Abstract
The physical basis for protein partitioning into lipid rafts remains an outstanding question in membrane biology that has previously been addressed only through indirect techniques involving differential solubilization by nonionic detergents. We have used giant plasma membrane vesicles, a plasma membrane model system that phase separates to include an ordered phase enriching for raft constituents, to measure the partitioning of the transmembrane linker for activation of T cells (LAT). LAT enrichment in the raft phase was dependent on palmitoylation at two juxtamembrane cysteines and could be enhanced by oligomerization. This palmitoylation requirement was also shown to regulate raft phase association for the majority of integral raft proteins. Because cysteine palmitoylation is the only lipid modification that has been shown to be reversibly regulated, our data suggest a role for palmitoylation as a dynamic raft targeting mechanism for transmembrane proteins.
Keywords: phase separation, raft partitioning, posttranslational modification, GPI-anchored protein
Posttranslational modifications allow for rapid modulation of protein structure, localization, and function. An important class is lipid modifications, which includes the addition of GPI anchors, sterols, as well as single saturated and/or unsaturated fatty acids. The cellular purpose of protein lipidation is often to anchor the modified polypeptide to membranes; however, the functional significance of the widespread S acylation of transmembrane (TM) proteins remains unclear. Additionally, S acylation, often referred to as “palmitoylation” due to the addition of a cysteine-linked palmitate, is the only protein lipidation under dynamic enzymatic regulation, implying a potentially important regulatory role (1).
Adapting a recently developed experimental system for measuring protein partitioning between coexisting fluid domains in cell-derived isolated plasma membranes, we find that palmitoylation regulates raft phase affinity of LAT (linker for activation of T cells), a critical adaptor in immune system signaling. This finding extends to the majority of raft phase partitioning proteins, suggesting a general role for protein palmitoylation in dynamic regulation of raft association. Although previous studies (2–7) have implicated palmitoylation in regulation of detergent resistance of TM proteins, the indirect and controversial nature of these experiments has limited their applicability in assigning raft affinity. The results presented here directly demonstrate and quantify the vital role of palmitoylation in partitioning of TM proteins to raft phase domains of isolated plasma membranes (PM).
Results
LAT Partitioning Is Disrupted by DTT.
Giant plasma membrane vesicles (GPMVs) are cell- and cytoskeleton-detached, organelle-free PM blebs that maintain the protein (8) and lipid (9) diversity of the native membrane and separate into two liquid phases (10) with different order (11) mirroring the behavior of pure lipid liposomes (12). Remarkably, phase separation sorts membrane components in accordance with their predicted raft affinity, as lipidic and lipid-anchored raft components (10, 13, 14) partition to the ordered phase (therefore referred to here as “raft phase”). In intriguing contrast, raft-predicted TM proteins are consistently excluded from the raft phase (10, 13, 14), echoing a similar discrepancy in simple lipid systems (15–17).
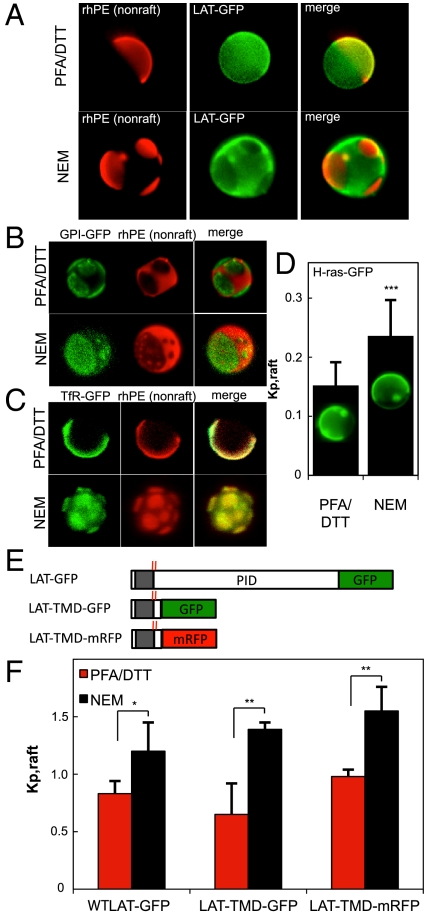
In GPMVs produced using the standard protocol of 25 mM paraformaldehyde (PFA) + 2 mM DTT (pdGPMVs), LAT-GFP was enriched in the nonraft phase [counterstained with an unsaturated lipid dye (rhodamine-dioleoyl phosphatidylethanolamine—rhPE)] (Fig. 1A). In contrast, this protein was highly enriched in the raft phase of vesicles produced using N-ethylmaleimide (NEM) (nGPMVs), raising the question of the mechanism of the observed perturbation and LAT’s raft affinity. The striking difference in phase partitioning of LAT-GFP between pdGPMVs and nGPMVs was not observed for either a raft phase-enriched GPI-anchored protein (GPI-GFP, Fig. 1B) or the canonical nonraft TM transferrin receptor (TfR, Fig. 1C). However, a significant reduction in raft phase affinity was observed for the lipid-anchored intracellular GTPase H-ras (Fig. 1D) (quantification details in Fig. S1). Although H-ras was not raft phase enriched, there was significant raft phase fluorescence in nGPMVs, emphasizing that lack of raft enrichment does not equate to lack of raft localization and underlining the importance of partitioning quantification rather than qualitative evaluation of enrichment.
