Abstract
Ig class-switch recombination (CSR) is a region-specific process that exchanges the constant Ig heavy-chain region and thus modifies an antibody's effector function. DNA lesions in switch (S) regions are induced by activation-induced cytidine deaminase (AID) and uracil-DNA glycosylase 2 (UNG2), subsequently processed to DNA breaks, and resolved by either the classical nonhomologous end-joining pathway or the alternative end-joining pathway (XRCC4/DNA ligase 4– and/or Ku70/Ku80-independent and prone to increased microhomology usage). We examined whether the induction of DNA lesions influences DNA end-joining during CSR by analyzing Sμ–Sα recombination junctions in various human Ig CSR defects of DNA lesion induction. We observed a progressive trend toward the usage of microhomology in Sμ–Sα recombination junctions from AID-heterozygous to AID–autosomal dominant to UNG2-deficient B lymphocytes. We thus hypothesize that impaired induction of DNA lesions in S regions during CSR leads to unusual end-processing of the DNA breaks, resulting in microhomology-mediated end-joining, which could be an indication for preferential processing by alternative end-joining rather than by classical nonhomologous end-joining.
Keywords: antibody maturation, switch junction, DNA repair
During immune responses, mature B cells diversify their Ig genes through class-switch recombination (CSR) and somatic hypermutation (SHM). The latter mechanism introduces nontemplated point mutations in the variable region of Ig genes and thereby enables the selection of antibodies with increased affinity for the antigen. CSR modulates antibody effector function by replacing one constant region with another in a deletion-mediated recombination process, while retaining the binding specificity (variable region) of the B-cell receptor. Both processes are initiated by the enzyme activation-induced cytidine deaminase (AID), which deaminates cytosine to produce U:G mismatches in target DNA (1–3). GC-rich regions, so-called “switch” (S) regions, present in front of each constant region and of varying lengths, are the target DNAs for AID during CSR. The main route for processing AID-induced lesions involves uracil excision through several pathways, primarily the pathway involving uracil-DNA glycosylase (UNG2) (4, 5). Abasic sites are further processed to generate DNA double-strand breaks (DSBs), which are obligate intermediates in CSR (6, 7). The DSBs activate damage response proteins, such as PI3-like protein kinase ataxia-telangiectasia mutated, the phosphorylated histone variant H2AX, the MRN complex (MRE11, RAD50, and NBS1), MDC1, and 53BP1, all of which are known to play roles in CSR in promoting appropriate repair and efficient long-range, region-specific recombination (8–14). In CSR, the resolution step is normally mediated by the classical nonhomologous end-joining pathway (c-NHEJ); however, recent reports strongly suggest that resolution also can be mediated (albeit at a lower frequency) by Ku 70-, Ku 80-, and/or XRCC4/DNA ligase 4–independent alternative end-joining (AEJ) pathways, which are biased toward microhomology usage, and which components are not yet well defined (15–18). The low frequency of microhomologies in S junctions from healthy individuals suggests that under normal conditions, AEJ is inefficient or even excluded from the CSR process, raising the question of how the choice between these pathways is made.
In the other lymphocyte-specific antigen receptor gene diversification process [V(D)J recombination], DNA breaks are induced by the recombinase-activating gene 1/2 (RAG1/2) in a site-specific manner. RAG1/2 core or RAG2 truncated protein (with a frameshift mutation at aa 361) enables V(D)J recombination in the absence of components of the c-NHEJ pathway, providing evidence for (i) the existence of an AEJ pathway and (ii) a role for WT RAG1/2 in recruiting c-NHEJ for repair and/or excluding AEJ components (19). This observation suggests that AEJ is excluded from the V(D)J process by the time that DNA lesions are induced.
AID and UNG can be viewed as the CSR “counterparts” of RAG1/2, given that they are responsible for inducing DNA lesions in the S regions. AID activity is essential for CSR, and the expression level of this enzyme influences the efficiency of the process. Haploinsufficiency has been reported for AID-deficient mice, although no evidence for this has been reported in humans on the basis of serum Ig levels and in vitro CSR experiments (20–22). Conversely, overexpression of AID in the CH12 murine B lymphoma cell line and in murine B lymphocytes increases the frequency of cells undergoing CSR (3, 23). We have found evidence of defective CSR in patients bearing a heterozygous deletion in which the C-terminal part of AID, including the nuclear export signal, is missing, resulting in an autosomal dominant form of AID (due to a dominant-negative effect) (20). AID+/C-termΔ mutants, together with the UNG2-deficient setting that we described previously (4), provide a unique opportunity to study DSB repair pathways in human CSR with impaired AID activity or in the absence of UNG. Using a PCR-based assay, we established that the nature of Sμ–Sα CSR junctions differs in AID-heterozygous (AID+/−), AID–autosomal dominant, and UNG2-deficient B cells compared with controls, with a progressive, marked shift toward the use of microhomologies (≥10 bp), indicating unusual processing of altered DNA lesions during CSR.
