Abstract
Bacteria frequently possess two type IIA DNA topoisomerases, gyrase and topo IV, which maintain chromosome topology by variously supercoiling, relaxing, and disentangling DNA. DNA recognition and functional output is thought to be controlled by the C-terminal domain (CTD) of the topoisomerase DNA binding subunit (GyrA/ParC). The deeply rooted organism Aquifex aeolicus encodes one type IIA topoisomerase conflictingly categorized as either DNA gyrase or topo IV. To resolve this enzyme’s catalytic properties and heritage, we conducted a series of structural and biochemical studies on the isolated GyrA/ParC CTD and the holoenzyme. Whereas the CTD displays a global structure similar to that seen in bone fide GyrA and ParC paralogs, it lacks a key functional motif (the “GyrA-box”) and fails to wrap DNA. Biochemical assays show that the A. aeolicus topoisomerase cannot supercoil DNA, but robustly removes supercoils and decatenates DNA, two hallmark activities of topo IV. Despite these properties, phylogenetic analyses place all functional domains except the CTD squarely within a gyrase lineage, and the A. aeolicus GyrB subunit is capable of supporting supercoiling with Escherichia coli GyrA, but not DNA relaxation with E. coli ParC. Moreover, swapping the A. aeolicus GyrA/ParC CTD with the GyrA CTD from Thermotoga maritima creates an enzyme that negatively supercoils DNA. These findings identify A. aeolicus as the first bacterial species yet found to exist without a functional gyrase, and suggest an evolutionary path for generation of bacterial type IIA paralogs.
Keywords: chromosome dynamics, DNA binding proteins, protein evolution
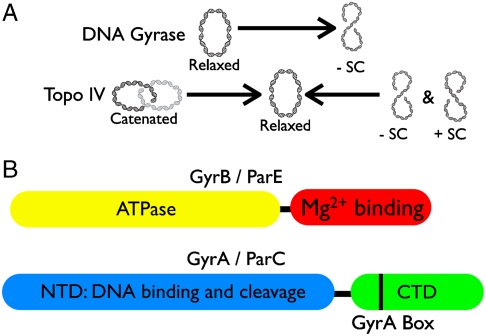
Cellular DNA topology is managed by topoisomerases, molecular machines that both regulate supercoiling and unlink tangled chromosomes (1). The bacterial/archaeal type IIA topoisomerase superfamily, which includes DNA gyrase and topoisomerase IV (topo IV), couples ATP binding and hydrolysis with DNA binding, cleavage, and strand transport to physically move one duplex through another (2, 3) (Fig. 1A). DNA gyrase negatively supercoils DNA, an activity that helps contribute to the underwinding of many bacterial genomes (4, 5). Conversely, topo IV preferentially relaxes DNA supercoils and is a robust DNA decatenase (6, 7). Gyrase and topo IV share significant structural homology and are thought to be paralogs that resulted from gene duplication of an ancestral type IIA topoisomerase (8, 9).
Fig. 1.
Hallmark activities (A) and primary domain structures (B) of bacterial type IIA topoisomerases. Domains are colored. Domain functions are indicated with black labels.
Both gyrase and topo IV are A2B2 heterotetramers. Their functional differences are thought to be predicated upon a C-terminal DNA binding domain (the CTD) that is appended to the homologous A protomers (gyrA and parC) (Fig. 1B) (10–14). The gyrase CTD is believed to constrain a positive supercoil by bending DNA around its surface, allowing the enzyme to introduce a negative supercoil upon strand passage (15–17). Loss of the GyrA CTD, or the GyrA box, a conserved motif found in the first blade of the GyrA CTD (Fig. 1B and Fig. S1), eliminates gyrase’s ability to negatively supercoil DNA (10, 18, 19). The topo IV CTD has been proposed to control enzyme activity not by wrapping DNA around its surface, but by helping to capture a DNA segment in trans for strand passage (14). Removal of the topo IV CTD ablates the holoenzyme’s preference for its favored substrates, positively supercoiled and catenated DNAs (14, 20).
