Abstract
The G protein-coupled receptor (GPCR), chemokine CXC-type receptor 4 (CXCR4), and its ligand, CXCL12, mediate the retention of polymorphonuclear neutrophils (PMNs) and hematopoietic stem and progenitor cells (HSPCs) in the bone marrow. Agents that disrupt CXCL12-mediated chemoattraction of CXCR4-expressing cells mobilize PMNs and HSPCs into the peripheral circulation and are therapeutically useful for HSPC collection before autologous bone marrow transplantation (ABMT). Our aim was to develop unique CXCR4-targeted therapeutics using lipopeptide GPCR modulators called pepducins. A pepducin is a synthetic molecule composed of a peptide derived from the amino acid sequence of one of the intracellular (IC) loops of a target GPCR coupled to a lipid tether. We prepared and screened a small CXCR4-targeted pepducin library and identified several pepducins with in vitro agonist activity, including ATI-2341, whose peptide sequence derives from the first IC loop. ATI-2341 induced CXCR4- and G protein-dependent signaling, receptor internalization, and chemotaxis in CXCR4-expressing cells. It also induced dose-dependent peritoneal recruitment of PMNs when administered i.p. to mice. However, when administered systemically by i.v. bolus, ATI-2341 acted as a functional antagonist and dose-dependently mediated release of PMNs from the bone marrow of both mice and cynomolgus monkeys. ATI-2341–mediated release of granulocyte/macrophage progenitor cells from the bone marrow was confirmed by colony-forming assays. We conclude that ATI-2341 is a potent and efficacious mobilizer of bone marrow PMNs and HSPCs and could represent a previously undescribed therapeutic approach for the recruitment of HSPCs before ABMT.
Chemokine CXC-type receptor 4 (CXCR4) belongs to the heptahelical G protein-coupled receptor (GPCR) superfamily and couples to Gi-mediated signaling pathways. CXCR4 plays a prominent role in organogenesis, hematopoiesis, vascularization, and stem cell homing (1). Disruption of normal CXCR4 signaling contributes to a number of pathophysiological conditions such as inflammatory diseases, HIV entry, and cancer metastasis (2, 3). CXCR4 is found in many tissues, including the bone marrow, where it is expressed by hematopoietic stem cells, myeloid progenitors, and immature neutrophils. The natural ligand for CXCR4 is the CXC chemokine, CXCL12, or stromal cell-derived factor 1α (SDF-1α). CXCL12 is produced by bone marrow stromal cells and is a potent chemoattractant of CXCR4-expressing cells (3).
CXCR4 and CXCL12 play a critical role in the retention of polymorphonuclear neutrophils (PMNs) and hematopoietic stem and progenitor cells (HSPCs) in the bone marrow niche. Agents that disrupt the CXCR4/CXCL12 axis result in the mobilization of hematopoietic cells from the bone marrow into the peripheral circulation. This is therapeutically exemplified by the CXCR4 antagonist, AMD-3100 (Mozobil), which has been approved for the mobilization and collection of HSPCs before autologous bone marrow transplantation (ABMT) (4–6). Notably, however, HSPCs can also be released from the bone marrow by CXCR4 agonists, including CXCL12 itself. For example, i.v. injection of an adenoviral vector expressing CXCL12 resulted in a 10-fold increase in leukocytes in the peripheral circulation as well as in the mobilization of colony-forming unit granulocyte-macrophage (CFU-GM) (7). Furthermore, s.c. administration of a CXCR4-cyclized peptide agonist, CTCE-0021, resulted in the mobilization of PMNs and CFU-GM, among other HSPCs (8). CXCR4 agonists are hypothesized to mobilize hematopoietic cells through functional antagonism, mediated through disruption of the normal CXCL12 gradient in the bone marrow niche, and/or down-regulation of the CXCR4 receptor. Thus, antagonists and agonists of CXCR4 can both elicit stem cell mobilization from the bone marrow milieu.
There has been continued interest in developing novel therapeutic agents that target disruption of the CXCL12/CXCR4 axis. In this study, a pepducin-based approach was used to identify agonists of CXCR4. Pepducins are synthetic molecules that are composed of a peptide derived from the amino acid sequence of one of the four intracellular (IC) loops of a target GPCR coupled to a lipid tether (8). The peptide component of the pepducin confers receptor-modulating activity and the lipid component facilitates cell penetration and access to the intracellular face of the target GPCR (9). The IC loop sequences of GPCRs are targeted in pepducin design because it is well known that these regions of the receptor interact with the cellular signaling machinery (10–12) and are known to undergo considerable conformational rearrangement during receptor activation (13). Pepducins are hypothesized to interact with the intracellular face of the target GPCR and stabilize the receptor in one of a spectrum of receptor conformations, from the inactive to fully activated state, resulting in modulation of signal transduction (14). Pepducin agonists and antagonists have been identified for a number of receptors (14–16) and pepducins have been shown to have in vivo efficacy in several disease models (9, 15, 17).
