Abstract
The accumulation of metal ions and amyloid-β (Aβ) aggregates found in the brain of patients with Alzheimer’s disease (AD) has been suggested to be involved in AD pathogenesis. To investigate metal-Aβ-associated pathways in AD, development of chemical tools to target metal-Aβ species is desired. Only a few efforts, however, have been reported. Here, we report bifunctional small molecules, N-(pyridin-2-ylmethyl)aniline (L2-a) and N1,N1-dimethyl-N4-(pyridin-2-ylmethyl)benzene-1,4-diamine (L2-b) that can interact with both metal ions and Aβ species, as determined by spectroscopic methods including high-resolution NMR spectroscopy. Using the bifunctional compound L2-b, metal-induced Aβ aggregation and neurotoxicity were modulated in vitro as well as in human neuroblastoma cells. Furthermore, treatment of human AD brain tissue homogenates containing metal ions and Aβ species with L2-b showed disassembly of Aβ aggregates. Therefore, our studies presented herein demonstrate the value of bifunctional compounds as chemical tools for investigating metal-Aβ-associated events and their mechanisms in the development and pathogenesis of AD and as potential therapeutics.
Keywords: amyloid-β peptide, copper, zinc, reactive oxygen species, rational structure-based design
More than 24 million people worldwide have Alzheimer’s disease (AD), a devastating and fatal form of dementia (1–3). The key pathological markers in AD are amyloid-β (Aβ) plaques and neurofibrillary tangles, the accumulation of which is accompanied by oxidative stress, inflammation, and neurodegeneration. The “amyloid hypothesis” in AD states that Aβ, generated via cleavage of the amyloid precursor protein (APP) by β- and γ-secretases, is a proximal causative agent (1–4). It is, however, still unclear which morphological Aβ species, from soluble small oligomers to large fibrils, are central to AD pathogenesis (1–5).
In addition to Aβ aggregate deposits, dyshomeostasis and miscompartmentalization of metal ions such as Fe, Cu, and Zn ions clearly occur in AD brains (1–4, 6–10). Some studies suggest that highly concentrated metal ions play an important role in Aβ aggregate deposition and neurotoxicity including the formation of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2) (1–4, 6–13). The role of metal ions in AD and molecular mechanisms of metal-Aβ-associated pathological pathways, however, are not fully understood. Using traditional metal chelating agents, potential regulation of metal-induced Aβ aggregation and neurotoxicity has been shown in vitro and in vivo (1–4, 6, 10, 13–17). Some compounds such as clioquinol (CQ) and an 8-hydroxyquinoline derivative (PBT2) have moved into clinical trials and showed improved cognition. Long-term use of CQ is, however, limited by an adverse side effect, subacute myelo-optic neuropathy (18). Although these traditional metal chelators have yet to be available as therapeutic agents, the studies using these compounds show the possible involvement of metal ions in AD pathogenesis.
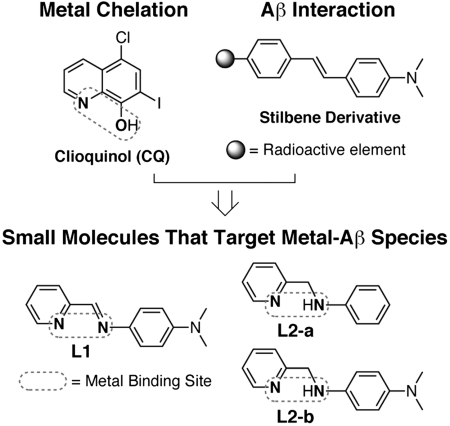
To elucidate the pathological pathways associated with both metal ions and Aβ in AD, chemical reagents that are capable of targeting metal-Aβ species would be valuable. To date, only limited efforts have been made toward this goal (2, 13, 19–24). Recently, we reported small molecules having bifunctionality (metal chelation and Aβ interaction) (22). One of the compounds, N1,N1-dimethyl-N4-(pyridin-2-ylmethylene)benzene-1,4-diamine (L1, Fig. 1), can modulate metal-induced Aβ aggregation and ROS production, leading to reduction of metal-Aβ neurotoxicity in living cells (22). Limited stability of L1 in water, however, prevents further biological applications.
Fig. 1.
Chemical structures of small molecules having bifunctionality (metal chelation and Aβ interaction).
Here, we present the small molecules N-(pyridin-2-ylmethyl)aniline (L2-a) and N1,N1-dimethyl-N4-(pyridin-2-ylmethyl)benzene-1,4-diamine (L2-b) which have greater stability than the previous generation while maintaining bifunctionality (Fig. 1). Using these compounds, metal-Aβ-triggered pathways for AD neuropathology (e.g., Aβ aggregation and ROS production) could be controlled. More importantly, the small molecule L2-b was able to target and disassemble Aβ aggregates that existed in a complex heterogeneous environment such as brain tissue homogenates of human AD patients. Our structural and reactivity studies demonstrate that bifunctional compounds could be used as chemical tools for studying metal-Aβ chemistry and biology and contribute to the development of potential therapeutic agents for AD.
Results and Discussion
Design Consideration and Preparation of N-(Pyridin-2-ylmethyl)aniline (L2-a) and N1,N1-Dimethyl-N4-(pyridin-2-ylmethyl)benzene-1,4-diamine (L2-b).
In the rational structure-based design principle, small molecules targeted to metal-Aβ species must have primary structural moieties for metal chelation and Aβ interaction (Fig. 1). In addition, for potential applications in the brain, the blood-brain barrier (BBB) permeability of compounds is essential (2, 25, 26). For BBB penetration, molecules should satisfy the restrictive terms of Lipinski’s rules [low molecular weight (MW ≤ 450); relatively lipophilic (clogP, calculated logarithm of the octanol/water partition coefficient, ≤ 5); hydrogen-bond donor atoms (HBD ≤ 5); hydrogen-bond acceptor atoms (HBA ≤ 10); small polar surface area (PSA ≤ 90 Å2)] along with calculated logBB values (Table S1). Selection of structural frameworks that meet all of these criteria should be an essential step for the rational structure-based design of small molecules to be used in the brain.