Fig. 1.
LAT enriches in raft phase in NEM derived GPMVs. (A) LAT-GFP segregates away from the nonraft marker rhPE in nGPMVs (induced with 2 mM NEM), in contrast to slight depletion in pdGPMVs (25 mM PFA and 2 mM DTT). (B) Preparation-dependent partitioning is not observed for either (B) the raft-enriched GPI-GFP or (C) the nonraft TfR, but is significantly different for (D) H-ras-GFP (rhPE marker images not included; the marker is enriched in the H-ras-GFP-rich phase in both cases). Average + SD from 7–10 vesicles/condition; representative of two independent experiments. (E) Graphic of LAT constructs with palmitoylated cysteines highlighted by red lines and TMD in the gray region. (F) LAT is enriched in the raft phase of nGPMVs regardless of FP oligomerization or PID (average + SD from three independent experiments; 7–10 vesicles/condition/experiment. *p < 0.05; ** p < 0.01; p > 0.1 between all constructs in nGPMVs). Quantifications from 6–10 vesicles/condition; vesicles shown in all figures are 5- to 10-μm diameter.
These effects were quantified by a fluorescence-based scheme to measure Kp,raft, the raft phase partition coefficient (Fig. S1). Using this technique, the influence of the GPMV isolation agent is quantitatively evident: three different LAT constructs [LAT-GFP, LAT-TMD-GFP including only the ectodomain, transmembrane domain (TMD), and a short cytosolic sequence of LAT, and LAT-TMD-mRFP (trLAT); Fig. 1E] partitioned significantly differently in nGPMVs compared to pdGPMVs (p < 0.03 for each construct) with each enriching in the raft phase of nGPMVs (Fig. 1F). In contrast, no significant differences were observed between the various constructs in nGPMVs (p > 0.1), suggesting that neither the choice of fluorescent tag nor the protein interaction domain (PID) of LAT is required for raft partitioning.
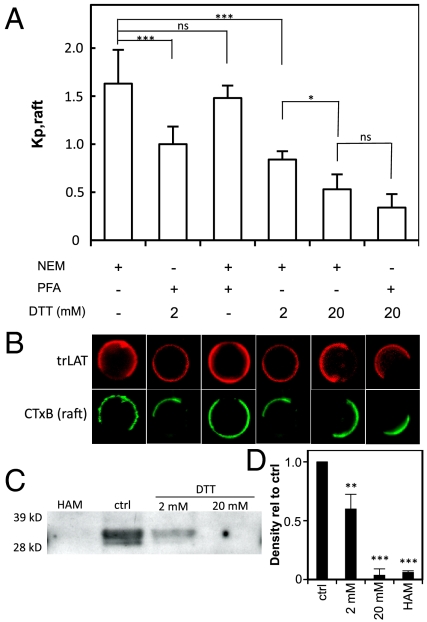
Vesicles were treated postisolation with PFA or DTT individually to determine which of these was responsible for missorting of LAT to the nonraft phase. Treatment of nGPMVs with DTT induced a significant, concentration-dependent reduction in raft phase partitioning of trLAT, with partitioning in 2 mM DTT-treated nGPMVs similar to pdGPMVs (which are derived with 2 mM DTT) and 20 mM DTT essentially eliminating trLAT from the raft phase in both preparations (Fig. 2 A and B). In contrast, 25 mM PFA had no significant effect on trLAT partitioning. Thus, the aberrant nonraft phase partitioning observed in pdGPMVs is directly attributable to DTT present in this preparation.
Fig. 2.
DTT induces LAT translocation to nonraft phase independent of cross-linking. (A) Kp,raft as a function of GPMV treatment. GPMVs derived with NEM (2 mM ; lanes 1, 3, 4, and 5) or PFA + DTT (25 mM + 2 mM, respectively; lanes 2 and 6), then treated for 2 h at the condition shown (*** p < 0.001; * p < 0.05; nsp > 0.05 of 7–10 vesicles/condition and representative of three independent experiments). (B) Representative images of LAT-TMD-mRFP (red) with respect to the raft phase marker CTxB-A488. (C) Western blot against native LAT after pulldown of palmitoylated proteins by acyl-biotinyl exchange. Treatment of lysates with 2 mM DTT leads to ∼50% loss of LAT signal, whereas signal from 20 mM DTT treatment is equivalent to complete depalmitoylation by HAM. (D) Densitometric quantification is average + SD from three independent experiments (** p < 0.01; *** p < 0.001 compared to control).