Results
Sμ–Sα Junctions Exhibit Trends Toward Microhomology in AID-Heterozygous, AID–Autosomal Dominant, and UNG2-Deficient B Cells.
To study the impact of DNA lesion induction on DSB repair during CSR, we purified genomic DNA from peripheral blood from healthy donors (controls), AID+/− patients, AID+/C-termΔ patients carrying the previously described R190X mutation (20) or the recently identified V186X mutation, and the UNG-deficient (UNG2−/−) patients described earlier. The various mutations analyzed are listed in Materials and Methods. Both AID+/C-termΔ mutations resulted in a dominant-negative form of AID. Individual Sμ–Sα fragments from the various patients were amplified using a nested PCR assay, as described previously (24). The amplification efficiency of Sμ–Sα fragments was similar for the AID+/−, AID+/C-termΔ, and control samples, in contrast to the UNG2−/− samples, in which fewer bands were amplified by nested PCR. In particular, only a few Sμ–Sα fragments were amplified in UNG2−/− patient P1 (as identified in ref. 4) (Fig. S1). To define the S junctions, the amplified Sμ–Sα fragments were sequenced and aligned with the germline Sμ and Sα sequences. In all, we characterized 67 Sμ–Sα recombination junctions in blood samples from five AID+/− patients, 84 from five AID+/C-termΔ patients, and 88 from three UNG2−/− patients. The distribution of Sμ–Sα junctions for each individual patient is shown in Fig. S2. Of note, α1 junctions were more prevalent than α2 junctions in controls (83%) compared with AID+/−, AID+/C-termΔ, and UNG2−/− patients (AID+/−, 66%, P < 0.05, χ2 test; AID+/C-termΔ, 58%, P < 0.001; UNG2−/−, 53%, P < 0.0001). Because the Sα2 sequence does not exhibit enhanced microhomology with Sμ compared with the Sα1 sequence, our observation might be related to increased IgA2 production in the patients, based on recurrent bacterial intestinal stimulation (25, 26).
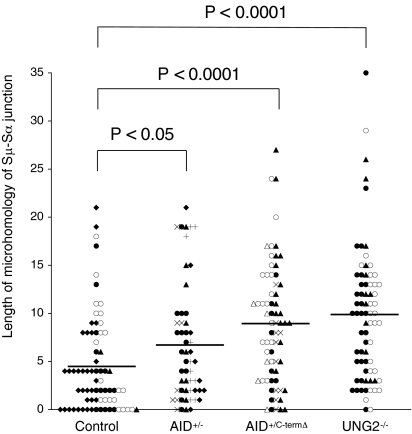
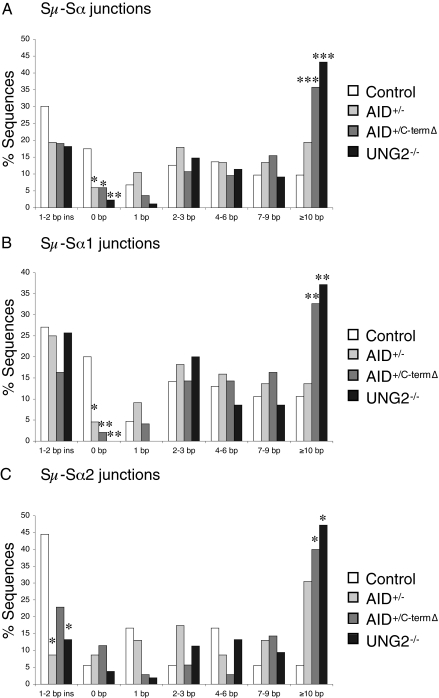
There was a trend toward an increased overlap between the Sμ and Sα sequences in recombination junctions derived from AID+/− cells (6.7 ± 0.8 nucleotides vs. 4.5 ± 0.6 in controls; P < 0.05, Student t test) (Fig. 1). A more pronounced increase in overlap between the Sμ and Sα sequences was observed with recombination junctions derived from AID+/C-termΔ and UNG2−/− patients (8.9 ± 0.8 and 9.8 ± 0.8 nucleotides, respectively) compared with controls (P < 0.0001) (Fig. 1). The increase in microhomology between Sμ and Sα sequences in recombination junctions from AID+/−, AID+/C-termΔ, and UNG2−/− patients was associated with (i) a significant decrease in the proportion of Sμ–Sα junctions with no microhomology (6% in both AID groups and 2% in UNG2−/− patients vs. 17% in controls; P < 0.05 and < 0.01, respectively, χ2 test) (Fig. 2A) and (ii) a significantly greater proportion of junctions exhibiting a long microhomology (≥10 bp) for the AID+/C-termΔ and UNG2−/− patients (36% and 43%, respectively, vs. 10% in controls; P < 0.001, χ2 test; AID+/−, 19%, P < 0.09) (Fig. 2A). We excluded the possibility that the increased microhomology observed in the junctions from the patients was the consequence of enhanced Sα2 usage, because when analyzed separately, Sμ–Sα1 and Sμ–Sα2 from AID+/−, AID+/C-termΔ, and UNG2−/− patients demonstrated the same preferential microhomology usage (Fig. 2 B and C).