To achieve the rational structure-based development of chemical reagents that are capable of targeting metal-Aβ species, we have fashioned small molecules by direct introduction of a metal binding site into the structure of an Aβ aggregate-imaging agent (Fig. 1) (21–24). This approach can afford bifunctionality (metal chelation and Aβ interaction) in the compounds. The stilbene derivative was chosen as the basic structure of bifunctional small molecules (Fig. 1) because of its properties for the rational structure-based design principle such as strong binding affinity to Aβ species, BBB penetration, and easy removal from normal brain tissue (27). We recently reported the compound L1 that incorporates two nitrogen donor atoms into the structure of the stilbene derivative without major structural modifications (Fig. 1) (22). Although L1 is capable of regulating metal-induced Aβ events, its biocompatibility is poor due to limited stability in water, which hinders its biological applications. To solve this drawback, an amine derivative of L1 (L2-b) was prepared following the previously reported procedure [reduction of imine to amine using sodium borohyride (NaBH4), Fig. S1] (28). In addition, L2-a, which does not have a dimethylamino moiety, was synthesized by the same procedure of L2-b (Fig. S1) to compare the structure-interaction-reactivity relationship. The dimethylamino functionality has been suggested to be important for Aβ interaction (27, 29, 30).
Prediction of BBB Permeability of L2-a and L2-b.
The small molecules, L2-a and L2-b, fulfill the criteria defined by Lipinski’s rules (Table S1). As a measure of possible in vivo BBB permeability of L2-a and L2-b, the parallel artificial membrane permeability assay (PAMPA-BBB) was performed. This assay measures permeability by passive diffusion of small molecules through an artificial lipid membrane that was designed to mimic the BBB (31). Each compound can be classified as BBB permeable (CNS+) or BBB impermeable (CNS-) based on measured permeability (- log Pe). Previous reports suggest the limit for CNS+ compounds is - log Pe < 5.4 and for CNS- compounds, - log Pe > 5.7 (31). The - log Pe for L2-a, L2-b, and CQ were found to be 4.24( ± 0.01), 4.04( ± 0.06), and 4.80( ± 0.08), respectively, suggesting that these compounds may be BBB permeable (Table S2). Because of modifications to previously reported protocols (e.g., no stirring during incubation), the - log Pe values for the highly permeable known compounds verapamil and quinidine varied from published values, but based on the above classification, they were still CNS+ (32). For the low permeable test compound theophylline (CNS-), permeability was not dependent on the stirring condition and fitted well with the expected value. Overall, these results are consistent with the CNS+/CNS- classification of the known compounds and, more importantly, suggest that our compounds may be able to cross the BBB (31, 32).
Metal Binding Properties of L2-a and L2-b.
Our compounds, L2-a and L2-b, were able to chelate metal ions such as Cu2+ or Zn2+, indicated by the observation of new optical bands using UV-visible spectroscopy (UV-vis) (Fig. S2A). To understand more details regarding metal binding properties of L2-b in solution and evaluate its ability to compete with Aβ for Cu2+ and Zn2+, solutions of L2-b in the absence and presence of metal ions were investigated by UV-vis. Spectrophotometric titrations of L2-b were first completed at room temperature (I = 0.10 M NaCl) to determine its acidity constants (Ka) from pH 2.0 to 10.0. Two pKa values for L2-b (pKa1 = 4.844(4); pKa2 = 6.684(5)) were observed based on the dataset of variable pH UV-vis experiments (Fig. S2B), indicating the formation of the diprotonated species H2L2-b2+. Using these acidity constants, a solution speciation diagram was calculated showing that the neutral molecule L2-b exists predominantly (ca. 84%) at physiological pH (ca. 7.4) (Fig. S2B). Solution equilibria studies of L2-b with Cu2+ and Zn2+ were performed under the same condition (2∶1 [L2-b]/[M], I = 0.10 M NaCl, room temperature) by spectrophotometric pH titrations that were simulated by assuming the existence of 1∶1 and 1∶2 metal-ligand complexes (ML and ML2; L = ligand, L2-b) (Fig. S2C). The pKa values for L2-b as well as the hydrolysis reactions of free metal ions were included as constants in the calculations (33, 34). The stability constants of the metal-L2-b complexes suggest that L2-b has a stronger binding affinity for Cu2+ over Zn2+ (Fig. S2C, M + L⇌ML (log β1); M + 2L⇌ML2 (log β2); for Cu2+, log β1 = 10.20(7); log β2 = 15.30(9); for Zn2+, log β1 = 6.167(5); log β2 = 10.727(8)). On the basis of the stability constants, solution speciation diagrams were calculated for Cu2+ and Zn2+ with L2-b (Fig. S2C). These diagrams suggest that a mixture of 1∶1 and 1∶2 metal-ligand complexes exist at physiological pH. In addition, the concentration of free Cu2+ above pH 4.0 with the ligand was negligible, while free Zn2+ was visible up to pH 7.0. From these solution speciation diagrams, the concentrations of unchelated Cu2+ and Zn2+ (pM = - log[Munchelated]) at a specific pH value can be calculated (pCu = 9.9 and 10.2 at pH 6.6 and pH 7.4, respectively; pZn = 6.1 at pH 7.4). The pM values represent the metal binding affinity of L2-b at a specific pH value as well as an estimate of its ability to sequester metal ions bound to Aβ peptide (21, 35). The pCu and pZn values for L2-b, corresponding to approximate dissociation constants (Kd), indicated that these Kd values were comparable to the reported ones of Cu-Aβ (picomolar to nanomolar) and Zn-Aβ (micromolar) (2, 3, 8, 9, 21, 35–37). Thus, the affinities of L2-b for Cu2+ and Zn2+ at the relevant pHs [for metal-induced Aβ aggregation (2–4, 7–9)] suggest that this compound is able to chelate metal ions from soluble Aβ species, which could support the results from reactivity studies of L2-b with metal-treated Aβ species (vide infra).
To explore metal binding properties of L2-b with other divalent metal ions and its metal selectivity, the optical responses of L2-b in the presence of other divalent metal ions (Mg2+, Ca2+, Mn2+, Fe2+, Co2+, and Ni2+) were first investigated in 20 mM HEPES, pH 7.4, 150 mM NaCl, as depicted in Fig. S2D. Only minor optical changes were observed upon incubation of L2-b with biologically relevant alkaline earth metal salts, CaCl2 and MgCl2, and divalent transition metal salts, MnCl2 and FeCl2, even at high concentrations (ligand to metal ions, 1 mM/1 mM). On the other hand, treatment of L2-b (1 mM) with CoCl2 and NiCl2 (1 mM) exhibited distinct optical band shifts (Fig. S2D). These results, shown in Fig. S2 A and D, suggest noticeable optical responses of L2-b for divalent metal ions Co2+, Ni2+, Cu2+, and Zn2+. Secondly, the selectivity of L2-b for Cu2+ in the presence of other divalent metal ions in 20 mM HEPES, pH 7.4, 150 mM NaCl was investigated by UV-vis (Fig. S2E). The optical intensity at 450 nm (absorption band for Cu2+-treated L2-b, Fig. S2A) was monitored when the appropriate metal ions (40 μM and 1 mM) were added to a 40-μM solution of L2-b followed by the subsequent treatment with 40 μM Cu2+. As shown in Fig. S2E, binding of L2-b (40 μM) to Cu2+ (40 μM) was observed in the presence of other metal ions (40 μM and even 1 mM), which indicated that L2-b was selective to Cu2+ at physiological pH.