DTT Removes S-Linked Fatty Acids.
LAT is palmitoylated at two juxtamembrane intracellular cysteines (marked by red lines in the graphic in Fig. 1E), and this palmitoylation has been shown to be required for its enrichment in detergent resistant membranes (DRMs) (7). Additionally, DTT has previously been shown to remove S-linked palmitates from proteins in vitro (18), leading us to hypothesize that DTT induces nonraft missorting of trLAT by removal of its fatty acid modifications. To evaluate the S acylation of LAT, we used acyl-biotinyl exchange (ABE) (19), which involves chemical treatments that substitute a biotinyl moiety for every S-linked palmitoylation followed by specific pulldown of biotinylated (i.e., previously palmitoylated) proteins.
DTT treatment of cell lysates prior to palmitoylation analysis by acyl-biotinyl exchange led to a concentration-dependent reduction of LAT palmitoylation (Fig. 2 C and D). There was an approximately 50% reduction of palmitoylated LAT following treatment with 2 mM DTT, whereas 20 mM DTT removed essentially all palmitates, as did the well-characterized chemical depalmitoylation agent, hydroxylamine (HAM) (18). These results identify a clear quantitative correlation between LAT palmitoylation and raft phase partitioning in GPMVs, as treatment with 2 mM DTT led to partial loss of both palmitoylation and raft phase association, whereas complete elimination of S-linked acylation by 20 mM DTT was concomitant with nearly complete nonraft phase partitioning of trLAT (Fig. 2).
Raft Partitioning Is Enhanced by Oligomerization.
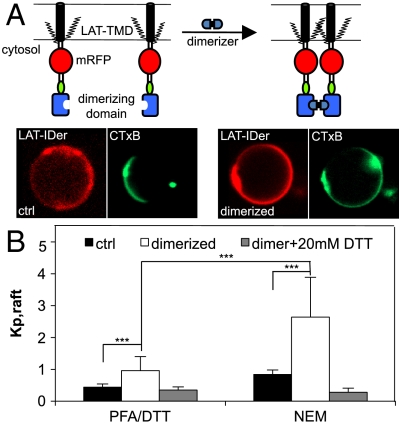
Treatment with PFA had no significant effect on trLAT partitioning (Fig. 2A), a surprising result in light of the nonspecific protein cross-linking expected from this treatment. To measure the effect of specific cross-linking and quantitatively evaluate the hypothesis that oligomerization of raft components enhances raft enrichment (20), we employed the trLAT-IDer (trLAT-inducible dimer) containing a dimerization domain whose homointeraction is inducible by a specific membrane-permeable agent (Fig. 3A) (21). Dimerization induced a striking and significant enhancement of raft phase localization, reversing the phase preference of the trLAT-IDer from slightly depleted to almost 3-fold raft phase enriched (Fig. 3 A and B). These results emphasize the importance of specific oligomerization, rather than nonspecific cross-linking, in determining the extent of raft association of raft partitioning components. As for nonoligomerized LAT, raft phase enrichment was strictly dependent on palmitoylation, as 20 mM DTT completely eliminated raft phase partitioning of trLAT-IDer regardless of dimerization (Fig. 3B).
Fig. 3.
Specific homodimerization enhances raft phase partitioning. (A) Schematic of LAT-IDer inducible dimerization. Representative images show LAT-IDer reverses phase preference from slightly nonraft to highly raft enriched following dimerization. (B) Dimerization enhances raft partitioning of LAT-IDer in both pdGPMVs and nGPMVs, dependent on palmitoylation (average + SD of 7–12 vesicles/condition; *** p < 0.001; p > 0.05 between the two 20 mM DTT-treated conditions; results representative of three independent experiments). Average + SD of 6–11 vesicles/condition; results representative of three independent experiments (*** p < 0.001; ** p < 0.01; nsp > 0.05).
Disruption of Raft Partitioning by Palmitoylation-Deficient Mutants and Palmitoylation Inhibitor.
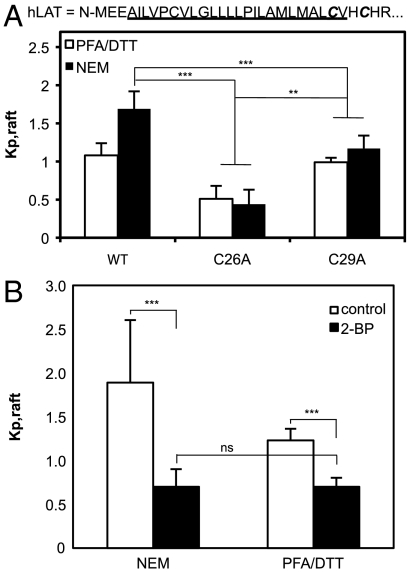
To confirm the link between palmitoylation and raft partitioning established by the DTT experiments, we evaluated raft phase association of (1) two palmitoylation-deficient mutants (C26A and C29A) of trLAT (Fig. 4A); and (2) trLAT partitioning after inhibition of palmitoylation by 2-bromopalmitate (2-BP).