Herein, we describe the identification of a unique CXCR4 agonist, ATI-2341, which was derived from a small CXCR4-targeted pepducin library. ATI-2341 induces CXCR4-dependent calcium flux, receptor internalization, and chemotaxis in cell-based assays. ATI-2341 also displays potent efficacy in the mobilization of PMNs and HSPCs in mice and nonhuman primates. ATI-2341 represents a unique CXCR4-targeted agonist with unique therapeutic potential. Because ATI-2341 was designed on the basis of only the primary structure of CXCR4, our work also provides proof of concept for the rapid development of GPCR-specific pepducin-based agonists.
Results
Identification and Characterization of the CXCR4 Pepducin Agonist, ATI-2341.
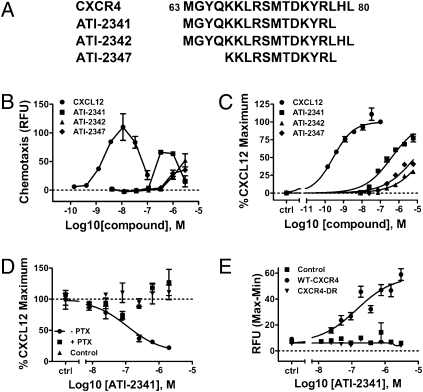
The goal of this study was to identify pepducin agonists of the human CXCR4 receptor and to characterize their pharmacology relative to the endogenous ligand, CXCL12. A library of 72 pepducins was designed based on the sequences of each of the four intracellular loops of human CXCR4. This library was screened in CCRF-CEM cells, a human T lymphoblastic leukemia cell line that endogenously expresses CXCR4. Three pepducins, ATI-2341, ATI-2342, and ATI-2347 were identified on the basis of their ability to elicit a positive chemotactic response in CCRF-CEM cells (Fig. S1 and Fig. 1A). These pepducin agonists were all derived from overlapping sequences of IC loop 1 (i1). Upon dose ranging, these pepducin agonists stimulated chemotaxis, with the most potent, ATI-2341, eliciting a bell-shaped chemotactic profile in CCRF-CEM cells similar to that observed with the more potent natural agonist, CXCL12 (Fig. 1B). The agonist activity of ATI-2341 was further characterized in human PMNs where it was shown to induce a bell-shaped chemotactic response (Fig. S2). All three pepducins induced a dose-dependent calcium mobilization in CCRF-CEM cells (Fig. 1C). ATI-2341 was the most potent agonist with an EC50 value of 194 ± 16 nM and an intrinsic activity of 81 ± 4%.
Fig. 1.
Identification and characterization of the CXCR4 agonist, ATI-2341. (A) Sequences of ATI-2341, 2342, and 2347. Depiction of the sequences of ATI-2341, ATI-2342, and ATI-2347 within the i1 loop region of human CXCR4. (B) ATI-2341, -2342, and -2347 dose-dependently elicit chemotaxis. Chemotaxis of CCRF-CEM cells was assessed in 96-well Transwell plates in response to a dose-range of CXCL12 or test articles in the bottom well. Cell migration was quantified using Cyquant dye. The averages of relative fluorescent intensity of duplicate wells were plotted. Data are representative of n = 3 independent experiments. (C) ATI-2341, -2342, and -2347 dose-dependently induce an increase in intracellular calcium in CCRF-CEM cells. CCRF-CEM cells preloaded with calcium 4 dye and incubated with varying concentrations of CXCL12 or test article and fluoresence intensity was recorded on a FlexStation III. Data were normalized to CXCL12-stimulated maximal calcium response. The averages of duplicate wells were plotted. Data are representative of n = 3 independent experiments. (D) ATI-2341 inhibits NKH477-stimulated cAMP in a CXCR4- and Gi-dependent manner. HEK-293 cells stably transfected with CXCR4, were pretreated (+PTX) or not pretreated (−PTX) with pertussis toxin overnight before the experiment. Control cells represent naïve HEK-293 cells. All cells were preincubated for 15 min with 10 μM of NKH477 before a 30-min incubation with ATI-2341. cAMP levels were then determined by HTRF assay. Data were normalized to the NKH477-stimulated intracellular cAMP level in the absence of compounds. The averages of triplicate wells were plotted. Data are representative of n = 3 independent experiments. (E) ATI-2341 stimulates calcium response in U87 cells transfected with human CXCR4 but not a loss-of-function mutant. U87 cells transfected with wild-type CXCR4, a CXCR4 with a mutation in the DRY motif to the sequence RDY (CXCR4-DR), or mock transfected were loaded with calcium 4 dye and incubated with varying levels of AT-2341. Fluoresence intensity was recorded on a FlexStation III. Data were normalized to CXCL12-stimulated maximal calcium response and the averages of triplicate wells were plotted. Data are representative of n = 3 independent experiments.