Interactions of L2-a and L2-b with Amyloid-β (Aβ) Species.
To determine the ability of L2-a and L2-b to interact directly with the monomeric form of Aβ, 2D 1H-15N TROSY-HSQC NMR spectroscopy was conducted (TROSY = transverse relaxation optimized spectroscopy; HSQC = heteronuclear single quantum correlation) (38, 39). In order to stabilize the monomeric form of the peptide and reduce aggregation, SDS-d25 micelles (SDS = sodium dodecyl sulfate) were employed (39–42). At this condition, the Aβ monomer adopts an α-helical conformation, which has been observed in solution and in contact with other biological molecules and may contribute to initial aggregation pathways leading to the formation of oligomers that have been proposed as the neurotoxic species in AD (5, 43–47). Therefore, the investigation of the possible interactions with the Aβ monomer using this model is valuable in evaluating the efficiency of our compounds to target and interact with Aβ species.
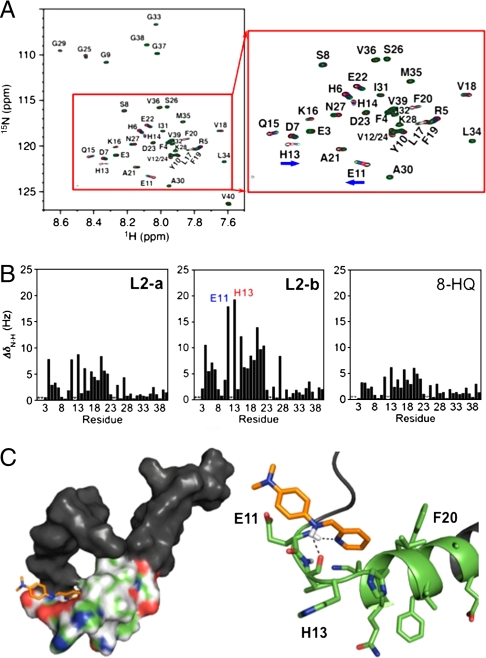
With the addition of L2-b to Aβ1-40 in SDS-d25 micelles, a significant effect on portions of the peptide was observed upon increasing the ligand concentration from five to 15 equivalents (Fig. 2A), while L2-a showed more modest effects (Fig. 2B and Fig. S3A). Additionally, the traditional metal chelator, 8-hydroxyquinoline (8-HQ) presented a smaller effect on Aβ than L2-a and L2-b (Fig. 2B and Fig. S3B), indicating that our bifunctional scaffold has greater interaction ability with the Aβ monomer. Residues that were most affected by the treatment with L2-b are E11 and H13, which are close to the putative metal binding site of Aβ (H6, H13, and H14) (Fig. 2) (2–4, 8, 9, 36, 37, 48). This is similar to previous results for L1, the imine based derivative of L2-b (22). This close contact of L2-b on the metal binding site of Aβ may facilitate chelation of metal ions surrounded by Aβ. A portion of the helical region of the Aβ peptide between Q15 and E22 was also influenced by L2-b. This helical domain of the peptide retains the most rigidity in solution, whereas the N and C termini are far more dynamic (38). The significance of the dimethylamino group in the Aβ interaction framework was exemplified by contrasting spectra of L2-a and L2-b (Fig. 2 and Fig. S3A). Adding 10 equivalents of L2-a with the Aβ monomer resulted in similar shifting of residues as L2-b but to a much lesser extent (Fig. 2B and Fig. S3A). Overall, the NMR studies suggest more direct interaction of L2-b with Aβ than L2-a and 8-HQ, which clearly displays greater bifunctionality.
Fig. 2.
Interaction of small molecules with Aβ by NMR and docking studies. (A) 2D 1H-15N TROSY-HSQC spectrum of ca. 308 μM 15N-labeled Aβ1-40 in black (900 MHz, 200 mM SDS-d25, 20 mM sodium phosphate, 7% D2O (v/v), pH 7.3, 25 °C) and the addition of 5 (red), 10 (blue), and 15 (green) equivalents of L2-b. (B) Combined 1H and 15N chemical shifts of Aβ upon the addition of 10 equivalents of L2-a (left), L2-b (middle), and 8-HQ (right), indicating the significant residues involved in small molecule recognition. *Denotes absent or overlapping peaks. (C) Docking studies of L2-b with Aβ (PDB 1BA4). Surface (left) and cartoon (right) representations of L2-b interacting with Aβ in one possible binding conformation near E11 and H13 (Conformation A, Fig. S4). Residues E11–E22, which are shown to be most affected by NMR analysis, are depicted in color. The dashed lines indicate possible hydrogen-bond contacts (2.1–2.2 Å).
The interaction of Aβ aggregates with L2-b (which has contact with the monomeric Aβ form) was also investigated employing an enzyme-linked immunosorbent assay (ELISA) (49). Small molecules that are able to be in contact with Aβ aggregates block the antibody binding, which can be detected optically. Using this method, the interaction of Aβ aggregates, generated at 37 °C for 72 h, with L2-b was compared to that with thioflavin-T (ThT), a well-known fluorescent probe for Aβ aggregates (50). As depicted in Fig. S3C, L2-b exhibited interaction with Aβ aggregates, similarly to ThT. Thus, the above studies suggest that the small molecule L2-b can interact with both Aβ monomer and aggregates.
Docking Studies for Possible Conformations of L2-a and L2-b with Aβ.