Fig. 4.
Effect of palmitoylation-deficient mutants and palmitoylation inhibitor on raft phase partitioning. (A) Loss of palmitoylation at membrane-embedded C26 leads to complete reversal of raft phase partitioning while lack of (juxtamembrane) C29 palmitoylation leads to approximately equal partitioning between the two phases. (B) Pretreatment of cells with the palmitoylation inhibitor 2-BP abrogates LAT raft phase partitioning regardless of isolation agents. Average + SD of 8–11 vesicles/condition; results representative of two independent experiments (*** p < 0.001; ** p < 0.01; nsp > 0.05).
As expected, both palmitoylation mutants led to a significant reduction of raft phase partitioning with a significantly greater reduction observed for the C26 compared to C29 mutant (in nGPMVs: C26A = 0.4; C29A = 1.2; p < 0.01). An intriguing observation was that trLAT-C29A partitioning in nGPMVs was essentially identical to wild-type trLAT in pdGPMVs, suggesting that the 2 mM DTT in the pdGPMV preparation removes only the palmitate from the cytoplasmic C29, whereas higher concentrations can also remove the membrane-embedded palmitate at C26, as evidenced by an almost complete lack of raft phase partitioning of trLAT both for the C26A mutant (Fig. 4A) and in 20 mM DTT-treated GPMVs (Fig. 2A).
Cells treated with the palmitoylation inhibitor 2-BP prior to GPMV isolation and quantification of trLAT partitioning also showed completely reversed trLAT phase preference from raft phase enriched in nGPMVs to more than 2× nonraft phase enriched in both pd and nGPMVs (Fig. 4B). Significantly, there was no difference between partitioning in these two preparations from 2-BP treated (palmitoylation-inhibited) cells, as expected if the difference in preparations is dependent on perturbation of palmitoylation.
Palmitoylation Is Required for Raft Partitioning of Majority of Integral Raft Proteins.
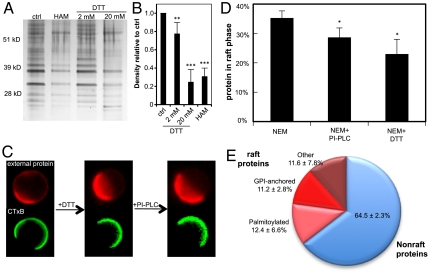
As for LAT (Fig. 2 C and D), DTT-induced depalmitoylation was observed for a large number of proteins, as demonstrated by silver staining of treated acyl-biotinyl exchange pulldowns (Fig. 5 A and B); treatment with 20 mM DTT prior to ABE led to complete depalmitoylation (silver signal at the same level as completely depalmitoylating treatment of 0.5 M HAM). Given the importance of palmitoylation in the raft association of LAT, we sought to exploit the nonspecific removal of S-linked protein palmitoylation by DTT to evaluate the generality of palmitoylation as a requirement for raft partitioning.
Fig. 5.
Majority of integral raft phase proteins in nGPMVs are palmitoylated. (A) Depalmitoylating effect of DTT is observed for most palmitoylated proteins as evidenced by reduction of silver staining of acyl-biotinyl exchanged pulldowns in DTT-treated lysates. (B) Densitometric quantification suggests that treatment with 20 mM DTT removes all S-linked palmitates, at parity with 0.5 M HAM (average plus SD of the seven major bands between 28–51 kDa, representative of three separate experiments; ** p < 0.01; *** p < 0.001). (C) Representative images of total external membrane protein (stained with monomeric fluorescent anti-biotin following nonspecific biotinylation) in GPMVs following treatment with 20 mM DTT followed by 0.1 U/mL PI-PLC. (D) External plasma membrane protein is somewhat depleted from raft phase in nGPMVs; treatment with either DTT or PI-PLC reduces raft phase signal (average + SD of three independent experiments each with 7–14 vesicles/condition; * p < 0.05). (E) Fluorescent quantification of raft protein abundance following removal of palmitoylated TM proteins by 20 mM DTT or GPI-APs by PI-PLC allows quantification of the composition of the nGPMVs (average + SD from three independent experiments). Nonraft outnumber raft proteins ∼2∶1 while palmitoylated proteins comprise > 50% of integral raft proteins.