Thus far, it is unclear how specific evolutionary modifications to the CTD, such as blade number and CTD shape, help fine-tune the substrate selectivity and functional output of prokaryotic type IIA topoisomerases. In this regard, one particularly intriguing, and uncharacterized, type IIA topoisomerase derives from Aquifex aeolicus, a member of one of the most thermophilic and deeply branched families within the bacterial domain (21). Determination of the A. aeolicus genome sequence led to the initial annotation of this enzyme as a DNA gyrase (21), a conclusion supported by subsequent phylogenetic comparisons, which found that the B subunit aligns with gyrB orthologs (8, 22). However, these later studies also noted that the A. aeolicus CTD lacked a canonical GyrA-box motif within its putative gyrA gene, suggesting that this open reading frame might in fact code for ParC, a subunit of topo IV (8, 22). This dichotomy was further accentuated by: (i) the lack of either a clear ParE homolog, or a GyrA subunit containing an appropriate GyrA box in A. aeolicus, and (ii) the present understanding that all bacterial species are believed to minimally retain and require DNA gyrase for viability (8, 23). These observations not only raised a basic question as to whether the A. aeolicus enzyme functions as a gyrase or topo IV, but also suggested that inspection of the Aquifex system could afford a new general perspective into the evolution of bacterial type IIA topoisomerases.
To address these issues, we carried out structural and biochemical analyses of the sole type II topoisomerase of A. aeolicus. We first determined the crystal structure of the A. aeolicus GyrA/ParC CTD to 1.3 Å resolution, and characterized the ability of the domain to introduce writhe into DNA. We then purified the holoenzyme and characterized its ability to relax, supercoil, or decatenate DNA. We find that the A. aeolicus CTD bears physical hallmarks of both GyrA and ParC CTDs, but, like topo IV, it is unable to alter DNA writhe. Similarly, the activity profiles of the A. aeolicus holoenzyme follow those exhibited by topo IV, and not gyrase. However, subunit-mixing experiments show that the A. aeolicus GyrB subunit can support DNA supercoiling with E. coli GyrA, but not DNA relaxation with E. coli ParC. Moreover, replacing the A. aeolicus CTD with the CTD from Thermotoga maritima DNA gyrase bestowed a negative supercoiling activity on the A. aeolicus holoenzyme. These findings, together with bioinformatic analyses, indicate that A. aeolicus originally possessed a DNA gyrase that later converted directly into an enzyme with topo IV-like properties. Our findings provide a demonstration that bacterial species can exist without gyrase, and further highlight a unique path for the evolution of functionally distinct bacterial type IIA topoisomerases.
Results
Structure of GyrA C-Terminal Domain.
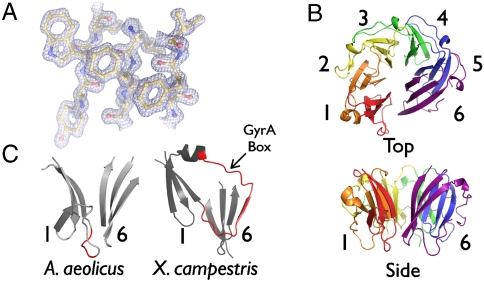
To assess the functionality of the A. aeolicus type IIA topoisomerase, we first examined the structure of the CTD of the GyrA subunit. Crystals of the isolated A. aeolicus GyrA CTD (residues 489–769) were obtained in the spacegroup P1 and diffracted to 1.3 Å, the highest resolution yet reported for any topoisomerase region. The structure was solved using multiple isomorphous replacement. Interpretable density was present for amino acids 494–769 for each of the three A. aeolicus GyrA CTD chains present in the asymmetric unit (Fig. 2A). The final model was refined to an Rwork/Rfree of 17.46/19.20% and shows excellent stereochemistry (Table S1).
Fig. 2.
A. aeolicus CTD structure. (A) Representative electron density from a refined 2Fo-Fc map contoured at 1.8σ. (B) Ribbon model of A. aeolicus CTD. Blades are numbered. (C) Comparison of blades 1 and 6 of the A. aeolicus and X. campestris CTDs highlights the missing A. aeolicus GyrA-box, shown in red in both structures.
As seen in other GyrA and ParC CTDs, the A. aeolicus CTD forms a planar disc comprising a multibladed “β-pinwheel” structure similar to, but topologically distinct from, canonical β-propellers (Fig. 2B) (17). Analogous to other GyrA/ParC CTDs, the A. aeolicus CTD retains a positively charged band around the disc’s perimeter that is thought to assist with DNA binding and/or bending (Fig. S2A) (17, 24). The A. aeolicus CTD most closely superposes with the CTD of Borrelia burdorferi GyrA (1.48 Å rmsd), although the A. aeolicus CTD adopts a partially open configuration seen in other CTD structures such as E. coli GyrA and Bacillus stearothermophilus ParC (Fig. S2B) (16, 24). Comparison of the A. aeolicus CTD with the Xanthomonas campestris GyrA CTD reveals that this open configuration is due to the loss of the GyrA-box in the A. aeolicus domain, which in X. campestris, closes off the pinwheel by pairing the first blade with the sixth (Fig. 2C) (25). X campestris was used in this comparison as it is the only structure to date that includes a complete GyrA-box. By comparison, the E. coli ParC CTD also lacks the GyrA box loop (as well as the entire sixth blade), forming a C-shaped structure that is even more open than that seen for the A. aeolicus CTD (3.01 Å rmsd).