Fig. 1.
Distribution of microhomology lengths for Sμ–Sα recombination junctions from AID+/−, AID+/C-termΔ, and UNG2−/− patients. Each symbol indicates a unique Sμ–Sα junction. The average length for each group and P values (two-tailed unpaired Student t test) are indicated. Junctions with insertions were excluded from this representation. The figure represents 72 control, 54 AID+/−, 68 AID+/C-termΔ, and 72 UNG2−/− Sμ–Sα junctions. The Sμ–Sα junctions obtained from the same patient in each group are marked with the same symbol. Open circles and open triangles indicate Sμ–Sα junctions from children.
Fig. 2.
Characterization of Sμ–Sα recombination junctions. The bar graphs show perfectly matched microhomology at junctions of PCR products cloned from the indicated B cells. White bars indicate sequences from control cells, light-gray bars indicate sequences from AID+/− cells, dark-gray bars indicate sequences from AID+/C-termΔ cells, and black bars indicate sequences from UNG2−/− cells. Statistical analyses were performed with the χ2 test. Statistically significant differences from control cells are shown. *P < 0.05; **P < 0.01; ***P < 0.001. (A) Characterization of Sμ–Sα recombination junctions for microhomology. Data are based on 103 junctions from controls, 67 junctions from AID+/− patients, 84 junctions from AID+/C-termΔ patients, and 88 junctions from UNG2−/− patients. (B) Analysis of Sμ–Sα1 recombination junctions. Shown are bar graphs based on 85 junctions from controls, 44 junctions from AID+/− patients, 49 junctions from AID+/C-termΔ patients, and 35 junctions from UNG2−/− patients from the pool of junctions shown in A. (C) Bar graphs for Sμ–Sα2 recombination junctions based on 18 junctions from controls, 23 junctions from AID+/− patients, 35 junctions from AID+/C-termΔ patients, and 53 junctions from UNG2−/− patients from the pool of junctions shown in A.
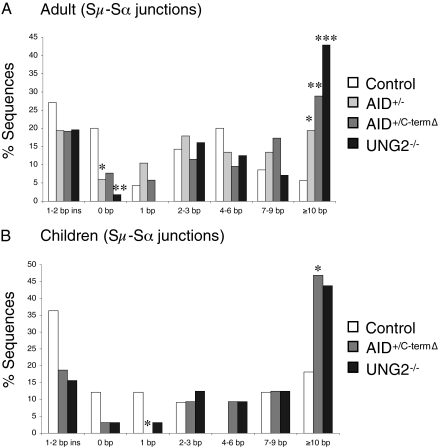
A recent study of Sμ–Sα junctions recommended the use of aged-matched controls, given that S junctions from young subjects are biased toward microhomology (27). In an aged-matched analysis, we confirmed the trend toward microhomology in separately studied adult and young AID+/C-termΔ and UNG2−/− patients and adult AID+/− patients; young AID+/− patients were not available (Fig. 3).
Fig. 3.
Age-dependent characterization of Sμ–Sα junctions. The bar graphs show perfectly matched microhomology at junctions of PCR products cloned from B cells. White bars indicate sequences from control cells, light-gray bars indicate sequences from AID+/− cells, dark-gray bars indicate sequences from AID+/C-termΔ cells, and black bars indicate sequences from UNG2−/− cells. Statistical analyses were performed with the χ2 test. Statistical significant differences are shown. *P < 0.05; **P < 0.01; ***P < 0.001. (A) Characterization of Sμ–Sα recombination junctions for microhomology derived from adult patients. Data are based on 70 junctions from controls, 67 junctions from AID+/− adult patients, 52 junctions from AID+/C-termΔ adult patients, and 56 junctions from UNG2−/− adult patients from the pool of junctions shown in Fig. 2A. (B) Analysis of Sμ–Sα recombination junctions from children (age 4–14 y). Shown are bar graphs based on 33 junctions from controls, 32 junctions from AID+/C-termΔ patients, and 32 junctions from UNG2−/− patients from the pool of junctions shown in Fig. 2A. No AID+/− children were available.