To gain a structural understanding and visualize the interaction of the monomeric Aβ species with L2-a and L2-b, docking studies were performed using AutoDock4 (51, 52). Using the previously determined structure of Aβ1-40 in SDS-d25 micelles by NMR (PDB 1BA4) (38), peptide-ligand interactions were modeled with the Lamarckian genetic algorithm with a grid box spanning the entire peptide (52). In an attempt to account for the dynamic nature of Aβ in solution, multiple conformations of the peptide were tested. Overall, five conformations agree with the NMR results, which suggests that L2-b most affects the region from E11 to E22 (Fig. 2 and Fig. S4). Ligand conformations with Aβ were selected from clusters with the highest occurrence and the lowest energy (Fig. 2C and Fig. S4). Binding energies ranged from -3.49 to -3.78 and -2.61 to -5.68 kcal/mol for L2-a and L2-b, respectively (Fig. S4). Interaction of L2-a with Aβ by docking studies was also in agreement with NMR results, where the hydrophobic region from L17 to A21 was preferred.
The dimethylamino functionality, which distinguishes L2-b from L2-a, appears to act in an amphiphilic manner, with two conformations positioning the head group toward the hydrophilic E11 and H13 residues, while the other three conformations suggest interaction with the hydrophobic region from L17 to A21 (Fig. 2C and Fig. S4). These two orientations hint at multiple binding modes in which the dimethylamino group can act as an anchor through either hydrophobic or hydrophilic contacts, which is in accordance with previous results studying the significance of this functionality (30). Inclusion of two N donor atoms for metal coordination in the structure of the stilbene derivative (Fig. 1) could facilitate hydrogen bonding with the amino acid residues or the peptide backbone affording better Aβ interaction. As expected, possible hydrogen bonding interactions of L2-a and L2-b, using N donor atoms and amine functionality (N-H), with Aβ were detected (Fig. 2C and Fig. S4). Taken with NMR investigations, our docking studies demonstrate direct Aβ interaction of L2-a and L2-b with different contacts due to the dimethylamino functionality.
Modulation of Metal-Induced Aβ Aggregation by L2-a and L2-b (Inhibition and Disaggregation Experiments).
Using L2-a and L2-b, two different reaction pathways of metal-Aβ species were investigated, as shown in Fig. 3 and Fig. S5 (inhibition: prevention of formation of metal-induced Aβ aggregates; disaggregation: transformation of metal-involved Aβ aggregates) (22). The degree of Aβ aggregation was determined by transmission electron microscopy (TEM) and native gel electrophoresis followed by Western blotting with an anti-Aβ antibody 6E10 (22). General methods [e.g., fluorescence (ThT) and turbidity measurements] to monitor the degree of Aβ aggregation were not employed because of the interference of the analysis windows with the optical bands of compounds and their corresponding metal complexes (Fig. S2A) (17, 22).
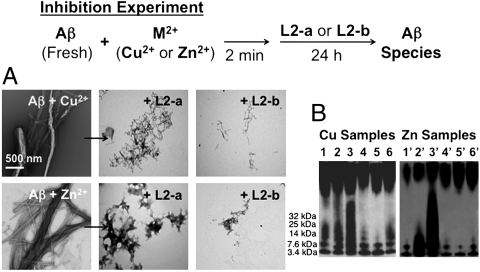
Fig. 3.
Visualization of Aβ species from inhibition experiments. Top: Scheme of the inhibition experiment. Bottom: (A) TEM images of samples of Cu2+- or Zn2+-treated fresh Aβ incubated with the compound L2-a or L2-b ([Aβ] = 25 μM, [M2+] = 25 μM, [compound] = 50 μM, 24 h, 37 °C, constant agitation). (B) Native gel electrophoresis and Western blot of the Aβ species. All samples were incubated for 24 h at 37 °C with constant agitation and anaylzed by native gel electrophoresis followed by Western blotting using the anti-Aβ antibody 6E10. Left: (1) [Aβ + Cu2+]; (2) [1 + L2-a]; (3) [1 + L2-b]; (4) [1 + CQ]; (5) [1 + phen]; (6) [1 + EDTA]. Right: (1′) [Aβ + Zn2+]; (2′) [1′ + L2-a]; (3′) [1′ + L2-b]; (4′) [1′ + CQ]; (5′) [1′ + phen]; (6′) [1′ + EDTA].
First, the formation of metal-triggered Aβ aggregates in the absence and presence of the compounds was monitored (inhibition experiment, Fig. 3). The Cu2+- or Zn2+-treated solutions of freshly prepared Aβ showed fibrillogenesis after 24 h (Fig. 3) (17, 22). On the other hand, upon 24 h incubation of freshly prepared Aβ with a metal ion with L2-a or L2-b, less Aβ aggregation was presented with different morphology from that of metal-Aβ aggregates (Fig. 3A). More importantly, L2-b, which has better bifunctionality, was able to control metal-mediated Aβ aggregation more noticeably than L2-a. These TEM results were consistent with those visualized by native gel electrophoresis and Western blotting using the anti-Aβ antibody 6E10 (Fig. 3B). Compared to other metal chelators such as CQ, phen, and EDTA [phen = 1,10-phenanthroline; EDTA = 2,2′,2′′,2′′′ - (ethane-1,2-diyldinitrilo)tetraacetic acid] (17, 22), the sample including L2-b from inhibition experiments indicated more soluble low MW Aβ species. Shortly, these inhibition experiment results could reflect that better bifunctionality of compounds (e.g., L2-b) allows for effective prevention of metal-induced Aβ aggregation.
Secondly, along with the inhibition experiments, influence of L2-a and L2-b on transformation of metal-associated Aβ aggregates, generated by 24 h incubation of freshly prepared Aβ with Cu2+ or Zn2+, was also probed (disaggregation experiment, Fig. S5A). Although metal-induced Aβ aggregates were well formed, L2-b was capable of altering their structural organization. Similar to the inhibition experiments, L2-a only slightly altered disaggregation of metal-induced Aβ aggregates, compared to L2-b. In addition, significant transformation of preformed metal-free Aβ aggregates by treatment with L2-a or L2-b was not visible (Fig. S5B). Overall, these molecules, designed for targeting metal-Aβ species, exhibit regulation of forming metal-involved Aβ aggregates, demonstrating that metal ions could be key players in Aβ aggregation.
Investigation of the Reaction of Metal-Treated Aβ with L2-b.