A. aeolicus Holoenzyme Activity.
Because the A. aeolicus CTD structure contained hallmarks of both GyrA and ParC CTDs, we next sought to determine how the intact topoisomerase acts on different DNA substrates. To accomplish this, we prepared and reconstituted the heterotetrameric holoenzyme. Because A. aeolicus is a hyperthermophile, we initially screened for activity over a range of temperatures (37–90 °C). Although activity was detected across this range, optimal activity was observed at 70 °C.
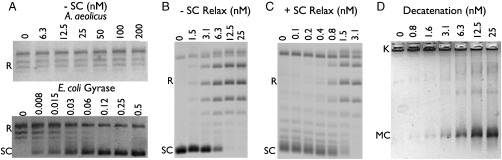
The first reaction we tested was negative supercoiling, the defining hallmark of gyrase (2). In this reaction, relaxed circular DNA is incubated with enzyme and ATP, after which the reaction products are resolved by agarose-gel electrophoresis. Significantly, the A. aeolicus enzyme failed to produce any sign of negatively-supercoiled DNA from the starting substrate, even when present at a > 30-fold molar excess (Fig. 3A). This result provided clues that, contrary to its annotated status, the type II topoisomerase present in A. aeolicus is not a gyrase.
Fig. 3.
A. aeolicus holoenzyme assays. (A) Negative supercoiling activity. E. coli gyrase activity is shown for comparison. Negative (B) and positive (C) supercoil relaxation. (D) Decatenation. DNA species are labeled as follows: SC—supercoiled, R—relaxed, K—intact kDNA network, MC—released kDNA minicircles.
We next assessed the ability of the enzyme to catalyze DNA relaxation. In the presence of ATP, A. aeolicus DNA gyrase was able to remove both negative and positive DNA supercoils. Interestingly, positive supercoil relaxation was more robust than the relaxation of negative supercoils (∼5-fold), a characteristic of topo IV enzymes (Fig. 3 B and C) (6, 14). The ability to relax negative supercoils in the presence of ATP directly conflicts with the canonical activity of DNA gyrase. Together, these data demonstrate that, at least functionally, the A. aeolicus type II topoisomerase serves as a topo IV.
To further test this claim, we examined DNA decatenation, a known specialty of topo IV. Activity was quantified by incubating the reconstituted enzyme with kinetoplast DNA (kDNA) and ATP, and the reaction again resolved by electrophoresis. As a network of catenated DNA minicircles, intact kDNA is too large to enter the gel, whereas the small minicircles can be liberated by the A. aeolicus topoisomerase and detected. The observed decatenation activity of the A. aeolicus topoisomerase was similar in efficiency to the relaxation activities of the enzyme, although not as robust as seen for positive supercoil relaxation (Fig. 3D). Moreover, the released minicircles showed no sign of supercoiling, a reaction known to be catalyzed by gyrase in this assay (18).
DNA Writhe Introduction by the A. aeolicus ParC C-Terminal Domain.
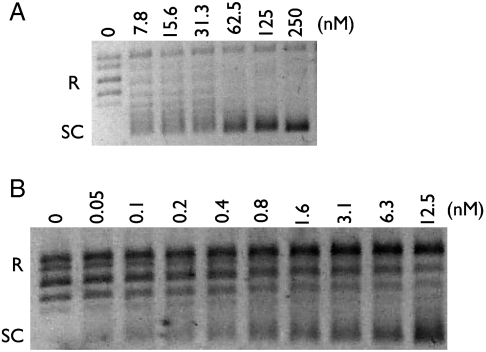
The distinguishing ability of gyrase to negatively supercoil DNA relies on the DNA wrapping properties of the GyrA CTD (10). These properties can be observed experimentally and quantitated by a “topology footprint” (15, 16). In this type of experiment, the isolated CTD is mixed with relaxed plasmid DNA, after which a type IB topoisomerase (e.g., eukaryotic topo I) is added to remove compensatory supercoils formed by CTD binding and wrapping. The subsequent stripping of bound CTD protomers by SDS/proteinase K leaves the DNA with an “afterimage” of the number of supercoils originally sequestered by the CTDs; the assay also reports on the handedness of the wrap, which is known to be positive for the isolated CTD fold (15, 26). Examination of the A. aeolicus CTD by this method showed it was only marginally capable of introducing writhe into DNA, to a similar degree as the E. coli ParC CTD (Fig. S3A). By contrast, control experiments using the E. coli GyrA CTD show robust positive supercoiling of the DNA, consistent with its known chrial wrapping properties. Together with the activities seen for the holoenzyme, this result further supports the proposition that the type IIA topoisomerase genes of A. aeolicus code for a functional topo IV enzyme.