Together, our results indicate end-processing biased toward long microhomologies in UNG2−/−, AID+/C-termΔ, and, to a lesser extent, AID+/− B cells.
Position of Sμ and Sα Breakpoints in Switch Junctions from AID-Heterozygous, AID–Autosomal Dominant, and UNG2-Deficient B Cells.
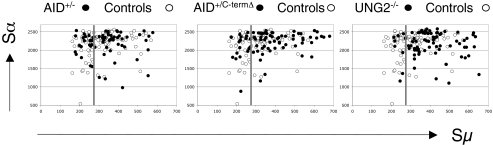
We next analyzed whether the end-processing biased toward microhomologies in UNG2−/−, AID+/C-termΔ, and AID+/− cells also affects the position of the Sμ and Sα breakpoints. In DNA ligase 4–deficient B cells, significantly more Sμ breakpoints were located in the stretch of the Sμ region (position 275–760) that has the greatest homology with Sα1 and Sα2 (17). We observed this phenomenon for Sμ breakpoints in UNG2−/− and AID+/C-termΔ patients (82% and 75%, respectively, of all Sμ breakpoints vs. 54% in controls; P < 0.0001 and < 0.01, respectively, χ2 test), indicating the need for donor/acceptor homology in DSB resolution, but not in AID+/− patients (63%) (Fig. 4). We conclude that the recombination of Sμ with Sα regions in UNG2−/− and AID+/C-termΔ cells depends in part on the presence of microhomologous sequences.
Fig. 4.
Scatterplot analysis of Sμ and Sα breakpoints. The x-axis and y-axis indicate the position of the Sμ and Sα breakpoints, respectively, with respect to the annotated S sequences. The vertical line indicates the start of the Sμ region displaying the highest degree of homology with Sα1 and Sα2. For clarity, only the Sμ breakpoints upstream of position 700 are shown. One AID+/− switch-fragment and three AID+/C-termΔ, seven UNG2−/−, and five control switch fragments were located in the more 3′ part of Sμ (i.e., downstream of position 800) and are thus not shown.
Mutations in Sμ–Sα Junctions from AID-Heterozygous, AID–Autosomal Dominant, and UNG2-Deficient B Cells.
Point mutations occurring close to the recombination junction are supposedly generated during the resolving step of CSR, given that they are generally more frequent and mostly affect GC residues, in comparison with point mutations in the germline Sμ region, which exhibit a pattern similar to SHM in V regions and thus likely involve a different mechanism (28, 29). A lower number of mutations around the Sμ–Sα junctions (±15 bp) has been reported in patients with Artemis deficiency and Ataxia-telangiectasia compared with controls, suggesting that DNA ends are not normally processed in these individuals (27). We examined the Sμ–Sα junctions from the UNG2−/−, AID+/C-termΔ, and AID+/− cells for mutations and found significantly fewer mutations located close to the junctions in the UNG2−/− and AID+/C-termΔ patients compared with controls (4.5% and 7.9%, respectively, vs. 14.2%; P < 0.01 and < 0.05, respectively, χ2 test), but not in the AID+/− patients (10.4%) (Table 1). We also noted a significant shift toward AT mutations in the AID+/C-termΔ patients (AT vs. GC; 45% in AID+/C-termΔ patients vs. 14% in controls; P < 0.01, χ2 test) (Table 1), possibly indicating a different resolution of Sμ–Sα junctions than in controls.
Table 1.
Mutations in Sμ–Sα fragments
| AT vs. GC | GC transition (G→A; C→T) | Total number of mutations | Number of bp sequenced | Frequency per 1,000 bp | |
| Close to the junction (± 15 bp) | |||||
| Controls | 6 vs. 38 (14% vs. 86%) | 22 (58%) | 44 | 3,090 | 14.2 |
| AID+/− | 6 vs. 15 (29% vs. 71%) | 10 (67%) | 21 | 2,010 | 10.4 |
| AID+/C-termΔ | 9 vs. 11(45% vs. 55%)** | 9 (82%) | 20 | 2,520 | 7.9* |
| UNG2−/− | 2 vs. 10 (17% vs. 83%) | 8 (80%) | 12 | 2,640 | 4.5** |
| Within the Sμ core (>15 bp upstream of the junction) | |||||
| Controls | 35 vs. 73 (32% vs. 68%) | 40 (55%) | 108 | 16,442 | 6.6 |
| AID+/− | 33 vs. 38 (46% vs. 53%) | 26 (68%) | 72 | 11,316 | 6.4 |
| AID+/C-termΔ | 44 vs. 36(55% vs. 45%)** | 25 (69%) | 80 | 16,650 | 4.8* |
| UNG2−/− | 34 vs. 55 (38% vs. 62%) | 52(95%)*** | 99 | 20,313 | 4.9* |
Statistical analyses were performed using the χ2 test. Statistically significant differences are shown in bold (*P < 0.05; **P < 0.01; ***P < 0.001). The Sμ core was defined as the 300-bp region starting from position Sμ 142 (X54713).