How do bifunctional small molecules control metal-induced Aβ aggregation? In order to understand this, the reaction of Zn2+-Aβ with L2-b was investigated by 2D NMR and UV-vis. The 2D 1H-15N TROSY-HSQC NMR spectrum of freshly prepared 15N-labeled Aβ presented well-resolved peaks in agreement with previous reports (Fig. S6A) (22, 39). Upon addition of one equivalent of ZnCl2 to Aβ, peak intensities were greatly reduced overall, with the N terminus being most affected due to metal binding to H6, H13, and H14 (Fig. S6A) (1–4, 8, 9, 36, 37). Interestingly, the NMR spectrum, obtained from the incubation of L2-b with Zn2+-bound Aβ for 4 h, was different from that of only Aβ, Aβ treated with L2-b, or Zn2+-Aβ (Fig. S6A). In particular, peaks from N-terminal residues began to grow back, but H6, H13, and H14 residues associated with metal binding were still invisible, suggesting that L2-b could not chelate Zn2+ out from Zn2+-Aβ completely. Different from L2-b, other NMR studies have shown that the metal chelating agents CQ and EDTA are able to extract Zn2+ or Cu2+ from samples of Zn2+-Aβ or Cu2+-Aβ as confirmed by the resemblance of NMR spectra of these samples with that of free Aβ (14, 37).
From the observation via NMR above, it seems apparent that L2-b is interacting with Aβ but not chelating metal ions completely from the peptide. To find out if the metal ion bound to Aβ would be interacting with the ligand or if any overall change of metal coordination would occur upon addition of L2-b, the NMR sample containing Aβ, ZnCl2, and L2-b was monitored by UV-vis. The optical band (ca. 500 nm) of the Zn-L2-b complex was observed from the NMR sample of Zn2+-Aβ incubated with L2-b (Fig. S6B), which was consistent with that shown in the reaction of ZnCl2 with L2-b at the NMR sample condition. This UV-vis evidence suggests that L2-b is interacting with Zn2+ surrounded by Aβ, which is also supported from its binding affinity for Zn2+ (described in the metal binding properties of L2-b, vide supra). Overall, L2-b is capable of being in contact with both Aβ and Zn2+, and upon the chelation of Aβ-bound Zn2+ by L2-b, the species generated is possibly a complex of L2-b-Zn2+-Aβ that is responsible for the control of metal-induced Aβ aggregation pathways.
Control of Metal-Aβ-Triggered ROS Production by L2-a and L2-b.
Binding of redox active metal ions such as Cu2+ to Aβ species is known to be involved in generation of ROS such as H2O2 and subsequent facilitation of Aβ aggregation and neurotoxicity (1, 2, 4, 6, 7, 9–12). The molecules L2-a and L2-b were tested to see if they could regulate ROS production by Cu-Aβ species. Using a horseradish peroxidase (HRP)/Amplex Red assay (12, 22, 48), the amounts of H2O2 in samples containing Cu2+, Aβ, and ascorbate in the absence and presence of L2-a and L2-b were monitored. The compounds, L2-a and L2-b, were capable of reducing Cu-Aβ-induced [H2O2] by 72( ± 3)% and 84( ± 2)%, respectively (Fig. S7). These results present that L2-a and L2-b (in particular, L2-b) are able to chelate metal ions and interact with Aβ, as well as regulate ROS production, which shows promise for their further applications.
Cytotoxicity of L2-a and L2-b in the Absence and Presence of Metal Ions in Living Cells.
Small molecules to be utilized as biological chemical tools and potential therapeutic agents must be minimally toxic. To investigate the potential toxicity of L2-a and L2-b, cell survival was monitored in two human neuroblastoma cell lines (SK-N-BE(2)-M17 and SK-N-AS) as a function of incubation of the compounds at various concentrations by the MTT assay (MTT = 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) (22). The compound L2-a (up to 100 μM) in human neuroblastoma cells showed no toxicity for 24 h and for 72 h (Fig. S8A). Compared to L2-a, L2-b indicated no toxicity for 24 h and ca. 70% or 85% cell survival (up to 100 μM) for 72 h in the SK-N-BE(2)-M17 or SK-N-AS cell line, which was higher cell viability than that observed using other metal chelators such as CQ (clinically tested) and phen (ca. 30–40% cell survival for 72 h, up to 100 μM, Fig. S8A). Furthermore, the cytotoxicity of L2-a or L2-b in the presence of CuCl2 or ZnCl2 at 1∶1 and 1∶2 ratios for 24 h was examined in the SK-N-BE(2)-M17 cell line. More than 95% cell survival was observed from cells treated with up to 20 μM metal chloride salts and 20 μM/40 μM compounds (Fig. S8B). Above 40 μM of metal salts, the cell survival rates decreased relative to the samples containing both only metal chloride salts and metal chloride salts/compounds [when CuCl2/ZnCl2 (120 μM) and L2-a/L2-b (120 μM or 240 μM) were treated with cells, approximately 30–50% cell survival was observed]. Taken together, these cytotoxicity studies suggest that the active molecule L2-b exhibits relatively minimal toxicity in the absence and presence of up to 20 μM metal ions, suggesting that it might be used as a chemical tool in biological systems or a potential therapeutic agent.
Regulation of Metal-Aβ Neurotoxicity by L2-a and L2-b in Living Cells.
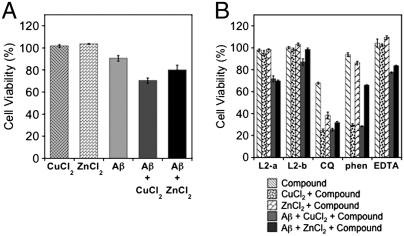
Metal-associated Aβ species in living cells are suggested to be neurotoxic (1, 2, 4, 6, 7, 10–12, 14–16, 19–24). Using relatively nontoxic concentrations of L2-a and L2-b with and without metal ions, the neurotoxicity of metal-Aβ species was monitored in living cells. First, the cytotoxicity from the cells treated with Aβ (20 μM) and either CuCl2 or ZnCl2 (20 μM) in the absence of L2-a and L2-b was investigated. As indicated in Fig. 4A, 70( ± 2) and 80( ± 4)% of cells incubated with Cu2+- and Zn2+-treated Aβ for 24 h survived, respectively, compared to 90( ± 3)% of those treated with metal-free Aβ. Secondly, to investigate the effect of our compounds on metal-Aβ neurotoxicity in living cells, cells were introduced with Aβ (20 μM) and metal chloride salts (20 μM) immediately followed by L2-b (40 μM). Noticeably, 24 h treatment of L2-b with the cells including Aβ and either Cu2+ or Zn2+ presented better cell survival [Fig. 4, cell survival: 87( ± 3)% for Cu2+-Aβ; 99( ± 1)% for Zn2+-Aβ]. In the case of L2-a, only a slight increase in the survival of cells incubated with Aβ and either Cu2+ or Zn2+ was observed, which may be expected as it revealed less modulation of metal-induced Aβ aggregation and ROS production than L2-b. Overall, this observation suggests that L2-b can target metal-Aβ species and control metal-induced Aβ aggregation and neurotoxicity.