The Type II Topoisomerase of A. aeolicus Is a Gyrase/topo IV Hybrid.
Although the reaction profiles of the A. aeolicus enzyme are clearly distinct from those seen for a gyrase, they did not immediately comport with phylogenetic analyses of the protein placing the type II topoisomerase in the gyrase clade (8). To examine this discrepancy in more detail, we constructed alignments using 46 representative bacterial GyrA/ParC and 49 GyrB/ParE protein sequences. Consistent with prior annotations (8, 21, 22), our neighbor-joining trees placed the complete A. aeolicus A subunit with ParC sequences and the intact B subunit with GyrB sequences (Figs. S4 and S5). However, when we separated and aligned the individual N- and C-terminal domains of the A. aeolicus A subunit with other gyrase/topo IV sequences, only the CTD fell within the ParC clade (Fig. S4). By contrast, the N-terminal domain (NTD), which houses the active site for DNA cleavage, aligned with known GyrA proteins. Moreover, the placement of the CTD within the type II topoisomerase phylogenetic tree resides close to the branch point that that separates the gyrase/topo IV clades. Thus, whereas A. aeolicus’s type IIA topoisomerase behaves functionally like a topo IV, its ancestry at the sequence level is overall more similar to DNA gyrase.
A. aeolicus GyrB Supports Negative Supercoiling Activity.
The discrepancy between sequence and function for the A. aeolicus type II topoisomerase suggested a direct means of typing its paternity. Specifically, if the A. aeolicus enzyme was truly of gyrase heritage, we reasoned that its individual subunits might work with those of E. coli gyrase. Conversely, if it descended from topo IV, then functional chimeras might be formed from E. coli topo IV. Such subunit mixing experiments have been shown to work for very distantly related gyrases, such as those found in chloroplasts (27).
To test this idea, we reconstituted A2B2 heterotetramers using E. coli GyrA and A. aeolicus GyrB, and measured their ability to negatively supercoil relaxed DNA in the presence of ATP (Fig. S6). As a control, E. coli GyrA also was mixed with E. coli ParE, the topo IV paralog of GyrB. As expected, the E. coli GyrA/ParE mixture failed to support supercoiling (Fig. S3B). By contrast, A. aeolicus GyrB functionally paired with E. coli GyrA to negatively supercoil the substrate (Fig. 4A), with an optimal activity observed between 37°–47 °C. Moreover, the addition of A. aeolicus GyrB to E. coli ParC did not give rise either to supercoiling or to DNA relaxation (Fig. S3C). These data support the concept that, despite its biochemical functions, the A. aeolicus enzyme is of direct gyrase ancestry.
Fig. 4.
Chimera activities. (A) Subunit mixing—E. coli GyrA with A. aeolicus GyrB. (B) A. aeolicus NTD fused to the T. maritima CTD.
A. aeolicu: T. maritima GyrA Fusion Supports Negative Supercoiling Activity.
Because the A. aeolicus GyrB subunit is able to support negative supercoiling activity and the CTD of the GyrA subunit was the only portion of the holoenzyme to align as a topo IV, we next asked whether the A. aeolicus GyrA CTD could be swapped with a bone fide GyrA CTD to restore gyrase function. Using the GyrA CTD from the thermophile T. maritima, which contains a canonical GyrA-box (Fig. S1), we constructed a chimera with the A. aeolicus GyrA/ParC NTD. We then reconstituted A2B2 heterotetramers using the chimera and A. aeolicus GyrB, and measured this enzyme’s ability to negatively supercoil relaxed DNA in the presence of ATP. Agarose gel electrophoresis of the products shows that the T. maritima CTD was sufficient to bestow negative supercoiling activity on the A. aeolicus holoenzyme (Fig. 4B). Taken with our prior observations, these results further suggest the A. aeolicus enzyme is of direct gyrase ancestry.