We found no differences between AID+/− patients and controls in terms of mutations in the Sμ core region, excluding mutations close to (i.e., within 15 bp) the S junction (Table 1). In contrast, the frequency of mutations in the Sμ core region was significantly lower in UNG2−/− and AID+/C-termΔ patients compared with controls (4.9% and 4.8%, respectively, vs. 6.6%; P < 0.05 for both groups, χ2 test) and indicated a shift toward AT mutations in the AID+/C-termΔ group (Table 1). This might indicate an increased number of lesions on which Polη was active (30). However, the significantly higher proportion of GC transitions observed in the rearranged UNG2−/− Sμ core region sequences (95% vs. 55% in controls; P < 0.001, χ2 test) (Table 1) was reminiscent of the UNG-deficient SHM pattern (4). In conclusion, the analysis of mutations in Sμ–Sα junctions derived from UNG2−/− and AID+/C-termΔ B cells is in agreement with an unusual end-processing.
Discussion
Our analysis of Sμ–Sα junctions in AID+/−, AID+/C-termΔ, and UNG2−/− patients provides evidence to suggest that the choice of the DNA repair pathway for S region end-joining is made at the start of the CSR process (i.e., as soon as DNA lesions have been induced). We chose to analyze Sμ–Sα junctions because these junctions are known to be strongly affected by deficiencies in DNA ligase 4 or PMS2, in contrast to Sμ–Sγ junctions, which are little influenced by these deficiencies (17, 31). We also attempted to amplify Sμ–Sα junctions from homozygous AID-deficient (AID−/−) patients but failed, most likely due to the complete absence of CSR in these patients (1).
We analyzed the frequency of mutations in the Sμ core region from recombined S regions and found that it was significantly lower in UNG2−/− and AID+/C-termΔ patients compared with controls. Contradictory data have been reported in mice, with retroviral overexpression of the AID+/C-termΔ (Δ189–198) murine mutant in AID−/− B cells showing normal in vitro cytidine deaminase activity and induction of mutations in the Sμ region, but leading to inefficient CSR (32). This discrepancy could be related to the sequencing of different regions in Sμ, or to the fact that the murine study analyzed mutations in germline (i.e., unrearranged) Sμ regions from B cells undergoing in vitro CSR, whereas we analyzed rearranged Sμ regions from AID+/C-termΔ patients that were successfully switched in vivo. A recent study suggested that AID participates via its C-terminal part in a multimolecular complex (supposedly at nuclear pores) necessary for its stability and CSR (33). Thus, the lower frequency in the Sμ core region from Sμ–Sα junctions derived from UNG2−/− and AID+/C-termΔ B cells could be explained by differences in the DNA repair/recombination environment.
The significantly lower frequency of mutations close to the junctions in UNG2−/− and AID+/C-termΔ patients compared with controls most likely reflects a different recombination and/or end-processing during CSR in these cells. The significantly higher AT:GC ratio of mutations in the AID+/C-termΔ group must be interpreted with caution. Indeed, the overall number of mutations was low, and mutations close to the junctions might be corrupted by mutations introduced into Sμ before recombination. The difference in the frequency of mutations, as well as the progressive trend toward microhomology usage observed in AID+/−, AID+/C-termΔ, and UNG2−/− patients, correlated well with the in vivo CSR deficiency based on IgG and IgA serum levels, with AID+/− normal, AID+/C-termΔ decreased, and UNG2−/− dramatically decreased or even absent (4, 20).