Fig. 4.
Influence of L2-a and L2-b on metal-Aβ neurotoxicity in living cells. (A) Cell viability (%) upon incubation of CuCl2, ZnCl2, Aβ, Aβ + CuCl2, or Aβ + ZnCl2 with SK-N-BE(2)-M17 cells for 24 h, which was determined by the MTT assay. Values of cell viability depicted in the figure are relative to that of the cells only containing 1% DMSO. (B) Modulation of metal-Aβ neurotoxicity by L2-a and L2-b for 24 h ([Aβ] = 20 μM; [Cu2+ or Zn2+] = 20 μM; [compound] = 40 μM).
Interaction of L2-a and L2-b with Human AD Brain Tissue Samples.
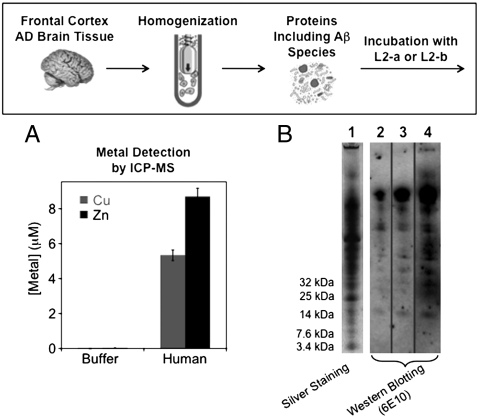
Moving forward, we explored the interaction of Aβ species from human AD brain tissues with L2-a and L2-b (Fig. 5). The frontal cortex section of the human brain was selected because it contains high amounts of Aβ aggregates (1, 2). First, we examined reactivity of L2-a and L2-b with the supernatants of homogenized human AD brain tissue samples, prepared following the method previously established (53). The metal contents (Cu and Zn) of the supernatants of the human brain homogenates were determined by inductively coupled plasma mass spectrometry (ICP-MS). The [Cu] and [Zn] found in 100 mg/mL of brain tissues were ca. 6 μM and ca. 9 μM, respectively (Fig. 5A), which may be sufficient to influence the pathways of Aβ aggregation. In addition to metal ions, the supernatants of homogenate samples exhibited a cocktail of various proteins including Aβ species, visualized by native gel electrophoresis/silver staining (Fig. 5B, lane 1). To envision the types of Aβ species in the supernatants of the human brain tissue samples, native gel electrophoresis followed by Western blotting using the anti-Aβ antibody 6E10 was performed indicating distributions of high MW Aβ species as major components (Fig. 5B, lane 2). Noticeably, as depicted in lane 4 (Fig. 5B), upon 24 h treatment of the supernatants of homogenates from the human AD brain tissue with L2-b (10 μM), more gel-permeable and lower MW Aβ species were visualized by native gel electrophoresis followed by Western blotting with 6E10. As expected, L2-b showed stronger ability to disassemble Aβ aggregates obtained from the human AD brain than L2-a. These results imply that L2-b may target and fragment existing Aβ aggregates that may be associated with metal ions in heterogeneous in vivo contexts.
Fig. 5.
Disaggregation experiments using human AD brain tissue homogenates (frontal cortex). Top: Scheme of disaggregation experiments of AD brain tissue homogenates with L2-a and L2-b. Bottom: (A) Metal concentrations from the supernatant of brain tissue homogenates (100 mg/mL) by ICP-MS. (B) Visualization of proteins including Aβ species by silver staining (lane 1) or native gel electrophoresis using Western blotting with the anti-Aβ antibody 6E10 (lane 2: only the supernatant of human homogenized AD brain tissue samples; lanes 3 and 4: the supernatants of homogenates incubated with L2-a and L2-b for 24 h, respectively).
Summary.
Small molecules having bifunctionality, such as metal chelation and Aβ interaction, were prepared based on the rational structure-based design principle. Their bifunctionality was characterized by spectroscopic methods and their capabilities to modulate metal-Aβ-involved aggregation and neurotoxicity in vitro and in living cells were revealed. The overall observations in this study suggest that bifunctional small molecules can be fashioned and used as chemical tools and potential therapeutic agents. Employing these chemical reagents that can target metal-Aβ species in the AD brain, the relationship between metal-Aβ-induced events and the development/pathogenesis of AD in vivo may be elucidated and furthermore provide new aspects of metal ions associated with Aβ species in this disease. Lastly, on the basis of the structure-interaction-reactivity relationship described herein, structural modifications of molecules (e.g., incorporation of a variety of substituents) to improve biocompatibility can move the field to a wide range of applications from diagnosis to therapeutic treatments in AD.
Materials and Methods
Details describing the chemical reagents and methods; synthesis and characterization of L2-a and L2-b; PAMPA-BBB assay; measurements of acidity and stability constants of L2-b and metal-L2-b complexes; 2D NMR and docking studies; reactivity of compounds with Aβ species using TEM, native gel electrophoresis, and other biological assays; cytotoxicity assays; studies of human AD brain tissue samples with compounds can be found in SI Text.
Supplementary Material
Acknowledgments.
We thank Dr. Subramanian Vivekanadan for help with NMR data analysis, Dr. Ted Huston for ICP-MS measurements, pION Inc. for helpful discussion on PAMPA-BBB studies, as well as Alaina DeToma and Kristin Ko for experimental assistance. We are grateful to the Neuropathology Core of the Michigan Alzheimer’s Disease Research Center for AD patients’ brain tissue samples (NIH P50 AG08671), and Srikanth Patury for helping with the docking studies. This work was supported by generous startup funding and a Rackham faculty grant from the Horace H. Rackham School of Graduate Studies from the University of Michigan as well as the Alzheimer’s Art Quilt Initiative (AAQI) (M.H.L.) and National Institutes of Health Grant DK078885 (A.R.). J.-S.C. thanks the National Research Foundation of Korea funded by the Korean government for a postdoctoral fellowship (NRF-2009-352-F00042). R.P.R.N. thanks a fellowship from the Department of Chemistry.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1006091107/-/DCSupplemental.