Discussion
The majority of bacteria code for two heterotetrameric (A2B2) type II topoisomerases (1, 8, 9). The underlying basis for this duplication may derive from a need to divide the labor of DNA supercoiling and chromosome decatenation between distinct enzymes. The topoisomerase required for supercoiling, DNA gyrase, is more widespread than its counterpart used in chromosome segregation, topo IV. Moreover, in those species that contain a single type II topoisomerase, such as Mycobacterium tuberculosis, this enzyme up until now always has been shown to be a gyrase (28). There has been significant debate as to whether topo IV preceded gyrase, or whether the enzyme is a degenerate form of gyrase (8, 14). A key point of distinction between these two paralogs, aside from general sequence divergence, derives from their CTD, which in some topo IV orthologs is missing entirely (14).
A. aeolicus possesses only one type II topoisomerase. Based on phylogenetic analyses, this enzyme was classified principally as gyrase (21); however, the A subunit also lacked defining signatures for the GyrA box, a CTD motif required for gyrase activity (19, 22). This odd confluence of characteristics prompted us to investigate the A. aeolicus enzyme further. Through structural methods, we first found that the A. aeolicus A-subunit CTD displayed hallmarks of both gyrase and topo IV CTDs (Fig. 2). We next biochemically characterized the purified A. aeolicus holoenzyme and found it to behave as a topo IV, with the ability to both decatenate kDNA and relax both positive and negative DNA supercoils (Fig. 3). Significantly, the protein showed no propensity to negatively supercoil DNA, the defining property of gyrase. Moreover, like that of topo IV, the isolated A. aeolicus CTD proved unable to introduce writhe into a relaxed DNA plasmid (Fig. S3A). These findings firmly establish the A. aeolicus type II topoisomerase as a functional topo IV, and distinguish this organism as the first bacterium found to lack gyrase functionality.
Despite these biochemical properties, the A. aeolicus protein nonetheless shows a generally closer kinship to gyrase at the amino acid sequence level (Fig. S4 and S5) (8, 22). In addition, subunit-mixing experiments demonstrate that A. aeolicus GyrB can pair functionally with the E. coli GyrA subunit, but not E. coli ParC (Fig. 4 and Fig. S3C). The only portion of this protein to align with topo IV enzymes is the CTD; however, this element also maps close to the GyrA/ParC branch point, and swapping the A. aeolicus CTD with the CTD from T. maritima DNA gyrase furnishes the A. aeolicus holoenzyme with the ability to negatively supercoil relaxed DNA. These observations suggest that the functional conversion of a DNA gyrase into a topo IV can require relatively modest modifications of the CTD, such as loss of the GyrA box (8, 23). Consistent with this idea, removal of the CTD or ablation of the GyrA box in E. coli GyrA eliminates negative supercoiling activity, but retains DNA relaxation and decatenation activities (10, 18).
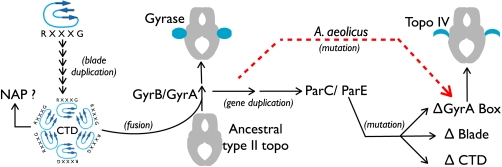
Together, these lines of evidence suggest a model for how gyrase and topo IV arose evolutionarily (Fig. 5). The ancestral protein likely was first formed as a consequence of a domain fusion between the CTD and topoisomerase core (29, 30). Because the CTD is itself composed of several repeats of a single subdomain, each of which contains remnants of the GyrA box motif present in the first blade, it is likely that this element descended from the duplication of a simple, ancestral greek-key type of β-sheet (31). The role of the CTD prior to its fusion with a type II topoisomerase A-subunit is unclear, as thus far the domain has been found only in gyrases and topo IVs; however, its ability to wrap DNA when the GyrA box is intact suggests that it may have originally acted as a type of nucleoid-associated protein that could assist in DNA compaction (32). The bacterium B. burgdorferi is interesting in this regard, in that the CTD region of its gyrA gene is expressed independently and can form higher-order nucleoprotein complexes with DNA (33). If this initial pathway is correct, then the first CTD-modified enzyme to have appeared in bacteria would have been a gyrase. Once in place, a gene duplication event of gyrB and gyrA, followed by divergence of the CTD, would result in the relative specialization of topo IV from gyrase (8, 14, 23).
Fig. 5.
Model for bacterial type IIA DNA topoisomerase evolution and specialization.