Several hypotheses, not exclusive, can be raised to explain why microhomology-mediated end-joining/recombination is favored in AID+/−, AID+/C-termΔ, and UNG2−/− B cells. Induction of DNA lesions is likely impaired in cases of reduced AID activity or in the absence of UNG. Although DSBs are detectable in B cells with AID+/C-termΔ mutations, they are undetectable in UNG-deficient B cells (4, 20). Fewer AID- and/or UNG-induced DNA lesions likely leads to staggered and long single-stranded DNA breaks that may require extensive processing by polymerases, such as polymerase μ or ζ (34, 35). Polymerase-mediated repair might lead to the insertion of nucleotides at the junctions due to template-independent synthesis, or to microhomologies due to fill-in synthesis (34, 36). The polymerase-mediated repair is likely associated with the components of c-NHEJ (34); however, the microhomology length of >10 nucleotides and the location of the Sμ breakpoints observed in the analyzed patients argues for another pathway involving DNA end-resection to search for donor/acceptor homology. DNA end-resection facilitates microhomology-mediated end-joining/recombination and has been recently associated with AEJ (37). Fewer AID- and/or UNG-induced DNA lesions also could result in the absence of abasic sites located close to the DSB, which could favor AEJ processing, as suggested by a recent report demonstrating that abasic sites close to DSBs can be processed by Ku70’s lyase activity and exclude AEJ (38).
Microhomology-mediated end-joining also could be the result of impaired recruitment of cofactor(s) involved in the processing/recombination of AID-induced lesions. Several studies have strongly suggested that the C-terminal part of AID could recruit a repair cofactor or target AID to specific areas, such as nuclear pores, thereby providing a special DNA repair/recombination environment (32, 33, 39). The detection of DSBs in the Sμ region from AID+/C-termΔ B cells supports the impaired recruitment hypothesis for the AID+/C-termΔ group. Other data suggest that UNG acts in CSR not only through its enzymatic activity, but also as an important docking protein (40). Interestingly, AID, UNG, and PMS2 involved in the generation of DNA lesions or breaks seem to exert further activity during S region end-joining. Why microhomology-mediated repair occurs in PMS2-deficient cells remains unclear (31, 41). A recent study with PMS2 endonuclease–deficient mice indicated that the presence of the PMS2 protein, but not its endonuclease activity, was required for proper S region end-joining, but that PMS2 endonuclease activity was still necessary for efficient CSR (42). These results suggest that an as-yet unknown interaction of PMS2 with a putative partner could recruit the c-NHEJ pathway and/or counteract microhomology-mediated repair. Thus, AID, UNG, and PMS2 could be involved in cofactor recruitment to facilitate c-NHEJ, a hypothesis reminiscent of the known complex role of RAG1/2 in V(D)J recombination (19). The nature of the cofactor(s) required for proper end-joining during CSR remains unknown; 53BP1 is a possible candidate, given the recent demonstration of its involvement in the decision between AEJ and c-NHEJ during the CSR process (37).
Whether the microhomology-mediated end-joining that we observed in AID+/−, AID+/C-termΔ, and UNG2−/− cells is the actual result of AEJ (Ku70/Ku80- and/or XRCC4/DNA ligase 4–independent) or the consequence of modified c-NHEJ remains unresolved. The extent to which AEJ reflects a distinct bona fide repair mechanism or a compensatory pathway when c-NHEJ is defective is unclear (16, 36, 43).
In conclusion, the present study strongly suggests a direct link between DNA lesion induction in S regions during CSR and the DNA repair machinery subsequently involved in the end-joining process.
Materials and Methods
Patients.
The study included the three UNG patients described previously (4), five AID-heterozygous adults (with a G203A, C441A, or C70T mutation or deletion of the entire coding region) from four independent families, three AID–autosomal dominant patients (with the previously described heterozygous R190X mutation) from two unrelated families [including patients 2-I-1 and 2-II-2 from a previous study (20)], and two patients from the same family with a heterozygous V186X mutation as the result of a 4-nucleotide insertion at the beginning of exon 5. Blood samples were drawn after informed consent was obtained from the patient or parents. The study was approved by the local institutional review board (CCPPRB 05632; Paris Saint Antoine) and was conducted in accordance with the Declaration of Helsinki.
Amplification and Analysis of Sμ–Sα Recombination Junctions.
Genomic DNA was purified from peripheral blood, and 30 ng of DNA was used as a template in each individual PCR. Amplification was performed as described previously (24), but using Go-Taq (Promega). Sμ–Sα fragments were gel-purified (Roche), cloned into Topo-TA vector (Invitrogen), and sequenced with an automated fluorescent sequencer (MilleGen). CSRs were identified by aligning the S fragment sequences with Sμ (X54713), Sα1 (L19121), and Sα2 (AF030305). Microhomology was defined as successive nucleotides shared by Sμ and Sα at the S junction, with no mismatches. A nucleotide at the breakpoints that was not identical to either of the S regions was defined as an insertion. Polymorphisms in the S region were excluded from the mutation analysis.
Supplementary Material
Acknowledgments
We thank M. Forveille for excellent technical assistance. This work was funded by grants from Institut National de la Santé et de la Recherche Médicale, European Union Framework Programme 7 EUROPAD (Primary Antibody Deficiencies) Contract 201549, and Association Contre Le Cancer.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012591108/-/DCSupplemental.
