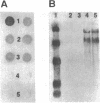
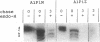Abstract
Protein degradation in the exocytic pathway was studied in Saccharomyces cerevisiae using human alpha-1-protease inhibitor (A1Pi) as a reporter molecule. Yeast cells transformed with A1Pi cDNA genes synthesized A1Pi that entered the secretion pathway and accumulated in the endoplasmic reticulum (ER). Cells expressing A1PiM (wild-type) accumulated about 10-fold more A1Pi than cells expressing A1PiZ (secretion defective variant). Analyses of A1Pi mRNA indicated that the low level of A1PiZ relative to A1PiM was not the result of differential gene transcription. Pulse-chase A1Pi radiolabeling showed that A1PiM and A1PiZ were degraded at different rates and suggested a rapid specific turnover of newly synthesized A1PiZ in the ER. Accumulated A1Pi was degraded at comparable rates in both wild-type cells and cells deficient in vacuolar protease activity, indicating that degradation of A1Pi did not occur in the vacuole. Studies to investigate the intracellular location of the degradative process, using temperature-sensitive secretion defective yeast strains, suggested the possibility that degradation occurs not only in the ER but at a second site accessed by vesicle transport. Together, these results demonstrate that a selective protein degradation process operates early in the yeast cell exocytic pathway.
Full text
PDF


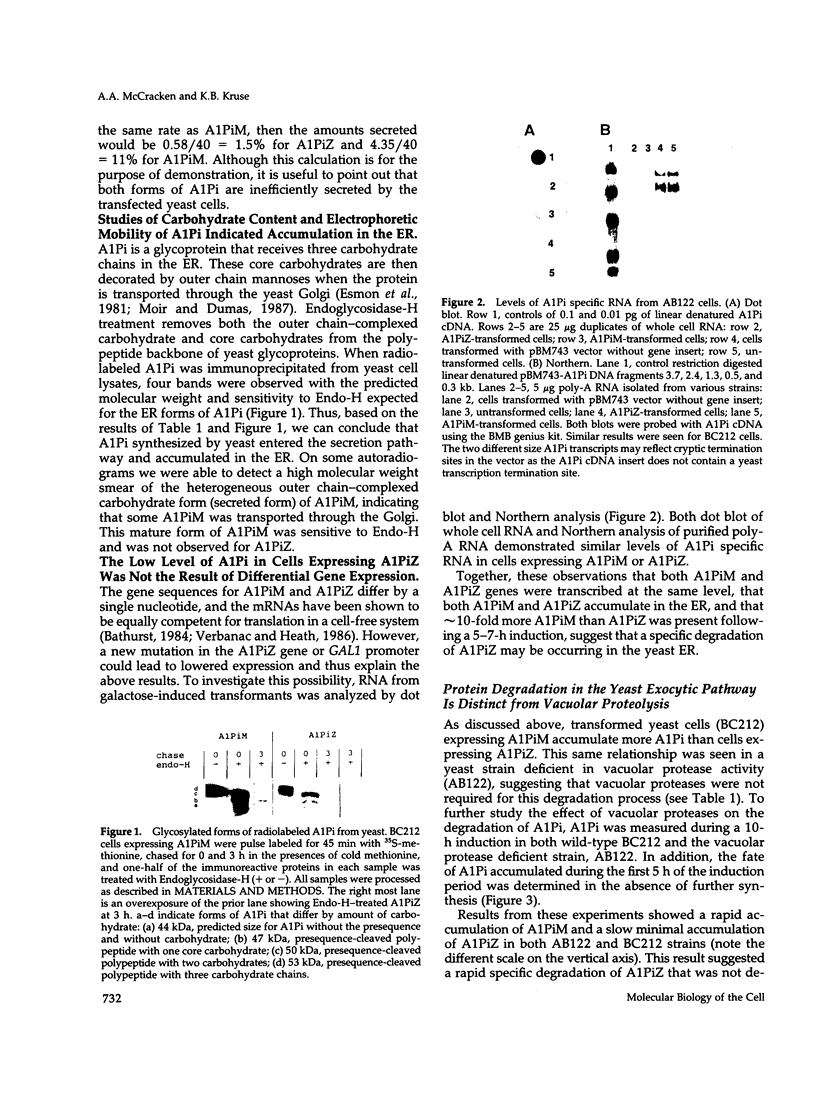
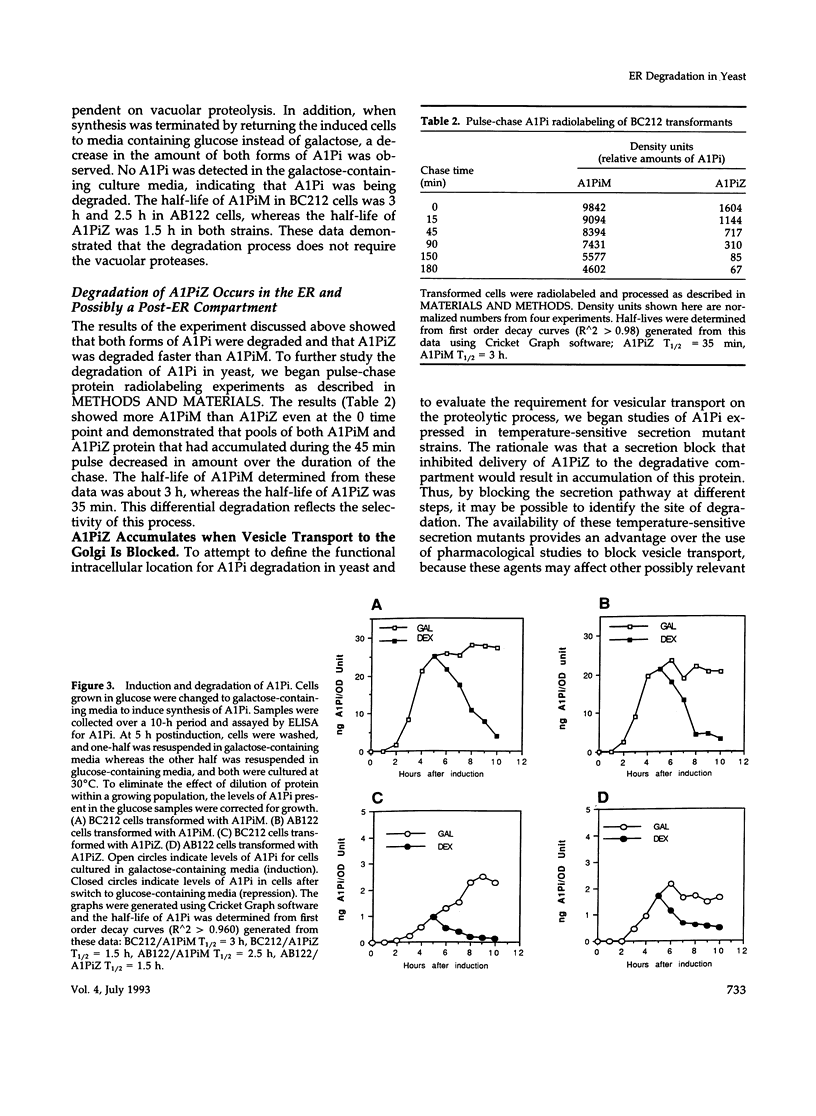



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bathurst I. C., Travis J., George P. M., Carrell R. W. Structural and functional characterization of the abnormal Z alpha 1-antitrypsin isolated from human liver. FEBS Lett. 1984 Nov 19;177(2):179–183. doi: 10.1016/0014-5793(84)81279-x. [DOI] [PubMed] [Google Scholar]
- Bienkowski R. S. Intracellular degradation of newly synthesized secretory proteins. Biochem J. 1983 Jul 15;214(1):1–10. doi: 10.1042/bj2140001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino J. S., Suzuki C. K., Klausner R. D. A peptide sequence confers retention and rapid degradation in the endoplasmic reticulum. Science. 1990 Jan 5;247(4938):79–82. doi: 10.1126/science.2294595. [DOI] [PubMed] [Google Scholar]
- Botstein D., Fink G. R. Yeast: an experimental organism for modern biology. Science. 1988 Jun 10;240(4858):1439–1443. doi: 10.1126/science.3287619. [DOI] [PubMed] [Google Scholar]