References
- 1.Jakob-Roetne R, Jacobsen H. Alzheimer’s disease: From pathology to therapeutic approaches. Angew Chem Int Ed. 2009;48:3030–3059. doi: 10.1002/anie.200802808. [DOI] [PubMed] [Google Scholar]
- 2.Scott LE, Orvig C. Medicinal inorganic chemistry approaches to passivation and removal of aberrant metal ions in disease. Chem Rev. 2009;109:4885–4910. doi: 10.1021/cr9000176. [DOI] [PubMed] [Google Scholar]
- 3.Gaggelli E, Kozlowski H, Valensin D, Valensin G. Copper homeostasis and neurodegenerative disorders (Alzheimer’s, prion, and Parkinson’s diseases and amyotrophic lateral sclerosis) Chem Rev. 2006;106:1995–2044. doi: 10.1021/cr040410w. [DOI] [PubMed] [Google Scholar]
- 4.Rauk A. The chemistry of Alzheimer’s disease. Chem Soc Rev. 2009;38:2698–2715. doi: 10.1039/b807980n. [DOI] [PubMed] [Google Scholar]
- 5.Haass C, Selkoe DJ. Soluble protein oligomers in neurodegeneration: Lessons from the Alzheimer’s amyloid β-peptide. Nat Rev Mol Cell Biol. 2007;8:101–112. doi: 10.1038/nrm2101. [DOI] [PubMed] [Google Scholar]
- 6.Bush AI, Tanzi RE. Therapeutics for Alzheimer’s disease based on the metal hypothesis. Neurotherapeutics. 2008;5:421–432. doi: 10.1016/j.nurt.2008.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnham KJ, Curtain CC, Bush AI. Part B: Molecular mechanisms of conformational diseases. In: Uversky VN, Fink A, editors. Protein Misfolding, Aggregation and Conformational Diseases. Media, NY: Springer Science+Business; 2007. pp. 31–47. [Google Scholar]
- 8.Faller P, Hureau C. Bioinorganic chemistry of copper and zinc ions coordinated to amyloid-β peptide. Dalton Trans. 2009:1080–1094. doi: 10.1039/b813398k. [DOI] [PubMed] [Google Scholar]
- 9.Faller P. Copper and zinc binding to amyloid-β: Coordination, dynamics, aggregation, reactivity and metal-ion transfer. ChemBioChem. 2009;10:2837–2845. doi: 10.1002/cbic.200900321. [DOI] [PubMed] [Google Scholar]
- 10.Zatta P, Drago D, Bolognin S, Sensi SL. Alzheimer’s disease, metal ions and metal homeostatic therapy. Trends Pharmacol Sci. 2009;30:346–355. doi: 10.1016/j.tips.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 11.Zhu X, Su B, Wang X, Smith MA, Perry G. Causes of oxidative stress in Alzheimer disease. Cell Mol Life Sci. 2007;64:2202–2210. doi: 10.1007/s00018-007-7218-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hureau C, Faller P. Aβ-mediated ROS production by Cu ions: Structural insights, mechanisms and relevance to Alzheimer’s disease. Biochimie. 2009;91:1212–1217. doi: 10.1016/j.biochi.2009.03.013. [DOI] [PubMed] [Google Scholar]
- 13.Perez LR, Franz KJ. Minding metals: Tailoring multifunctional chelating agents for neurodegenerative disease. Dalton Trans. 2010:2177–2187. doi: 10.1039/b919237a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherny RA, et al. Treatment with a copper-zinc chelator markedly and rapidly inhibits β-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron. 2001;30:665–676. doi: 10.1016/s0896-6273(01)00317-8. [DOI] [PubMed] [Google Scholar]
- 15.Faux NG, et al. PBT2 rapidly improves cognition in Alzheimer's disease: Additional phase II analyses. J Alzheimers Dis. 2010;20:509–516. doi: 10.3233/JAD-2010-1390. [DOI] [PubMed] [Google Scholar]
- 16.Chen T, et al. Effects of cyclen and cyclam on zinc(II)- and copper(II)-induced amyloid β-peptide aggregation and neurotoxicity. Inorg Chem. 2009;48:5801–5809. doi: 10.1021/ic900025x. [DOI] [PubMed] [Google Scholar]
- 17.Mancino AM, Hindo SS, Kochi A, Lim MH. Effects of clioquinol on metal-triggered amyloid-β aggregation revisited. Inorg Chem. 2009;48:9596–9598. doi: 10.1021/ic9014256. [DOI] [PubMed] [Google Scholar]
- 18.Arbiser JL, et al. Clioquinol-zinc chelate: A candidate causative agent of subacute myelo-optic neuropathy. Mol Med. 1998;4:665–670. [PMC free article] [PubMed] [Google Scholar]
- 19.Dedeoglu A, et al. Preliminary studies of a novel bifunctional metal chelator targeting Alzheimer’s amyloidogenesis. Exp Gerontol. 2004;39:1641–1649. doi: 10.1016/j.exger.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Wu W-h, et al. Sequestration of copper from β-amyloid promotes selective lysis by cyclen-hybrid cleavage agents. J Biol Chem. 2008;283:31657–31664. doi: 10.1074/jbc.M804722200. [DOI] [PubMed] [Google Scholar]
- 21.Rodríguez-Rodríguez C, et al. Design, selection, and characterization of thioflavin-based intercalation compounds with metal chelating properties for application in Alzheimer’s disease. J Am Chem Soc. 2009;131:1436–1451. doi: 10.1021/ja806062g. [DOI] [PubMed] [Google Scholar]
- 22.Hindo SS, et al. Small molecule modulators of copper-induced Aβ aggregation. J Am Chem Soc. 2009;131:16663–16665. doi: 10.1021/ja907045h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hureau C, Sasaki I, Gras E, Faller P. Two functions, one molecule: A metal-binding and a targeting moiety to combat Alzheimer’s disease. ChemBioChem. 2010;11:950–953. doi: 10.1002/cbic.201000102. [DOI] [PubMed] [Google Scholar]
- 24.Braymer JJ, DeToma AS, Choi J-S, Ko KS, Lim MH. Recent development of bifunctional small molecules to study metal-amyloid-β species in Alzheimer's disease. Int J Alzheimers Dis. 2011 doi: 10.4061/2011/623051. doi: 10.4061/2011/623051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clark DE, Pickett SD. Computational methods for the prediction of ‘drug-likeness’. Drug Discov Today. 2000;5:49–58. doi: 10.1016/s1359-6446(99)01451-8. [DOI] [PubMed] [Google Scholar]