References
- 1.Revy P, et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2) Cell. 2000;102:565–575. doi: 10.1016/s0092-8674(00)00079-9. [DOI] [PubMed] [Google Scholar]
- 2.Petersen-Mahrt SK, Harris RS, Neuberger MS. AID mutates E. coli, suggesting a DNA deamination mechanism for antibody diversification. Nature. 2002;418:99–103. doi: 10.1038/nature00862. [DOI] [PubMed] [Google Scholar]
- 3.Muramatsu M, et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA-editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 4.Imai K, et al. Human uracil-DNA glycosylase deficiency associated with profoundly impaired immunoglobulin class-switch recombination. Nat Immunol. 2003;4:1023–1028. doi: 10.1038/ni974. [DOI] [PubMed] [Google Scholar]
- 5.Rada C, Di Noia JM, Neuberger MS. Mismatch recognition and uracil excision provide complementary paths to both Ig switching and the A/T-focused phase of somatic mutation. Mol Cell. 2004;16:163–171. doi: 10.1016/j.molcel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 6.Petersen S, et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature. 2001;414:660–665. doi: 10.1038/414660a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Guikema JE, et al. APE1- and APE2-dependent DNA breaks in immunoglobulin class switch recombination. J Exp Med. 2007;204:3017–3026. doi: 10.1084/jem.20071289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manis JP, et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nat Immunol. 2004;5:481–487. doi: 10.1038/ni1067. [DOI] [PubMed] [Google Scholar]
- 9.Reina-San-Martin B, Chen HT, Nussenzweig A, Nussenzweig MC. ATM is required for efficient recombination between immunoglobulin switch regions. J Exp Med. 2004;200:1103–1110. doi: 10.1084/jem.20041162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Celeste A, et al. Genomic instability in mice lacking histone H2AX. Science. 2002;296:922–927. doi: 10.1126/science.1069398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kracker S, et al. Nibrin functions in Ig class-switch recombination. Proc Natl Acad Sci USA. 2005;102:1584–1589. doi: 10.1073/pnas.0409191102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reina-San-Martin B, Nussenzweig MC, Nussenzweig A, Difilippantonio S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc Natl Acad Sci USA. 2005;102:1590–1595. doi: 10.1073/pnas.0406289102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Franco S, et al. H2AX prevents DNA breaks from progressing to chromosome breaks and translocations. Mol Cell. 2006;21:201–214. doi: 10.1016/j.molcel.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 14.Dinkelmann M, et al. Multiple functions of MRN in end-joining pathways during isotype class switching. Nat Struct Mol Biol. 2009;16:808–813. doi: 10.1038/nsmb.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yan CT, et al. IgH class switching and translocations use a robust nonclassical end-joining pathway. Nature. 2007;449:478–482. doi: 10.1038/nature06020. [DOI] [PubMed] [Google Scholar]
- 16.Boboila C, et al. Alternative end-joining catalyzes class switch recombination in the absence of both Ku70 and DNA ligase 4. J Exp Med. 2010;207:417–427. doi: 10.1084/jem.20092449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pan-Hammarström Q, et al. Impact of DNA ligase IV on nonhomologous end joining pathways during class switch recombination in human cells. J Exp Med. 2005;201:189–194. doi: 10.1084/jem.20040772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Soulas-Sprauel P, et al. Role for DNA repair factor XRCC4 in immunoglobulin class switch recombination. J Exp Med. 2007;204:1717–1727. doi: 10.1084/jem.20070255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corneo B, et al. Rag mutations reveal robust alternative end joining. Nature. 2007;449:483–486. doi: 10.1038/nature06168. [DOI] [PubMed] [Google Scholar]
- 20.Imai K, et al. Analysis of class switch recombination and somatic hypermutation in patients affected with autosomal dominant hyper-IgM syndrome type 2. Clin Immunol. 2005;115:277–285. doi: 10.1016/j.clim.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 21.Takizawa M, et al. AID expression levels determine the extent of cMyc oncogenic translocations and the incidence of B cell tumor development. J Exp Med. 2008;205:1949–1957. doi: 10.1084/jem.20081007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sernández IV, de Yébenes VG, Dorsett Y, Ramiro AR. Haploinsufficiency of activation-induced deaminase for antibody diversification and chromosome translocations both in vitro and in vivo. PLoS ONE. 2008;3:e3927. doi: 10.1371/journal.pone.0003927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robbiani DF, et al. AID produces DNA double-strand breaks in non-Ig genes and mature B cell lymphomas with reciprocal chromosome translocations. Mol Cell. 2009;36:631–641. doi: 10.1016/j.molcel.