- Carlson J. A., Rogers B. B., Sifers R. N., Finegold M. J., Clift S. M., DeMayo F. J., Bullock D. W., Woo S. L. Accumulation of PiZ alpha 1-antitrypsin causes liver damage in transgenic mice. J Clin Invest. 1989 Apr;83(4):1183–1190. doi: 10.1172/JCI113999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chun K. T., Bar-Nun S., Simoni R. D. The regulated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase requires a short-lived protein and occurs in the endoplasmic reticulum. J Biol Chem. 1990 Dec 15;265(35):22004–22010. [PubMed] [Google Scholar]
- Danishefsky K., Hartwig R., Banerjee D., Redman C. Intracellular fate of fibrinogen B beta chain expressed in COS cells. Biochim Biophys Acta. 1990 Apr 6;1048(2-3):202–208. doi: 10.1016/0167-4781(90)90057-9. [DOI] [PubMed] [Google Scholar]
- Davis R. A., Thrift R. N., Wu C. C., Howell K. E. Apolipoprotein B is both integrated into and translocated across the endoplasmic reticulum membrane. Evidence for two functionally distinct pools. J Biol Chem. 1990 Jun 15;265(17):10005–10011. [PubMed] [Google Scholar]
- Dycaico M. J., Grant S. G., Felts K., Nichols W. S., Geller S. A., Hager J. H., Pollard A. J., Kohler S. W., Short H. P., Jirik F. R. Neonatal hepatitis induced by alpha 1-antitrypsin: a transgenic mouse model. Science. 1988 Dec 9;242(4884):1409–1412. doi: 10.1126/science.3264419. [DOI] [PubMed] [Google Scholar]
- Eakle K. A., Bernstein M., Emr S. D. Characterization of a component of the yeast secretion machinery: identification of the SEC18 gene product. Mol Cell Biol. 1988 Oct;8(10):4098–4109. doi: 10.1128/mcb.8.10.4098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmon B., Novick P., Schekman R. Compartmentalized assembly of oligosaccharides on exported glycoproteins in yeast. Cell. 1981 Aug;25(2):451–460. doi: 10.1016/0092-8674(81)90063-5. [DOI] [PubMed] [Google Scholar]
- Griff I. C., Schekman R., Rothman J. E., Kaiser C. A. The yeast SEC17 gene product is functionally equivalent to mammalian alpha-SNAP protein. J Biol Chem. 1992 Jun 15;267(17):12106–12115. [PubMed] [Google Scholar]
- Inoue S., Bar-Nun S., Roitelman J., Simoni R. D. Inhibition of degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase in vivo by cysteine protease inhibitors. J Biol Chem. 1991 Jul 15;266(20):13311–13317. [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston M., Davis R. W. Sequences that regulate the divergent GAL1-GAL10 promoter in Saccharomyces cerevisiae. Mol Cell Biol. 1984 Aug;4(8):1440–1448. doi: 10.1128/mcb.4.8.1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Sitia R. Protein degradation in the endoplasmic reticulum. Cell. 1990 Aug 24;62(4):611–614. doi: 10.1016/0092-8674(90)90104-m. [DOI] [PubMed] [Google Scholar]