On the basis of its potency and efficacy, AT-2341 was chosen for further characterization. ATI-2341 was assessed for its ability to inhibit cAMP accumulation in HEK-293 cells that stably express recombinant human CXCR4 (CXCR4-HEK cells). ATI-2341 dose-dependently inhibited NKH477-induced cAMP accumulation in CXCR4-HEK cells, but had no effect in naïve HEK-293 parental cells (Fig. 1D). Pretreatment of CXCR4-HEK cells with pertussis toxin (PTX) completely abrogated the ability of ATI-2341 to inhibit cAMP accumulation, demonstrating an effect mediated by a Gi-dependent pathway (Fig. 1D). ATI-2341 had a similar potency and extent of efficacy in this pathway compared with that seen in calcium mobilization.
Further characterization of ATI-2341 was conducted in U87 glioblastoma cells transiently transfected with CXCR4. ATI-2341 induced a dose-dependent increase in intracellular calcium in cells transfected with wild-type CXCR4 (EC50 = 140 ± 36 nM) while having no effect on untransfected cells (Fig. 1E). Activation of this signaling pathway by ATI-2341 was dependent on a fully functional CXCR4 receptor as ATI-2341 failed to mobilize calcium in cells transfected with a CXCR4 receptor variant in which the DRY motif was mutated to RDY (Fig. 1E), a mutation known to prevent coupling to G proteins (18).
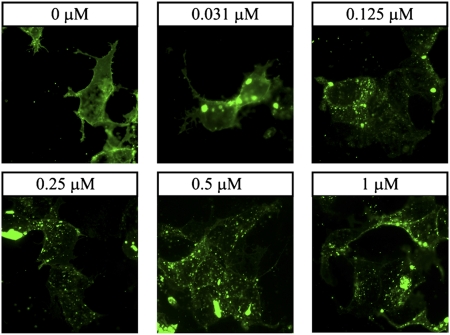
We next investigated the ability of ATI-2341 to induce receptor internalization. CXCL12 is well known to induce rapid internalization of CXCR4 (1). Similar to CXCL12, ATI-2341 induced the internalization of GFP-tagged CXCR4 transiently transfected in HEK-293 cells (Fig. 2). Following a 30-min incubation at 37 °C, ATI-2341 dose-dependently induced the loss of fluorescence on the cell surface and concomitantly promoted the appearance of punctate fluorescence in the cytosolic compartment. The potency of ATI-2341 to induce internalization was similar to that observed in calcium and cAMP signaling assays.
Fig. 2.
ATI-2341 induces internalization of CXCR4. HEK-293 cells transiently transfected with CXCR4–eGFP fusion construct were stimulated with indicated concentrations of ATI-2341 for 30 min at 37 °C. eGFP fluorescence was visualized directly using a Zeiss Axiovert inverted microscope.
Efficacy of ATI-2341 in Vivo.
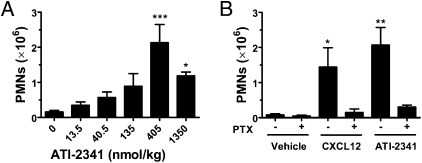
It was of interest to assess the ability of ATI-2341 to function as an agonist, in vivo. Intraperitoneal (i.p.) injection of ATI-2341 in BALB/c mice resulted in a dose-dependent recruitment of PMNs into the peritoneum, with a maximal effect seen at 405 nmol/kg (Fig. 3A). A higher concentration of ATI-2341 resulted in reduced recruitment, which is reminiscent of the bell-shaped curve generally seen with chemotactic agents. Intraperitoneal administration of CXCL12 also resulted in recruitment of PMNs into the peritoneum (Fig. 3B). As expected for a biological effect mediated by a Gi-coupled receptor and consistent with our in vitro findings, recruitment of PMNs by both ATI-2341 and CXCL12 was abrogated by pretreatment of mice with PTX (Fig. 3B). A separate study was conducted to determine whether i.p. administration of ATI-2341 was able to increase circulating levels of PMNs (through mobilization from the bone marrow) in addition to recruiting PMNs into the peritoneum. It was found that i.p. administration of ATI-2341 (or CXCL12) did not increase circulating levels of PMNs at doses that resulted in PMN recruitment into the peritoneum (Fig. S3).
Fig. 3.