Cell surface proteins were nonspecifically biotinylated with membrane-impermeable amine-reactive sulfo-NHS-biotin prior to GPMV isolation, followed by staining with a nonoligomerizing fluorescent anti-biotin Fab’ to evaluate their raft phase partitioning. Although anti-biotin signal (proportional to total cell surface protein) was enriched in the nonraft phase (Fig. 5C), there was significant fluorescence (Kp,raft = 0.59; Fig. S2) amounting to 38% of the surface protein in the raft phase (Fig. 5D). Treatment of nGPMVs with 20 mM DTT resulted in > 30% reduction of relative raft phase fluorescence, whereas hydrolysis of glycophosphatidyl anchored protein (GPI-APs) by a GPI-specific phospholipase (PI-PLC; Fig. S3) had a smaller, but still significant, effect (Fig. S2). These data were used to calculate the percentage of raft phase proteins remaining after these treatments (Fig. 5D—for calculation details see SI Methods; for caveats see Discussion), from which we calculated the fraction of proteins whose raft phase residence was due to (1) palmitoylation of TM proteins (DTT sensitive) or (2) a GPI-anchor (PI-PLC sensitive). Thirty-five percent of surface raft phase protein (13% of all surface protein) was found to be sensitive to DTT and hence to be anchored to the raft phase by palmitoylation, whereas ∼33% of raft phase proteins were GPI-anchored (Fig. 5E). The other 30% of raft phase proteins were sensitive to neither treatment; therefore, their mechanism of raft phase residence is unassigned, although it can be speculated to be based on binding to raft lipids such as cholesterol, as is the case for caveolin (22).
Discussion
Protein lipidations are an important class of posttranslational modifications that greatly modify protein hydrophobicity and conformation. Because of its dynamic nature, S acylation (typically by a saturated, 16-carbon fatty acid, i.e., palmitoylation) has the potential to serve a regulatory function by providing a stable membrane anchor for otherwise soluble proteins, e.g., Ras GTPases (23) and Src-family kinases (24). For integral membrane proteins, the function of this modification remains unclear.
Previous reports based on detergent resistance experiments have speculated that palmitoylation may impart protein association with ordered plasma membranes domains, i.e., lipid rafts (reviewed in ref. 25). However, this assertion remains controversial due to the inherently disruptive nature of detergent resistance experiments (26–28). The data presented here conclusively demonstrate that palmitoylation of the transmembrane adaptor LAT is essential for its partitioning to the ordered domain of phase-separated plasma membrane vesicles (GPMVs). In addition, these results quantify the magnitude of the effect and estimate the extent of the palmitoylation requirement as being necessary for the majority of integral raft proteins.
Because of their compositional complexity, large size (up to 10 μm), and microscopic phase separation, GPMVs provide an ideal system for measuring protein partitioning into a PM liquid ordered phase. However, several predicted raft proteins [e.g., LAT (14) and influenza hemagglutinin (13, 29)] failed to enrich in the ordered phase in vesicles prepared using the standard PFA/DTT preparation. Raft phase enrichment of LAT in nGPMVs resolves this discrepancy and suggests that the mechanism behind nonraft partitioning of raft TM proteins in pdGPMVs is depalmitoylation by the reducing agent present in this preparation. Experiments with palmitoylation-deficient mutants (Fig. 4A) and pharmacological inhibitors of palmitoylation (Fig. 4B) are fully consistent with this conclusion. Previous experiments with a synthetic LAT transmembrane peptide in model lipid vesicles measured disordered phase partitioning regardless of palmitoylation (16), likely reflecting the large-order difference between the coexisting lipid phases in artificial model membranes (11).
An interesting qualitative correlation was observed between the raft phase partitioning of the palmitoylation-deficient mutants (Fig. 4A) and their enrichment in DRMs (7) (i.e., WT > C29A > C26A in DRM association and Kp,raft). A similar correlation was observed for the dimerization of trLAT-IDer, where induced dimerization greatly enhanced raft partitioning (Fig. 3 A and B) and concomitantly increased the enrichment of this construct in DRMs (Fig. S4). These results suggest that raft phase partitioning in GPMVs and resistance to detergent solubilization are likely two manifestations of the same phenomenon, i.e., in vivo raft localization, as previously proposed (12).
Additionally, the nonparity in perturbation of raft phase partitioning between the mutants of the two available palmitoylation sites (mutation of membrane-embedded C26 perturbed raft phase partitioning more than the cytosolic C29) is closely correlated with a previously observed (30) trafficking defect in T cells. This defect was also correlated to raft partitioning in our cell system (Fig. S5), as we observed largely plasma membrane localization of the wild-type trLAT, whereas the C26A palmitoylation-deficient mutant seemed to localize more to intracellular membranes (the C29A-trLAT mutant showed an intermediate behavior). This result is suggestive of raft-dependent trafficking of LAT, in analogy with the recent observation of palmitoylation-dependent trafficking of Ras GTPases (4, 23) and raft-dependent trafficking in yeast (31). The trafficking defect of palmitoylation-deficient LAT was found to correlate with T-cell anergy (32), implicating dynamic posttranslational palmitoylation and subsequent raft association as a physiological control mechanism for regulation of LAT availability in T-cell activation.