- Le A., Ferrell G. A., Dishon D. S., Le Q. Q., Sifers R. N. Soluble aggregates of the human PiZ alpha 1-antitrypsin variant are degraded within the endoplasmic reticulum by a mechanism sensitive to inhibitors of protein synthesis. J Biol Chem. 1992 Jan 15;267(2):1072–1080. [PubMed] [Google Scholar]
- McCracken A. A., Fishman N. F. Secretion of rat serum albumin by COS cells transfected with a spliced cDNA gene. A system to study protein sorting. J Biol Chem. 1986 Jan 15;261(2):508–511. [PubMed] [Google Scholar]
- McCracken A. A., Kruse K. B., Brown J. L. Molecular basis for defective secretion of the Z variant of human alpha-1-proteinase inhibitor: secretion of variants having altered potential for salt bridge formation between amino acids 290 and 342. Mol Cell Biol. 1989 Apr;9(4):1406–1414. doi: 10.1128/mcb.9.4.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCracken A. A., Kruse K. B., Valentine J., Roberts C., Yohannes T. Z., Brown J. L. Construction and expression of alpha 1-proteinase inhibitor mutants and the effects of these mutations on secretion of the variant inhibitors. J Biol Chem. 1991 Apr 25;266(12):7578–7582. [PubMed] [Google Scholar]
- Miura O., Aoki N. Impaired secretion of mutant alpha 2-plasmin inhibitor (alpha 2 PI-Nara) from COS-7 and HepG2 cells: molecular and cellular basis for hereditary deficiency of alpha 2-plasmin inhibitor. Blood. 1990 Mar 1;75(5):1092–1096. [PubMed] [Google Scholar]
- Moir D. T., Dumais D. R. Glycosylation and secretion of human alpha-1-antitrypsin by yeast. Gene. 1987;56(2-3):209–217. doi: 10.1016/0378-1119(87)90138-7. [DOI] [PubMed] [Google Scholar]
- Naski M. C., Shafer J. A. Alpha-thrombin-catalyzed hydrolysis of fibrin I. Alternative binding modes and the accessibility of the active site in fibrin I-bound alpha-thrombin. J Biol Chem. 1990 Jan 25;265(3):1401–1407. [PubMed] [Google Scholar]
- Navarro D., Qadri I., Pereira L. A mutation in the ectodomain of herpes simplex virus 1 glycoprotein B causes defective processing and retention in the endoplasmic reticulum. Virology. 1991 Sep;184(1):253–264. doi: 10.1016/0042-6822(91)90842-y. [DOI] [PubMed] [Google Scholar]
- Novick P., Schekman R. Export of major cell surface proteins is blocked in yeast secretory mutants. J Cell Biol. 1983 Feb;96(2):541–547. doi: 10.1083/jcb.96.2.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotundo R. L., Thomas K., Porter-Jordan K., Benson R. J., Fernandez-Valle C., Fine R. E. Intracellular transport, sorting, and turnover of acetylcholinesterase. Evidence for an endoglycosidase H-sensitive form in Golgi apparatus, sarcoplasmic reticulum, and clathrin-coated vesicles and its rapid degradation by a non-lysosomal mechanism. J Biol Chem. 1989 Feb 25;264(6):3146–3152. [PubMed] [Google Scholar]