In vivo agonist activity of ATI-2341. (A) ATI-2341 induces chemotaxis of PMNs in vivo. BALB/c mice were injected intraperitoneally with increasing doses of ATI-2341 (eight mice per group). Peritoneal lavages were collected for determination of the number of PMNs. The data are presented as mean ± SEM (*P < 0.05, ***P < 0.001). (B) ATI-2341–mediated peritoneal recruitment of PMNs is Gi dependent. BALB/c mice received i.p. injections of either vehicle, CXCL12 (26 nmol/kg) or ATI-2341 (300 nmol/kg). Some mice were pretreated with PTX (4 μg per mouse) 2 h before i.p. injections of vehicle or chemoattractants. Three hours later peritoneal lavages were collected for determination of the number of PMNs. The data are presented as mean ± SEM (*P < 0.05; **P < 0.01).
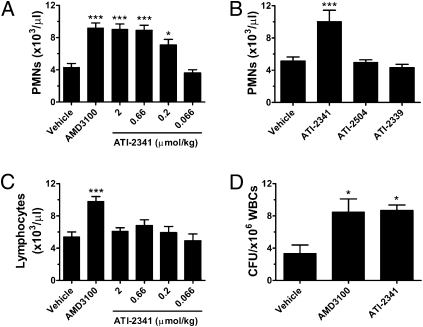
CXCR4 antagonists, CXCL12 itself, and analogs of CXCL12 are known to elicit mobilization of hematopoietic cells expressing CXCR4 from the bone marrow (4, 7, 8). The ability of ATI-2341 to mobilize bone marrow-derived PMNs and HSPCs was assessed in mice and cynomolgus monkeys. Intravenous (i.v.) administration of ATI-2341 in mice resulted in a dose-dependent increase in PMNs in the peripheral circulation (Fig. 4A), measured 90 min after administration of compound. The effect was maximal at 0.66 μmol/kg of ATI-2341, and the degree of efficacy was similar to that seen with 2 μmol/kg AMD-3100. Microscopic examination of Wright-Giemsa stained blood smears revealed the presence of immature granulocytes, consistent with a bone marrow origin of the mobilized PMNs. Intravenous administration of a nonlipidated analog of ATI-2341 (ATI-2504) had no effect on PMN mobilization (Fig. 4B), demonstrating the critical importance of the lipid moiety for the in vivo activity of ATI-2341. Intravenous administration of a control i1 pepducin (ATI-2339), that was inactive in cell-based signaling studies, similarly had no effect on PMN mobilization (Fig. 4B). In contrast to the increase in PMNs elicited by ATI-2341, ATI-2341 had no effect on the mobilization of lymphocytes at any dose tested (Fig. 4C). This is a clear distinction from AMD-3100, which elicited a significant release of lymphocytes into the plasma (Fig. 4C). Further studies demonstrated that AMD-3100 induced mobilization of B lymphocytes (B220 positive cells) but not T lymphocytes (CD4 and CD8 positive cells), whereas ATI-2341 had no effect on mobilization of either lymphocyte population (Fig. S4). To assess whether HSPCs were being released from the bone marrow milieu, ATI-2341 was administered i.v. to mice and the level of circulating HSPCs was assessed using a CFU-GM assay. Administration of ATI-2341 resulted in a greater than twofold increase in the number of CFU-GM compared with vehicle-treated mice, a level similar to that seen with the same dose of AMD-3100 (Fig. 4D).
Fig. 4.
ATI-2341 induces mobilization of PMNs and CFU-GM but not lymphocytes. (A) ATI-2341 induces mobilization of PMNs. BALB/c mice received tail vein injections of vehicle, AMD-3100 (2 μmol/kg) or varying doses of ATI-2341 (10 mice per group). PMN counts were determined 90 min after treatment. (B) Effect of control pepducins on PMNs mobilization. Four groups of BALB/c mice received tail vein injections of vehicle, ATI-2341, nonlipidated analog of ATI-2341 (ATI-2504), or inactive pepducin (ATI-2339), all at a dose of 2 μmol/kg. The number of PMNs in the circulation was determined 90 min later. (C) ATI-2341 does not induce mobilization of lymphocytes. BALB/c mice received tail vein injections of vehicle, AMD-3100 (2 μmol/kg) or ATI-2341 (10 mice per group). Lymphocyte counts were determined 90 min after treatment. (D) ATI-2341 induces mobilization of hematopoietic progenitors. Three groups of DBA/2 mice (four mice per group) were injected with vehicle, AMD-3100 (2 μmol/kg) or ATI-2341 (2 μmol/kg). Blood was collected at 60 min and processed in the CFU-GM assay. All data are presented as mean ± SEM (*P < 0.05; **P < 0.01; ***P < 0.001).