The type IIA topoisomerase of A. aeolicus is significant in this context, in that the overall phylogenetic relationships of its constituent subunits are consistent with the “gyrase-first” hypothesis. The enzyme also is noteworthy in that it suggests an alternative means for evolving a topo IV, namely, one that derives from direct modification of the gyrA gene. This “shortcut” to producing a topo IV-like function is more parsimonious than a gene duplication mechanism, in that the cell need not undergo multiple rounds of subunit gain, loss and fusion. The consequence of this approach is that A. aeolicus appears to have sacrificed its gyrase to gain topo IV. At present it is unclear why this organism has gone this route, although it is interesting to note that A. aeolicus is the most thermophilic bacteria identified to date, and it contains two copies of reverse gyrase, an enzyme that adds positive supercoils to DNA. As negatively supercoiled DNA is more prone to thermal denaturation than overwound DNA, A. aeolicus may have inactivated its gyrase as a consequence of adaptation to ultrahigh temperatures. This situation may be analogous to that seen in archaea, whose most thermophilic species also lack gyrase, and instead universally retain a specialized type IIB topoisomerase known as topo VI for decatenation (34, 35). Characterization of other deeply rooted organisms that are optimized for life’s extremes may provide further insights to the path topoisomerases traveled to become the highly specialized enzymes that exist today.
Materials and Methods
Details regarding all methods are presented in SI Materials and Methods.
Protein Purification.
The coding region of all constructs were amplified from genomic DNA and cloned into a derivative of pET28b behind an N-terminal, tobacco etch virus protease-cleavable hexahistidine tag that was removed during purification (36). Protein was expressed in E. coli and purified using Ni2+ affinity and size exclusion chromatography.
Crystallization, Data Collection, and Structure Solution.
A. aeolicus ParC CTD crystals were grown in hanging drop format at 25 °C. Crystals were equilibrated in harvest buffer and flash-frozen in liquid nitrogen for data collection. The structure was solved by MIR and the model built and refined using COOT, PHENIX, and MolProbity (Table S1 and Table S2) (37–39). The final model was deposited with the Protein Data Bank, ID code 3NO0.
DNA Relaxation, Decatenation, and Supercoiling Assays.
All activity assays were performed using appropriate DNA substrates in a buffer containing 300 ng plasmid. Reconstituted holoenzyme titrations were added to reaction mixtures, and run for 30 min at 70° or 37 °C, as indicated. Reactions were stopped, analyzed by agarose gel electrophoresis, stained with ethidium bromide (EtBr), and visualized by UV transillumination.
Topology Footprinting Assays.
DNA writhe assays were performed in a buffer containing 300 ng relaxed DNA substrate. Varying amounts of CTD were added to reaction mixtures that equilibrated at 25 °C for 20 min, 300 ng topo I was added to each mixture left at 25 °C for 20 min. Reactions were stopped, heated to 65 °C, and analyzed and visualized as described above.
Sequence Alignments and Phylogenetic Trees.
Alignments of 46 full-length, CTDs, and NTDs of GyrA and ParC sequences and 50 full-length GyrB and ParE sequences were performed using MAFFT (40). Neighbor-joining phylogenetic trees were created in Jalview and displayed using Fig Tree (41, 42).
Supplementary Material
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The crystallography, atomic coordinates, and structure factors have been deposited in the Protein Data Bank, www.pdb.org (PDB ID code 3NO0).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012938107/-/DCSupplemental.