- Sato R., Imanaka T., Takatsuki A., Takano T. Degradation of newly synthesized apolipoprotein B-100 in a pre-Golgi compartment. J Biol Chem. 1990 Jul 15;265(20):11880–11884. [PubMed] [Google Scholar]
- Stafford F. J., Bonifacino J. S. A permeabilized cell system identifies the endoplasmic reticulum as a site of protein degradation. J Cell Biol. 1991 Dec;115(5):1225–1236. doi: 10.1083/jcb.115.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoller T. J., Shields D. The propeptide of preprosomatostatin mediates intracellular transport and secretion of alpha-globin from mammalian cells. J Cell Biol. 1989 May;108(5):1647–1655. doi: 10.1083/jcb.108.5.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teichert U., Mechler B., Müller H., Wolf D. H. Lysosomal (vacuolar) proteinases of yeast are essential catalysts for protein degradation, differentiation, and cell survival. J Biol Chem. 1989 Sep 25;264(27):16037–16045. [PubMed] [Google Scholar]
- Tsao Y. S., Ivessa N. E., Adesnik M., Sabatini D. D., Kreibich G. Carboxy terminally truncated forms of ribophorin I are degraded in pre-Golgi compartments by a calcium-dependent process. J Cell Biol. 1992 Jan;116(1):57–67. doi: 10.1083/jcb.116.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulmer J. B., Palade G. E. Effects of brefeldin A on the processing of viral envelope glycoproteins in murine erythroleukemia cells. J Biol Chem. 1991 May 15;266(14):9173–9179. [PubMed] [Google Scholar]
- Valetti C., Grossi C. E., Milstein C., Sitia R. Russell bodies: a general response of secretory cells to synthesis of a mutant immunoglobulin which can neither exit from, nor be degraded in, the endoplasmic reticulum. J Cell Biol. 1991 Nov;115(4):983–994. doi: 10.1083/jcb.115.4.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbanac K. M., Heath E. C. Biosynthesis, processing, and secretion of M and Z variant human alpha 1-antitrypsin. J Biol Chem. 1986 Jul 25;261(21):9979–9989. [PubMed] [Google Scholar]
- Wikström L., Lodish H. F. Endoplasmic reticulum degradation of a subunit of the asialoglycoprotein receptor in vitro. Vesicular transport from endoplasmic reticulum is unnecessary. J Biol Chem. 1992 Jan 5;267(1):5–8. [PubMed] [Google Scholar]
- Zubenko G. S., Park F. J., Jones E. W. Genetic properties of mutations at the PEP4 locus in Saccharomyces cerevisiae. Genetics. 1982 Dec;102(4):679–690. doi: 10.1093/genetics/102.4.679. [DOI] [PMC free article] [PubMed] [Google Scholar]