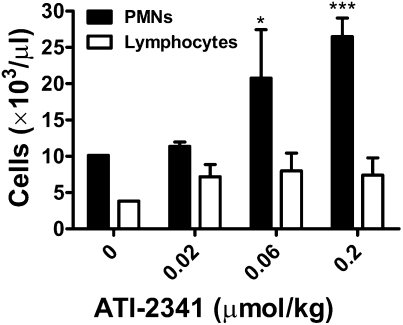
Studies were conducted to determine whether ATI-2341 was efficacious in mobilizing hematopoietic cells in non-human primates. ATI-2341 administered i.v. to cynomolgus monkeys elicited a dose-dependent mobilization of PMNs into plasma, with a maximal effect observed at 0.2 μmol/kg, although significant efficacy was observed at 0.06 μmol/kg (Fig. 5). The maximal release of PMNs occurred between 1 and 2 h; by 8 h, circulating PMN cell numbers had returned to basal levels (Table S1). Similar to what was observed in the mouse studies, there was no significant effect of ATI-2341 on the release of lymphocytes (Fig. 5). There was no dose-dependent effect of ATI-2341 on the release of lymphocytes at any dose. A CFU-GM assay confirmed that ATI-2341 was eliciting release of hematopoietic progenitor cells in cynomolgus monkeys (Fig. S5). A significant release of CFU-GM occurred at 1 h at a dose of 0.2 μmol/kg.
Fig. 5.
ATI-2341 induces PMN mobilization of PMNs but not lymphocytes in non-human primates. Nonnaïve cynomolgus monkeys received intravenous infusions of either vehicle (one monkey) or three different doses (0.2, 0.06, and 0.02 μmol/kg) of ATI-2341 (three monkeys per group). Blood was collected at 2 h and PMNs and lymphocytes were counted. Two-way repeat measurements ANOVA with the Bonferroni posttest were used for the statistical analysis. The data are presented as mean ± SD (*P < 0.05, ***P < 0.001).
Discussion
We report the identification and characterization of the CXCR4 agonist pepducin, ATI-2341. This compound, in addition to two other pepducin agonists, was identified through the screening of a small pepducin library targeting the IC loop sequences of human CXCR4 and represents a unique example of a pepducin agonist of a chemokine receptor. The three agonist pepducins identified in this study were all derived from overlapping sequences from the i1 loop (Fig. 1A). Interestingly, only pepducins from the i1 loop had agonist activity. The location of the sequence of ATI-2341 is shown in the context of a rhodopsin-based homology model of CXCR4 in Fig. S6. ATI-2341 spans the entire i1 loop and contains several juxtamembrane residues from both TM1 and TM2. Of note, each of the three agonist pepducins contains a C-terminal sequence motif, KYRL. Interestingly, this motif is not present in a previously published CXCR4 i1 pepducin, x4pal-1, that acts as an antagonist at the receptor (15), indicating the probable importance of this sequence motif for agonist activity.
ATI-2341 was found to be an agonist of CXCR4 whose effects were receptor dependent and therefore not mediated through nonspecific activation of G proteins. Similar to the endogenous agonist, CXCL12, ATI-2341 inhibited cAMP production and stimulated Ca2+ mobilization in host cells expressing recombinant human CXCR4, although the pepducin was less potent than CXCL12 and shown to be a partial agonist. Importantly, the agonist potency and efficacy of ATI-2341 was independent of host cell environment (U87 or HEK cells) and was similar in cells that naturally express CXCR4 (CCRF-CEM cells). As expected for an agonist signaling through a Gi-coupled receptor, ATI-2341–induced signaling was eliminated by pretreatment with PTX. The agonist activity of ATI-2341 was also dependent on the presence of a functional receptor as calcium mobilization induced by ATI-2341 was completely abrogated in U87 cells expressing a CXCR4 variant in which the DRY motif was mutated to RDY. This mutation has been shown to eliminate receptor coupling to G proteins (18). Agonists of GPCRs are well known for their ability to induce internalization of their cognate receptors (19) and studies have shown that CXCL12 rapidly promotes internalization of CXCR4 (1). Similar to CXCL12, ATI-2341 induced dose-dependent internalization of GFP-tagged CXCR4 with potency similar to that seen in calcium and cAMP signaling experiments. ATI-2341 is thus behaving as an agonist at CXCR4 with a pharmacology that resembles that of CXCL12. Studies are ongoing to determine the precise mode of action of ATI-2341, including efforts to map the site of interaction with the receptor and possible effects of ATI-2341 on receptor hetero- or homodimerization.