References
- 1.Schoeffler AJ, Berger JM. DNA topoisomerases: Harnessing and constraining energy to govern chromosome topology. Q Rev Biophys. 2008;41:41–101. doi: 10.1017/S003358350800468X. [DOI] [PubMed] [Google Scholar]
- 2.Gellert M, Mizuuchi K, O’Dea MH, Nash HA. DNA gyrase: An enzyme that introduces superhelical turns into DNA. Proc Natl Acad Sci USA. 1976;73:3872–3876. doi: 10.1073/pnas.73.11.3872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kato J, et al. New topoisomerase essential for chromosome segregation in E. coli. Cell. 1990;63:393–404. doi: 10.1016/0092-8674(90)90172-b. [DOI] [PubMed] [Google Scholar]
- 4.Zechiedrich EL, et al. Roles of topoisomerases in maintaining steady-state DNA supercoiling in Escherichia coli. J Biol Chem. 2000;275:8103–8113. doi: 10.1074/jbc.275.11.8103. [DOI] [PubMed] [Google Scholar]
- 5.Drlica K, Snyder M. Superhelical Escherichia coli DNA: Relaxation by coumermycin. J Mol Biol. 1978;120:145–154. doi: 10.1016/0022-2836(78)90061-x. [DOI] [PubMed] [Google Scholar]
- 6.Crisona NJ, Strick TR, Bensimon D, Croquette V, Cozzarelli NR. Preferential relaxation of positively supercoiled DNA by E. coli topoisomerase IV in single-molecule and ensemble measurements. Genes Dev. 2000;14:2881–2892. doi: 10.1101/gad.838900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng H, Marians KJ. Decatenation activity of topoisomerase IV during oriC and pBR322 DNA replication in vitro. Proc Natl Acad Sci USA. 1993;90:8571–8575. doi: 10.1073/pnas.90.18.8571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forterre P, Gribaldo S, Gadelle D, Serre MC. Origin and evolution of DNA topoisomerases. Biochimie. 2007;89:427–446. doi: 10.1016/j.biochi.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 9.Huang WM. Bacterial diversity based on type II DNA topoisomerase genes. Annu Rev Genet. 1996;30:79–107. doi: 10.1146/annurev.genet.30.1.79. [DOI] [PubMed] [Google Scholar]
- 10.Kampranis SC, Maxwell A. Conversion of DNA gyrase into a conventional type II topoisomerase. Proc Natl Acad Sci USA. 1996;93:14416–14421. doi: 10.1073/pnas.93.25.14416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morrison A, Cozzarelli NR. Site-specific cleavage of DNA by E. coli DNA gyrase. Cell. 1979;17:175–184. doi: 10.1016/0092-8674(79)90305-2. [DOI] [PubMed] [Google Scholar]
- 12.Liu LF, Rowe TC, Yang L, Tewey KM, Chen GL. Cleavage of DNA by mammalian DNA topoisomerase II. J Biol Chem. 1983;258:15365–15370. [PubMed] [Google Scholar]
- 13.Sander M, Hsieh T. Double strand DNA cleavage by type II DNA topoisomerase from Drosophila melanogaster. J Biol Chem. 1983;258:8421–8428. [PubMed] [Google Scholar]
- 14.Corbett KD, Schoeffler AJ, Thomsen ND, Berger JM. The structural basis for substrate specificity in DNA topoisomerase IV. J Mol Biol. 2005;351:545–561. doi: 10.1016/j.jmb.2005.06.029. [DOI] [PubMed] [Google Scholar]
- 15.Reece RJ, Maxwell A. The C-terminal domain of the Escherichia coli DNA gyrase A subunit is a DNA-binding protein. Nucleic Acids Res. 1991;19:1399–1405. doi: 10.1093/nar/19.7.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ruthenburg AJ, Graybosch DM, Huetsch JC, Verdine GL. A superhelical spiral in the Escherichia coli DNA gyrase A C-terminal domain imparts unidirectional supercoiling bias. J Biol Chem. 2005;280:26177–26184. doi: 10.1074/jbc.M502838200. [DOI] [PubMed] [Google Scholar]
- 17.Corbett KD, Shultzaberger RK, Berger JM. The C-terminal domain of DNA gyrase A adopts a DNA-bending beta-pinwheel fold. Proc Natl Acad Sci USA. 2004;101:7293–7298. doi: 10.1073/pnas.0401595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramlinger VM, Hiasa H. The “GyrA-box” is required for the ability of DNA gyrase to wrap DNA and catalyze the supercoiling reaction. J Biol Chem. 2006;281:3738–3742. doi: 10.1074/jbc.M511160200. [DOI] [PubMed] [Google Scholar]
- 19.Ward D, Newton A. Requirement of topoisomerase IV parC and parE genes for cell cycle progression and developmental regulation in Caulobacter crescentus. Mol Microbiol. 1997;26:897–910. doi: 10.1046/j.1365-2958.1997.6242005.x. [DOI] [PubMed] [Google Scholar]
- 20.Stone MD, et al. Chirality sensing by Escherichia coli topoisomerase IV and the mechanism of type II topoisomerases. Proc Natl Acad Sci USA. 2003;100:8654–8659. doi: 10.1073/pnas.1133178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deckert G, et al. The complete genome of the hyperthermophilic bacterium Aquifex aeolicus. Nature. 1998;392:353–358. doi: 10.1038/32831. [DOI] [PubMed] [Google Scholar]