- 26.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 27.Kung HF, et al. Novel stilbenes as probes for amyloid plaques. J Am Chem Soc. 2001;123:12740–12741. doi: 10.1021/ja0167147. [DOI] [PubMed] [Google Scholar]
- 28.Bloom SM, Borror AL, Greenwald RB. Precursors of β-aza-disubstituted amino styryl dyes. 4,006,151. US Patent. 1975
- 29.Zhuang Z-P, et al. Structure-activity relationship of imidazo[1,2-a]pyridines as ligands for detecting β-amyloid plaques in the brain. J Med Chem. 2003;46:237–243. doi: 10.1021/jm020351j. [DOI] [PubMed] [Google Scholar]
- 30.Leuma Yona R, Mazères S, Faller P, Gras E. Thioflavin derivatives as markers for amyloid-β fibrils: Insights into structural features important for high-affinity binding. ChemMedChem. 2008;3:63–66. doi: 10.1002/cmdc.200700188. [DOI] [PubMed] [Google Scholar]
- 31.Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood-brain barrier. Eur J Med Chem. 2003;38:223–232. doi: 10.1016/s0223-5234(03)00012-6. [DOI] [PubMed] [Google Scholar]
- 32.pION Inc. Report. Woburn, MA: pION Inc.; 2009. BBB protocol and test compounds. [Google Scholar]
- 33.Martell AE, Smith RM. Critical Stability Constants. New York: Plenum; 1974–1989. [Google Scholar]
- 34.Baes CF, Jr, Mesmer RE. The Hydrolysis of Cations. Malabar, FL: Krieger Publishing Co; 1986. [Google Scholar]
- 35.Storr T, et al. Synthesis, characterization, and metal coordinating ability of multifunctional carbohydrate-containing compounds for Alzheimer’s therapy. J Am Chem Soc. 2007;129:7453–7463. doi: 10.1021/ja068965r. [DOI] [PubMed] [Google Scholar]
- 36.Danielsson J, Pierattelli R, Banci L, Gräslund A. High-resolution NMR studies of the zinc-binding site of the Alzheimer’s amyloid β-peptide. FEBS J. 2007;274:46–59. doi: 10.1111/j.1742-4658.2006.05563.x. [DOI] [PubMed] [Google Scholar]
- 37.Hou L, Zagorski MG. NMR reveals anomalous copper(II) binding to the amyloid Aβ peptide of Alzheimer’s disease. J Am Chem Soc. 2006;128:9260–9261. doi: 10.1021/ja046032u. [DOI] [PubMed] [Google Scholar]
- 38.Coles M, Bicknell W, Watson AA, Fairlie DP, Craik DJ. Solution structure of amyloid β-peptide(1-40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry. 1998;37:11064–11077. doi: 10.1021/bi972979f. [DOI] [PubMed] [Google Scholar]
- 39.Jarvet J, Danielsson J, Damberg P, Oleszczuk M, Gräslund A. Positioning of the Alzheimer Aβ(1-40) peptide in SDS micelles using NMR and paramagnetic probes. J Biomol NMR. 2007;39:63–72. doi: 10.1007/s10858-007-9176-4. [DOI] [PubMed] [Google Scholar]
- 40.Shao H, Jao S, Ma K, Zagorski MG. Solution structures of micelle-bound amyloid β-(1-40) and β-(1-42) peptides of Alzheimer’s disease. J Mol Biol. 1999;285:755–773. doi: 10.1006/jmbi.1998.2348. [DOI] [PubMed] [Google Scholar]
- 41.Mandal PK, Pettegrew JW. Aβ peptide interactions with isoflurane, propofol, thiopental and combined thiopental with halothane: A NMR study. Biochim Biophys Acta. 2008;1778:2633–2639. doi: 10.1016/j.bbamem.2008.07.002. [DOI] [PubMed] [Google Scholar]
- 42.Wahlström A, Hugonin L, Perálvarez-Marín A, Jarvet J, Gräslund A. Secondary structure conversions of Alzheimer’s Aβ(1-40) peptide induced by membrane-mimicking detergents. FEBS J. 2008;275:5117–5128. doi: 10.1111/j.1742-4658.2008.06643.x. [DOI] [PubMed] [Google Scholar]
- 43.Shankar GM, et al. Amyloid-β protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jarvet J, et al. A left-handed 31 helical conformation in the Alzheimer Aβ(12-28) peptide. FEBS Lett. 2003;555:371–374. doi: 10.1016/s0014-5793(03)01293-6. [DOI] [PubMed] [Google Scholar]
- 45.Butterfield SM, Lashuel HA. Amyloidogenic protein-membrane interactions: Mechanistic insight from model systems. Angew Chem Int Ed. 2010;49:5628–5654. doi: 10.1002/anie.200906670. [DOI] [PubMed] [Google Scholar]
- 46.Walsh DM, et al. Amyloid β-protein fibrillogenesis. Structure and biological activity of protofibrillar intermediates. J Biol Chem. 1999;274:25945–25952. doi: 10.1074/jbc.274.36.25945. [DOI] [PubMed] [Google Scholar]
- 47.Kirkitadze MD, Condron MM, Teplow DB. Identification and characterization of key kinetic intermediates in amyloid β-protein fibrillogenesis. J Mol Biol. 2001;312:1103–1119. doi: 10.1006/jmbi.2001.4970. [DOI] [PubMed] [Google Scholar]
- 48.Himes RA, Park GY, Siluvai GS, Blackburn NJ, Karlin KD. Structural studies of copper(I) complexes of amyloid-β peptide fragments: Formation of two-coordinate bis(histidine) complexes. Angew Chem Int Ed. 2008;47:9084–9087. doi: 10.1002/anie.200803908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Inbar P, Bautista MR, Takayama SA, Yang J. Assay to screen for molecules that associate with Alzheimer’s related β-amyloid fibrils. Anal Chem. 2008;80:3502–3506. doi: 10.1021/ac702592f. [DOI] [PubMed] [Google Scholar]
- 50.LeVine H., III Quantification of β-sheet amyloid fibril structures with thioflavin T. Methods Enzymol. 1999;309:274–284. doi: 10.1016/s0076-6879(99)09020-5. [DOI] [PubMed] [Google Scholar]
- 51.Morris GM, et al. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Morris GM, et al. Automated docking using a Lamarckian genetic algorithm and an empirical binding free energy function. J Comput Chem. 1998;19:1639–1662. [Google Scholar]
- 53.Bolmont T, et al. Induction of tau pathology by intracerebral infusion of amyloid-β-containing brain extract and by amyloid-β deposition in APP x tau transgenic mice. Am J Pathol. 2007;171:2012–2020. doi: 10.2353/ajpath.2007.070403. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.