In addition to palmitoylation, it has been proposed that oligomerization of raft components can enhance raft affinity (20) and that this mechanism can be functionalized by cells in the formation of Golgi-to-PM sorting intermediates (33). In accordance with this prediction, raft phase enrichment of LAT was significantly enhanced by exogenous homodimerization (Fig. 3), fully consistent with previously observed cross-link-induced enhancement of ordered phase partitioning in model (34, 35) and plasma membranes (14).
The nonspecific protein labeling technique in Fig. 5 allows estimation of the lipid modification of raft-enriching proteins in isolated plasma membranes. Results suggest that the majority of integral raft proteins require palmitoylation for raft association, whereas ∼30% of raft-associated proteins are GPI-anchored. Several caveats must be considered in the interpretation of these data: (i) only protein susceptible to extracellular biotinylation is considered in this analysis; thus lipid-anchored intracellular proteins are not measured; (ii) strict lipid asymmetry is not maintained in either the NEM or PFA/DTT preparation, which may be a significant factor for proteins that require specific lipids for raft association; (iii) protein–protein interactions that may confer raft association to nonraft proteins may be disrupted by DTT, overestimating the effect of depalmitoylation; (iv) similarly, proteins whose raft phase residence is dependent on binding to either palmitoylated or GPI-anchored proteins would be indirectly included among the DTT/PI-PLC-sensitive fractions.
We emphasize that palmitoylation may be necessary for raft partitioning of TM proteins, but it is definitely not sufficient, as a number of palmitoylated proteins are likely not raft associated, including the TfR considered here (Fig. 1B). The other determinants of raft association remain to be characterized, but it is likely that the length and chemical nature of the TMD will be important factors, as suggested for the influenza spike protein hemagglutinin (36, 37).
In summary, the data presented here point to palmitoylation as a necessary posttranslational modification for raft phase partitioning of the important immune system TM adaptor LAT, in addition to implicating oligomerization in enhancement of raft association. Additionally, the values in Fig. 5E provide quantitative estimates of the abundance and lipidation of raft-resident proteins and suggest that lipid modifications, in particular palmitoylation, regulate raft affinity for the majority of integral raft proteins.
Methods
Giant Plasma Membrane Vesicle Isolation.
GPMVs were isolated, labeled, and imaged as described (10). Briefly, cells were washed 3 times with GPMV buffer (50 mM Hepes, 150 mM NaCl, 2 mM CaCl2, pH 7.4); then the GPMV induction agent (either 25 mM PFA + 2 mM DTT or 2 mM NEM) was added directly to the GPMV buffer. The cells were then incubated with GPMV buffer + induction agent for 1 h at 37 °C while shaking at 60 rpm. Phase separation behavior was somewhat dependent on induction agent, with the phase transition temperature ~7 ° higher in pdGPMVs (Fig. S6), requiring significant cooling of nGPMVs before phase separation was observed. This was not investigated further as protein partitioning rather than phase separation behavior was the focus of this study.
Partitioning Quantification.
A particular benefit of measuring protein partitioning in giant vesicles is the ease of quantification of the partition coefficient (Kp) (as in ref. 34). An example is shown in Fig. S1, where the fluorescent intensity of the protein of interest (in this case LAT-TMD-mRFP) is quantified in the two phases [raft phase marked by the GM1-binding cholera toxin (CTxB)] and the background-subtracted peak intensities are divided to derive the relative partitioning quotient of the protein in the raft phase (Kp,raft—in the example, ∼0.7). Variation between line scans within a single vesicle was very small (coefficient of variation < 5%) and Kp’s derived with this method were highly repeatable within a single population of vesicles. Only vesicles with clearly defined domains in the equatorial plane (as in Fig. S1) were used for partitioning quantification. Although fluorescent intensity quantification like this can be prone to artifacts from polarization and differences in quantum yield of the probe in different membrane environments (38), these issues are negligible in all experiments described here, where the fluorescent moiety is freely diffusive in 3D rather than embedded in the membrane.
Acyl-Biotinyl Exchange.
Acyl-biotinyl exchange to quantify the relative amount of palmitoylation in the DTT-treated samples was performed essentially as described (19) with the optional membrane purification steps (2 and 3 in the reference) included. All statistical evaluations were Student’s t tests for unpaired observations.
Supplementary Material
Acknowledgments.