CXCL12 is a potent chemoattractant of CXCR4-expressing cells (20). Like CXCL12, ATI-2341 was able to induce chemotaxis of CCRF-CEM cells, inducing the typical bell-shaped curve observed with chemotactic agents. ATI-2341 was also able to induce chemotaxis of human PMNs. The potency and efficacy of ATI-2341-induced chemotaxis was similar to that observed in calcium and cAMP signal transduction studies. To our knowledge, this is a unique demonstration of a pepducin acting as a chemoattractant, and indicates that CXCR4-expressing cells are able to orient and migrate toward a pepducin gradient.
Chemokines are known to induce migration of cells when administered in vivo (21). Thus, it was of interest to determine whether ATI-2341 could function, in vivo, as a chemoattractant. Analogous to CXLC12, ATI-2341 dose-dependently recruited PMNs into the peritoneum upon i.p. injection in BALB/c mice. At the concentration range tested, ATI-2341 did not increase circulating levels of PMNs, indicating that the PMNs recruited into the peritoneum were not mobilized from the bone marrow. As expected for an agonist of a Gi-coupled receptor, the chemoattractant activity of ATI-2341 was sensitive to pretreatment with PTX. This represents a unique in vivo demonstration of agonist-induced efficacy of a pepducin.
At present, the therapeutic utility of CXCR4-targeted drugs is centered on the mobilization and subsequent harvest of HSPCs before ABMT (22), primarily through disruption of the CXCL12-mediated retention of CXCR4-expressing cells in the bone marrow (23). This disruption can be achieved with an antagonist, such as AMD-3100 (Mozobil), which has been approved for this indication (4, 5), or through the administration of agonists that act to collapse the CXCL12 gradient and/or down-regulate CXCR4 receptor expression (8). We tested the ability of ATI-2341 to mobilize PMNs and HSPCs. Intravenous administration of ATI-2341 resulted in a dose-dependent increase of PMNs in the peripheral circulation, with potency and efficacy similar to that of AMD-3100. Functional activity at CXCR4 was essential for PMN release, as a pepducin (ATI-2339) that was inactive in the calcium and chemotaxis library screen was unable to elicit an increase in blood PMN levels. As has been observed previously (14), the lipid moiety is required for pepducin activity, as a nonlipidated analog of ATI-2341 (ATI-2504) had no effect on PMN mobilization. The PMNs released into the circulation originated in the bone marrow on the basis of the number of PMNs mobilized and their immature phenotype as determined by microscopic examination. Mobilization of committed bone marrow progenitor cells was definitively shown by CFU-GM assay and the extent of efficacy was again similar to that seen with AMD-3100. These results were extended to non-human primates where i.v. administration of ATI-2341 resulted in a dose- and time-dependent increase in PMNs in the peripheral circulation. Similar to the results in the mouse, ATI-2341–induced mobilization of committed bone marrow progenitor cells was demonstrated by CFU-GM assay. PMN levels in the circulation peaked at 2 h postdosing, and those of CFU-GM peaked at 1 h. Of note, the efficacy of ATI-2341 in PMN mobilization occurred at doses 10-fold lower in the monkey than that seen in the mouse. ATI-2341 thus appears to be an effective mobilizing agent for PMNs and HSPCs in both mice and cynomolgus monkeys. Interestingly, in contrast to AMD-3100, ATI-2341 did not result in lymphocyte mobilization in mice or in cynomolgus monkeys. AMD-3100 is known to mobilize PMNs, HPSCs, as well as B lymphocytes from the bone marrow (24). The mechanism by which ATI-2341 selectively mobilizes PMNs and HSPCs, but not lymphocytes, is unknown but suggests a potential differentiation from AMD-3100.
In conclusion, we have identified a unique pepducin agonist that like the endogenous agonist, CXCL12, activates CXCR4-dependent signaling pathways, induces receptor internalization, and promotes both in vitro and in vivo chemotaxis. Importantly, ATI-2341 acts as a functional antagonist to mobilize PMNs and HSPCs from the bone marrow niche in both mice and non-human primates. The efficacy and selectivity of ATI-2341 for CXCR4, and its capability of mobilizing PMNs and HSPCs, make it an interesting potential alternative therapeutic option for the mobilization of hematopoietic stem cells. Because ATI-2341 was designed solely on the basis of the sequence and predicted structure of a selected GPCR, our work provides proof of concept for the rapid reverse engineering of GPCR-specific, pepducin-based agonists.
Materials and Methods
Reagents and Cells.
CCRF-CEM and HEK-293 cells were purchased from ATCC. U87-CD4 cells were obtained from the National Institutes of Health AIDS reagent registry. CXCL12 was purchased from Peprotech. Cell culture reagents were from Invitrogen. AMD-3100 was from Sigma. The water soluble analog of forskolin, NKH477, was from Tocris.