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pan Q, et al. Alternative end joining during switch recombination in patients with ataxia-telangiectasia. Eur J Immunol. 2002;32:1300–1308. doi: 10.1002/1521-4141(200205)32:5<1300::AID-IMMU1300>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 25.He B, et al. Intestinal bacteria trigger T cell–independent immunoglobulin A(2) class switching by inducing epithelial cell secretion of the cytokine APRIL. Immunity. 2007;26:812–826. doi: 10.1016/j.immuni.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 26.Fagarasan S, Kinoshita K, Muramatsu M, Ikuta K, Honjo T. In situ class switching and differentiation to IgA-producing cells in the gut lamina propria. Nature. 2001;413:639–643. doi: 10.1038/35098100. [DOI] [PubMed] [Google Scholar]
- 27.Du L, et al. Involvement of Artemis in nonhomologous end-joining during immunoglobulin class switch recombination. J Exp Med. 2008;205:3031–3040. doi: 10.1084/jem.20081915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schrader CE, et al. Mutations occur in the Ig Smu region but rarely in Sgamma regions prior to class switch recombination. EMBO J. 2003;22:5893–5903. doi: 10.1093/emboj/cdg550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nagaoka H, Muramatsu M, Yamamura N, Kinoshita K, Honjo T. Activation-induced deaminase (AID)-directed hypermutation in the immunoglobulin Smu region: Implication of AID involvement in a common step of class switch recombination and somatic hypermutation. J Exp Med. 2002;195:529–534. doi: 10.1084/jem.20012144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faili A, et al. DNA polymerase eta is involved in hypermutation occurring during immunoglobulin class switch recombination. J Exp Med. 2004;199:265–270. doi: 10.1084/jem.20031831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Péron S, et al. Human PMS2 deficiency is associated with impaired immunoglobulin class switch recombination. J Exp Med. 2008;205:2465–2472. doi: 10.1084/jem.20080789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Barreto V, Reina-San-Martin B, Ramiro AR, McBride KM, Nussenzweig MC. C-terminal deletion of AID uncouples class switch recombination from somatic hypermutation and gene conversion. Mol Cell. 2003;12:501–508. doi: 10.1016/s1097-2765(03)00309-5. [DOI] [PubMed] [Google Scholar]
- 33.Geisberger R, Rada C, Neuberger MS. The stability of AID and its function in class-switching are critically sensitive to the identity of its nuclear-export sequence. Proc Natl Acad Sci USA. 2009;106:6736–6741. doi: 10.1073/pnas.0810808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gu J, et al. XRCC4:DNA ligase IV can ligate incompatible DNA ends and can ligate across gaps. EMBO J. 2007;26:1010–1023. doi: 10.1038/sj.emboj.7601559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schenten D, et al. Pol zeta ablation in B cells impairs the germinal center reaction, class switch recombination, DNA break repair, and genome stability. J Exp Med. 2009;206:477–490. doi: 10.1084/jem.20080669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lieber MR, Wilson TE. SnapShot: Nonhomologous DNA end-joining (NHEJ) Cell. 2010;142:496. doi: 10.1016/j.cell.2010.07.035. [DOI] [PubMed] [Google Scholar]
- 37.Bothmer A, et al. 53BP1 regulates DNA resection and the choice between classical and alternative end joining during class switch recombination. J Exp Med. 2010;207:855–865. doi: 10.1084/jem.20100244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roberts SA, et al. Ku is a 5′-dRP/AP lyase that excises nucleotide damage near broken ends. Nature. 2010;464:1214–1217. doi: 10.1038/nature08926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ta VT, et al. AID mutant analyses indicate requirement for class-switch specific cofactors. Nat Immunol. 2003;4:843–848. doi: 10.1038/ni964. [DOI] [PubMed] [Google Scholar]
- 40.Begum NA, et al. Further evidence for involvement of a noncanonical function of uracil DNA glycosylase in class switch recombination. Proc Natl Acad Sci USA. 2009;106:2752–2757. doi: 10.1073/pnas.0813252106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrader CE, Vardo J, Stavnezer J. Role for mismatch repair proteins Msh2, Mlh1, and Pms2 in immunoglobulin class switching shown by sequence analysis of recombination junctions. J Exp Med. 2002;195:367–373. doi: 10.1084/jem.20011877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van Oers JM, et al. PMS2 endonuclease activity has distinct biological functions and is essential for genome maintenance. Proc Natl Acad Sci USA. 2010;107:13384–13389. doi: 10.1073/pnas.1008589107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lieber MR. NHEJ and its backup pathways in chromosomal translocations. Nat Struct Mol Biol. 2010;17:393–395. doi: 10.1038/nsmb0410-393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.