The authors acknowledge funding from the Humboldt Foundation Postdoctoral Research Fellowship, EUFP6 “PRISM” LSHB-CT2007-037740, Deutsche Forschungsgemeinschaft Schwerpunktprogramm 1175 Contract SI459/2-1, Bundesministerium fuer Bildung und Forschung (BMBF) “BioChance Plus” Grant 0313827, and BMBF “ForMaT” Grant 03FO1212.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016184107/-/DCSupplemental.
References
- 1.Resh MD. Palmitoylation of ligands, receptors, and intracellular signaling molecules. Sci STKE. 2006;2006:re14. doi: 10.1126/stke.3592006re14. [DOI] [PubMed] [Google Scholar]
- 2.Melkonian KA, Ostermeyer AG, Chen JZ, Roth MG, Brown DA. Role of lipid modifications in targeting proteins to detergent-resistant membrane rafts. Many raft proteins are acylated, while few are prenylated. J Biol Chem. 1999;274:3910–3917. doi: 10.1074/jbc.274.6.3910. [DOI] [PubMed] [Google Scholar]
- 3.Moffett S, Brown DA, Linder ME. Lipid-dependent targeting of G proteins into rafts. J Biol Chem. 2000;275:2191–2198. doi: 10.1074/jbc.275.3.2191. [DOI] [PubMed] [Google Scholar]
- 4.Rocks O, et al. An acylation cycle regulates localization and activity of palmitoylated Ras isoforms. Science. 2005;307:1746–1752. doi: 10.1126/science.1105654. [DOI] [PubMed] [Google Scholar]
- 5.Webb Y, Hermida-Matsumoto L, Resh MD. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J Biol Chem. 2000;275:261–270. doi: 10.1074/jbc.275.1.261. [DOI] [PubMed] [Google Scholar]
- 6.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296:913–916. doi: 10.1126/science.1068539. [DOI] [PubMed] [Google Scholar]
- 7.Zhang W, Trible RP, Samelson LE. LAT palmitoylation: Its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity. 1998;9:239–246. doi: 10.1016/s1074-7613(00)80606-8. [DOI] [PubMed] [Google Scholar]
- 8.Scott RE, Perkins RG, Zschunke MA, Hoerl BJ, Maercklein PB. Plasma membrane vesiculation in 3T3 and SV3T3 cells. I. Morphological and biochemical characterization. J Cell Sci. 1979;35:229–243. doi: 10.1242/jcs.35.1.229. [DOI] [PubMed] [Google Scholar]
- 9.Fridriksson EK, et al. Quantitative analysis of phospholipids in functionally important membrane domains from RBL-2H3 mast cells using tandem high-resolution mass spectrometry. Biochemistry. 1999;38:8056–8063. doi: 10.1021/bi9828324. [DOI] [PubMed] [Google Scholar]
- 10.Baumgart T, et al. Large-scale fluid/fluid phase separation of proteins and lipids in giant plasma membrane vesicles. Proc Natl Acad Sci USA. 2007;104:3165–3170. doi: 10.1073/pnas.0611357104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaiser HJ, et al. Order of lipid phases in model and plasma membranes. Proc Natl Acad Sci USA. 2009;106:16645–16650. doi: 10.1073/pnas.0908987106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Levental I, et al. Cholesterol-dependent phase separation in cell-derived giant plasma-membrane vesicles. Biochem J. 2009;424:163–167. doi: 10.1042/BJ20091283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson SA, et al. Temperature-dependent phase behavior and protein partitioning in giant plasma membrane vesicles. Biochim Biophys Acta. 2010;1798:1427–1435. doi: 10.1016/j.bbamem.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sengupta P, Hammond A, Holowka D, Baird B. Structural determinants for partitioning of lipids and proteins between coexisting fluid phases in giant plasma membrane vesicles. Biochim Biophys Acta. 2008;1778:20–32. doi: 10.1016/j.bbamem.2007.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hammond AT, et al. Crosslinking a lipid raft component triggers liquid ordered-liquid disordered phase separation in model plasma membranes. Proc Natl Acad Sci USA. 2005;102:6320–6325. doi: 10.1073/pnas.0405654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shogomori H, et al. Palmitoylation and intracellular domain interactions both contribute to raft targeting of linker for activation of T cells. J Biol Chem. 2005;280:18931–18942. doi: 10.1074/jbc.M500247200. [DOI] [PubMed] [Google Scholar]
- 17.Bacia K, Schuette CG, Kahya N, Jahn R, Schwille P. SNAREs prefer liquid-disordered over “raft” (liquid-ordered) domains when reconstituted into giant unilamellar vesicles. J Biol Chem. 2004;279:37951–37955. doi: 10.1074/jbc.M407020200. [DOI] [PubMed] [Google Scholar]
- 18.Tu Y, Wang J, Ross EM. Inhibition ofbrain Gz GAP and other RGS proteins by palmitoylation of G protein alpha subunits. Science. 1997;278:1132–1135. doi: 10.1126/science.278.5340.1132. [DOI] [PubMed] [Google Scholar]