Synthesis of Pepducins.
The design of the pepducin library was based on the sequences of each of the four IC loops of human CXCR4. The peptide sequences differed on the basis of the starting and ending points within the individual loops and juxtamembrane regions with varied peptide length from 10 to 37 amino acids. The peptides were synthesized by standard Fmoc solid-phase synthetic methods (SI Materials and Methods) and capped with a palmitic acid on the amino terminus of the peptide.
Calcium Flux Assays.
Calcium flux studies were conducted in CCRF-CEM and U87 cells and measured on a Flexstation III by standard methods (SI Materials and Methods).
cAMP Assay.
cAMP studies in HEK-293 cells were conducted according to established protocols using the Cisbio homogenous time resolved fluorescence (HTRF) method (SI Materials and Methods).
Chemotaxis Assay.
CCRF-CEM cells were maintained in RPMI-1640 medium supplemented with 10% (wt/vol) FBS at density between 2 × 105 and 2 × 106 per mL. Cells were collected by centrifugation and resuspended in assay buffer [phenol red free RPMI-1640, 20 mM Hepes, pH 7.4, and 0.5% (wt/vol) BSA]. Transwell 96-well plates with 5.0-μm pore size polycarbonate membranes (Corning) were used for the assays. CXCL12 or test articles were diluted in assay buffer and were added to the bottom wells, whereas 1 × 105 cells were added to the top. Cells were allowed to move to bottom wells for 2 h in a 37 °C incubator with 5% CO2 before the receiver plates were separated from the inserts. Cells translocated to the bottom wells were quantified using Cyquant dye (Invitrogen). Fluorescence intensity was measured on a FlexStation III.
Microscopy.
CXCR4–eGFP fusion construct was transiently transfected into HEK-293 cells using Lipofectamine Plus reagent (Invitrogen). GFP fluorescence was visualized as described in SI Materials and Methods.
PMN Mobilization Assay.
Circulating levels of PMNs were determined in mice and cynomolgus monkeys following i.v. administration of ATI-2341 (SI Materials and Methods).
Colony-Forming Unit Assay.
Colony-forming units (CFU-GM) circulating in mouse blood were quantified 60 min following compound injection into DBA/2J mice. Total colonies per milliliter of mouse blood were calculated by multiplying the number of colonies identified by the volume of blood prepared (SI Materials and Methods).
PMN Peritoneal Recruitment Model in Mice.
BALB/c mice received 0.5 mL of i.p. injections of ATI-2341 (300 nmol/kg) or CXCL12 (26 nmol/kg). Mice from the control group were injected with 0.5 mL of endotoxin-free water. Some mice received tail vein injections of PTX (4 μg per mouse) 2 h before i.p. administration of CXCL12 or ATI-2341. After 3 h, mice were killed using CO2 asphyxia and the absolute number of peritoneal PMNs was determined as described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Athan Kuliopulos and Lidija Covic for helpful discussion about pepducin biology, Walter Newman and Steven Kunkel for their input on chemokine biology, Thomas Huber for preparing the CXCR4 homology model, Joseph Stevens for assistance with in vivo studies, Dan DeCollibus for assistance with formulations, and Amy Ying Lin for assistance with the receptor internalization assays. P.S. received support from the Murray Foundation.
Footnotes
Conflict of interest statement: B.T., Y.R., J.M.J., L.H., A.O., E.M., R.L., Q.D., T.M., S.H. and K.E.C. are employees of Anchor Therapeutics. T.M. is a cofounder of Anchor Therapeutics. T.P.S. serves on the scientific advisory board and receives research funding from Anchor Therapeutics.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009633108/-/DCSupplemental.