- 22.Guipaud O, Forterre P. DNA gyrase from Thermotoga maritima. Methods Enzymol. 2001;334:162–171. doi: 10.1016/s0076-6879(01)34465-8. [DOI] [PubMed] [Google Scholar]
- 23.Forterre P, Gadelle D. Phylogenomics of DNA topoisomerases: Their origin and putative roles in the emergence of modern organisms. Nucleic Acids Res. 2009;37:679–692. doi: 10.1093/nar/gkp032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsieh TJ, Farh L, Huang WM, Chan NL. Structure of the topoisomerase IV C-terminal domain: A broken beta-propeller implies a role as geometry facilitator in catalysis. J Biol Chem. 2004;279:55587–55593. doi: 10.1074/jbc.M408934200. [DOI] [PubMed] [Google Scholar]
- 25.Hsieh TJ, et al. Twisting of the DNA-binding surface by a β-strand-bearing proline modulates DNA gyrase activity. Nucleic Acids Res. 2010 doi: 10.1093/nar/gkq153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu LF, Wang JC. Micrococcus luteus DNA gyrase: Active components and a model for its supercoiling of DNA. Proc Natl Acad Sci USA. 1978;75:2098–2102. doi: 10.1073/pnas.75.5.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wall MK, Mitchenall LA, Maxwell A. Arabidopsis thaliana DNA gyrase is targeted to chloroplasts and mitochondria. Proc Natl Acad Sci USA. 2004;101:7821–7826. doi: 10.1073/pnas.0400836101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Manjunatha UH, et al. Functional characterisation of mycobacterial DNA gyrase: An efficient decatenase. Nucleic Acids Res. 2002;30:2144–2153. doi: 10.1093/nar/30.10.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Todd AE, Orengo CA, Thornton JM. Evolution of protein function, from a structural perspective. Curr Opin Chem Biol. 1999;3:548–556. doi: 10.1016/s1367-5931(99)00007-1. [DOI] [PubMed] [Google Scholar]
- 30.Chaudhuri I, Soding J, Lupas AN. Evolution of the beta-propeller fold. Proteins. 2008;71:795–803. doi: 10.1002/prot.21764. [DOI] [PubMed] [Google Scholar]
- 31.Schoeffler AJ, Berger JM. Recent advances in understanding structure-function relationships in the type II topoisomerase mechanism. Biochem Soc Trans. 2005;33:1465–1470. doi: 10.1042/BST0331465. [DOI] [PubMed] [Google Scholar]
- 32.Dillon SC, Dorman CJ. Bacterial nucleoid-associated proteins, nucleoid structure and gene expression. Nat Rev Microbiol. 2010;8:185–195. doi: 10.1038/nrmicro2261. [DOI] [PubMed] [Google Scholar]
- 33.Knight SW, Samuels DS. Natural synthesis of a DNA-binding protein from the C-terminal domain of DNA gyrase A in Borrelia burgdorferi. EMBO J. 1999;18:4875–4881. doi: 10.1093/emboj/18.17.4875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Forterre P, Bergerat A, Lopez-Garcia P. The unique DNA topology and DNA topoisomerases of hyperthermophilic archaea. FEMS Microbiol Rev. 1996;18:237–248. doi: 10.1111/j.1574-6976.1996.tb00240.x. [DOI] [PubMed] [Google Scholar]
- 35.Bergerat A, et al. An atypical topoisomerase II from Archaea with implications for meiotic recombination. Nature. 1997;386:414–417. doi: 10.1038/386414a0. [DOI] [PubMed] [Google Scholar]
- 36.Kapust RB, et al. Tobacco etch virus protease: Mechanism of autolysis and rational design of stable mutants with wild-type catalytic proficiency. Protein Eng. 2001;14:993–1000. doi: 10.1093/protein/14.12.993. [DOI] [PubMed] [Google Scholar]
- 37.Adams PD, et al. PHENIX: building new software for automated crystallographic structure determination. Acta Crystallogr D. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
- 38.Emsley P, Cowtan K. Coot: model-building tools for molecular graphics. Acta Crystallogr D. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 39.Lovell SC, et al. Structure validation by Calpha geometry: Phi, psi and Cbeta deviation. Proteins. 2003;50:437–450. doi: 10.1002/prot.10286. [DOI] [PubMed] [Google Scholar]
- 40.Katoh K, Asimenos G, Toh H. Multiple alignment of DNA sequences with MAFFT. Methods Mol Biol. 2009;537:39–64. doi: 10.1007/978-1-59745-251-9_3. [DOI] [PubMed] [Google Scholar]
- 41.Waterhouse AM, Procter JB, Martin DM, Clamp M, Barton GJ. Jalview Version 2—A multiple sequence alignment editor and analysis workbench. Bioinformatics. 2009;25:1189–1191. doi: 10.1093/bioinformatics/btp033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rambaut A. FigTree. Edinburgh: University of Edinburgh; 2009. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.