- 19.Wan J, Roth AF, Bailey AO, Davis NG. Palmitoylated proteins: purification and identification. Nat Protoc. 2007;2:1573–1584. doi: 10.1038/nprot.2007.225. [DOI] [PubMed] [Google Scholar]
- 20.Simons K, Vaz WL. Model systems, lipid rafts, and cell membranes. Annu Rev Biophys Biomol Struct. 2004;33:269–295. doi: 10.1146/annurev.biophys.32.110601.141803. [DOI] [PubMed] [Google Scholar]
- 21.Lingwood D, Schuck S, Ferguson C, Gerl MJ, Simons K. Generation of cubic membranes by controlled homotypic interaction of membrane proteins in the endoplasmic reticulum. J Biol Chem. 2009;284:12041–12048. doi: 10.1074/jbc.M900220200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Murata M, et al. VIP21/caveolin is a cholesterol-binding protein. Proc Natl Acad Sci USA. 1995;92:10339–10343. doi: 10.1073/pnas.92.22.10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rocks O, et al. The palmitoylation machinery is a spatially organizing system for peripheral membrane proteins. Cell. 2010;141:458–471. doi: 10.1016/j.cell.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Resh MD. Myristylation and palmitylation of Src family members: The fats of the matter. Cell. 1994;76:411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- 25.Levental I, Grzybek M, Simons K. Greasing their way: Lipid modifications determine protein association with membrane rafts. Biochemistry. 2010;49:6305–6316. doi: 10.1021/bi100882y. [DOI] [PubMed] [Google Scholar]
- 26.Munro S. Lipid rafts: Elusive or illusive? Cell. 2003;115:377–388. doi: 10.1016/s0092-8674(03)00882-1. [DOI] [PubMed] [Google Scholar]
- 27.Kenworthy AK. Have we become overly reliant on lipid rafts? Talking point on the involvement of lipid rafts in T-cell activation. EMBO Rep. 2008;9:531–535. doi: 10.1038/embor.2008.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lichtenberg D, Goni FM, Heerklotz H. Detergent-resistant membranes should not be identified with membrane rafts. Trends Biochem Sci. 2005;30:430–436. doi: 10.1016/j.tibs.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 29.Nikolaus J, et al. Hemagglutinin of influenza virus partitions into the nonraft domain of model membranes. Biophys J. 2010;99:489–498. doi: 10.1016/j.bpj.2010.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hundt M, et al. Palmitoylation-dependent plasma membrane transport but lipid raft-independent signaling by linker for activation of T cells. J Immunol. 2009;183:1685–1694. doi: 10.4049/jimmunol.0803921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bagnat M, Keranen S, Shevchenko A, Simons K. Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc Natl Acad Sci USA. 2000;97:3254–3259. doi: 10.1073/pnas.060034697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hundt M, et al. Impaired activation and localization of LAT in anergic T cells as a consequence of a selective palmitoylation defect. Immunity. 2006;24:513–522. doi: 10.1016/j.immuni.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Schuck S, Simons K. Polarized sorting in epithelial cells: raft clustering and the biogenesis of the apical membrane. J Cell Sci. 2004;117:5955–5964. doi: 10.1242/jcs.01596. [DOI] [PubMed] [Google Scholar]
- 34.Kahya N, Brown DA, Schwille P. Raft partitioning and dynamic behavior of human placental alkaline phosphatase in giant unilamellar vesicles. Biochemistry. 2005;44:7479–7489. doi: 10.1021/bi047429d. [DOI] [PubMed] [Google Scholar]
- 35.Dietrich C, Volovyk ZN, Levi M, Thompson NL, Jacobson K. Partitioning of Thy-1, GM1, and cross-linked phospholipid analogs into lipid rafts reconstituted in supported model membrane monolayers. Proc Natl Acad Sci USA. 2001;98:10642–10647. doi: 10.1073/pnas.191168698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Scheiffele P, Roth MG, Simons K. Interaction of influenza virus haemagglutinin with sphingolipid-cholesterol membrane domains via its transmembrane domain. EMBO J. 1997;16:5501–5508. doi: 10.1093/emboj/16.18.5501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tall RD, Alonso MA, Roth MG. Features of influenza HA required for apical sorting differ from those required for association with DRMs or MAL. Traffic. 2003;4:838–849. doi: 10.1046/j.1398-9219.2003.0138.x. [DOI] [PubMed] [Google Scholar]
- 38.Silvius JR. Partitioning of membrane molecules between raft and non-raft domains: Insights from model-membrane studies. Biochim Biophys Acta. 2005;1746:193–202. doi: 10.1016/j.bbamcr.2005.09.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.