References
- 1.Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochim Biophys Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Faber A, et al. The many facets of SDF-1alpha, CXCR4 agonists and antagonists on hematopoietic progenitor cells. J Biomed Biotechnol. 2007;2007:26065. doi: 10.1155/2007/26065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burger JA, Kipps TJ. CXCR4: A key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107:1761–1767. doi: 10.1182/blood-2005-08-3182. [DOI] [PubMed] [Google Scholar]
- 4.Broxmeyer HE, et al. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med. 2005;201:1307–1318. doi: 10.1084/jem.20041385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fricker SP, et al. Characterization of the molecular pharmacology of AMD3100: a specific antagonist of the G-protein coupled chemokine receptor, CXCR4. Biochem Pharmacol. 2006;72:588–596. doi: 10.1016/j.bcp.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Flomenberg N, Comenzo RL, Badel K, Calandra G. (2010) Plerixafor (Mozobil) alone to mobilize hematopoietic stem cells from multiple myeloma patients for autologous transplantation. Biol Blood Marrow Transplant. 16:695–700. doi: 10.1016/j.bbmt.2009.12.538. [DOI] [PubMed] [Google Scholar]
- 7.Hattori K, et al. Plasma elevation of stromal cell-derived factor-1 induces mobilization of mature and immature hematopoietic progenitor and stem cells. Blood. 2001;97:3354–3360. doi: 10.1182/blood.v97.11.3354. [DOI] [PubMed] [Google Scholar]
- 8.Pelus LM, et al. The CXCR4 agonist peptide, CTCE-0021, rapidly mobilizes polymorphonuclear neutrophils and hematopoietic progenitor cells into peripheral blood and synergizes with granulocyte colony-stimulating factor. Exp Hematol. 2005;33:295–307. doi: 10.1016/j.exphem.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 9.Wielders SJ, Bennaghmouch A, Reutelingsperger CP, Bevers EM, Lindhout T. Anticoagulant and antithrombotic properties of intracellular protease-activated receptor antagonists. J Thromb Haemost. 2007;5:571–576. doi: 10.1111/j.1538-7836.2007.02364.x. [DOI] [PubMed] [Google Scholar]
- 10.Nussenzveig DR, Thaw CN, Gershengorn MC. Inhibition of inositol phosphate second messenger formation by intracellular loop one of a human calcitonin receptor. Expression and mutational analysis of synthetic receptor genes. J Biol Chem. 1994;269:28123–28129. [PubMed] [Google Scholar]
- 11.Wu V, et al. First intracellular loop of the human cholecystokinin-A receptor is essential for cyclic AMP signaling in transfected HEK-293 cells. J Biol Chem. 1997;272:9037–9042. doi: 10.1074/jbc.272.14.9037. [DOI] [PubMed] [Google Scholar]
- 12.Hawes BE, Luttrell LM, Exum ST, Lefkowitz RJ. Inhibition of G protein-coupled receptor signaling by expression of cytoplasmic domains of the receptor. J Biol Chem. 1994;269:15776–15785. [PubMed] [Google Scholar]
- 13.Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459:356–363. doi: 10.1038/nature08144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covic L, Gresser AL, Talavera J, Swift S, Kuliopulos A. Activation and inhibition of G protein-coupled receptors by cell-penetrating membrane-tethered peptides. Proc Natl Acad Sci USA. 2002;99:643–648. doi: 10.1073/pnas.022460899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaneider NC, Agarwal A, Leger AJ, Kuliopulos A. Reversing systemic inflammatory response syndrome with chemokine receptor pepducins. Nat Med. 2005;11:661–665. doi: 10.1038/nm1245. [DOI] [PubMed] [Google Scholar]
- 16.Leger AJ, Covic L, Kuliopulos A. Protease-activated receptors in cardiovascular diseases. Circulation. 2006;114:1070–1077. doi: 10.1161/CIRCULATIONAHA.105.574830. [DOI] [PubMed] [Google Scholar]
- 17.Yang E, et al. Blockade of PAR1 signaling with cell-penetrating pepducins inhibits Akt survival pathways in breast cancer cells and suppresses tumor survival and metastasis. Cancer Res. 2009;69:6223–6231. doi: 10.1158/0008-5472.CAN-09-0187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Franke RR, König B, Sakmar TP, Khorana HG, Hofmann KP. Rhodopsin mutants that bind but fail to activate transducin. Science. 1990;250:123–125. doi: 10.1126/science.2218504. [DOI] [PubMed] [Google Scholar]
- 19.Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: The role in receptor desensitization and signaling. Pharmacol Rev. 2001;53:1–24. [PubMed] [Google Scholar]
- 20.Vicente-Manzanares M, et al. The chemokine SDF-1alpha triggers a chemotactic response and induces cell polarization in human B lymphocytes. Eur J Immunol. 1998;28:2197–2207. doi: 10.1002/(SICI)1521-4141(199807)28:07<2197::AID-IMMU2197>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 21.Mercer-Jones MA, Shrotri MS, Heinzelmann M, Peyton JC, Cheadle WG. Regulation of early peritoneal neutrophil migration by macrophage inflammatory protein-2 and mast cells in experimental peritonitis. J Leukoc Biol. 1999;65:249–255. doi: 10.1002/jlb.65.2.249. [DOI] [PubMed] [Google Scholar]
- 22.Stem Cell Trialists’ Collaborative Group Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: An individual patient data meta-analysis of nine randomized trials. J Clin Oncol. 2005;23:5074–5087. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Furze RC, Rankin SM. Neutrophil mobilization and clearance in the bone marrow. Immunology. 2008;125:281–288. doi: 10.1111/j.1365-2567.2008.02950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin C, et al. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity. 2003;19:583–593. doi: 10.1016/s1074-7613(03)00263-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.