to determine the molecular identity of this channel. The PKD2L1 protein itself seems not to be a promising candidate because this protein requires a second protein (PKD2L3) for appropriate targeting to the chemosensory membrane (16), a protein that is, however, not required for sour taste (17). The discovery of a proton channel in sour-taste cells adds an important impulse to proton-channel research, which is a comparably recent and exciting line of ion channel physiology. Voltage-dependent proton channels are already quite well understood (for review see ref. 18), but there are many other kinds of H+ conductances, and the search is on for candidate genes. The Liman article on sour-taste cells opens a new route of exploration into an aversive taste quality and into a unique type of proton channel.
Footnotes
The author declares no conflict of interest.
See companion article on page 22320.
References
- 1.Chang RB, Waters H, Liman ER. A proton current drives action potentials in genetically identified sour taste cells. Proc Natl Acad Sci USA. 2010;107:22320–22325. doi: 10.1073/pnas.1013664107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mattes RD. Is there a fatty acid taste? Annu Rev Nutr. 2009;29:305–327. doi: 10.1146/annurev-nutr-080508-141108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Steiner JE, Glaser D, Hawilo ME, Berridge KC. Comparative expression of hedonic impact: Affective reactions to taste by human infants and other primates. Neurosci Biobehav Rev. 2001;25:53–74. doi: 10.1016/s0149-7634(00)00051-8. [DOI] [PubMed] [Google Scholar]
- 4.Chaudari N, Roper SD. The cell biology of taste. J Cell Biol. 2010;190:285–296. doi: 10.1083/jcb.201003144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nelson G, et al. Mammalian sweet taste receptors. Cell. 2001;106:381–390. doi: 10.1016/s0092-8674(01)00451-2. [DOI] [PubMed] [Google Scholar]
- 6.Nelson G, et al. An amino-acid taste receptor. Nature. 2002;416:199–202. doi: 10.1038/nature726. [DOI] [PubMed] [Google Scholar]
- 7.Chandrashekar J, et al. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 8.Adler E, et al. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 9.Heck GL, Mierson S, DeSimone JA. Salt taste transduction occurs through an amiloride-sensitive sodium transport pathway. Science. 1984;223:403–405. doi: 10.1126/science.6691151. [DOI] [PubMed] [Google Scholar]
- 10.Chandrashekar J, et al. The cells and peripheral representation of sodium taste in mice. Nature. 2010;464:297–301. doi: 10.1038/nature08783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lyall V, et al. Decrease in rat taste receptor cell intracellular pH is the proximate stimulus in sour taste transduction. Am J Physiol Cell Physiol. 2001;281:C1005–C1013. doi: 10.1152/ajpcell.2001.281.3.C1005. [DOI] [PubMed] [Google Scholar]
- 12.Richter TA, Caicedo A, Roper SD. Sour taste stimuli evoke Ca2+ and pH responses in mouse taste cells. J Physiol. 2003;547:475–483. doi: 10.1113/jphysiol.2002.033811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang AL, et al. The cells and logic for mammalian sour taste detection. Nature. 2006;442:934–938. doi: 10.1038/nature05084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Béhé P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papilla. Evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang YA, Maruyama Y, Stimac R, Roper SD. Presynaptic (Type III) cells in mouse taste buds sense sour (acid) taste. J Physiol. 2008;586:2903–2912. doi: 10.1113/jphysiol.2008.151233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishimaru Y, et al. Interaction between PKD1L3 and PKD2L1 through their transmembrane domains is required for localization of PKD2L1 at taste pores in taste cells of circumvallate and foliate papillae. FASEB J. 2010;24:4058–4067. doi: 10.1096/fj.10-162925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson TM, et al. Taste function in mice with a targeted mutation of the pkd1l3 gene. Chem Senses. 2010;35:565–577. doi: 10.1093/chemse/bjq070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tombola F, Ulbrich MH, Isacoff EY. Architecture and gating of Hv1 proton channels. J Physiol. 2009;587:5325–5329. doi: 10.1113/jphysiol.2009.180265. [DOI] [PMC free article] [PubMed] [Google Scholar]