Abstract
HSVs enter cells in a receptor-dependent [nectin1 or herpesviruses entry mediator (HVEM)] fashion by fusion of the viral envelope with plasma membrane (neutral pH compartment), by endocytosis into neutral or acidic compartments, or by macropinocytosis/phagocytosis. The cellular determinants of the route of entry are unknown. Here, we asked what cellular factors determine the pathway of HSV entry. CHO cells lack β3-integrin and the respective α-subunits’ heterodimers. We report that, in the absence of αVβ3-integrin, HSV enters CHO-nectin1 cells through a pathway independent of cholesterol-rich rafts and dynamin2. In the presence of αVβ3-integrin, HSV enters CHO-nectin1 cells through a pathway dependent on cholesterol-rich rafts and dynamin2. HSV enters J-nectin1 and 293T cells through a neutral compartment independent of cholesterol-rich rafts and dynamin2. αVβ3-integrin overexpression in these cells modifies the route of entry to an acidic compartment dependent on cholesterol-rich rafts and dynamin2, hence similar to that in αVβ3-integrin–positive CHO-nectin1 cells. In some cells, the diversion of entry from an integrin- and raft-independent pathway to an acidic compartment requiring cholesterol-rich lipids rafts and dynamin2 is irreversible. Indeed, HSV cannot infect CHO-nectin1-αVβ3 cells through any compartment when the αvβ3-integrin–dependent pathway is blocked by anti-integrin antibody, anti-dynamin2, or anti-acidification drugs. We conclude that the αvβ3-integrin is a determinant in the choice of HSV entry pathway into cells. Because the pathway dictated by αvβ3-integrin is through lipid rafts, the platforms for a number of Toll-like receptors, current findings raise the possibility that αvβ3-integrin acts as a sentinel of innate immunity.
Keywords: virus entry, virus internalization
Studies of the past years highlighted that the same virus can enter different cells through different pathways and that the same cell enables entry of different viruses through different pathways. Each virus entry pathway is defined by a set of factors, among which are the coat proteins that wrap the endocytic invaginations/vesicles (e.g., clathrin and caveolin), scission factors that pinch off membrane invaginations (e.g., dynamin2), actin involvement, etc. (1). So far, little attention has been paid to the cellular determinants that route a given virus to one or another of the alternative entry pathways.
HSV is an important pathogen for the human population and is being developed as a tumor-specific oncolytic agent (2). It enters cells by fusion of the virion envelope with plasma membranes or after endocytosis through neutral or acidic compartments [insensitive or sensitive to bafilomycin A (BFLA), respectively]. In some cells, it may enter by macropinocytosis/phagocytosis (3–8). Four virion glycoproteins are required for HSV to enter cells (9). Glycoprotein D (gD) serves as the receptor-binding glycoprotein, which is able to bind alternatively the two major receptors nectin1 and herpesvirus entry mediator (HVEM) (10–12). gD also triggers gH/gL and ultimately, gB to execute fusion (13–15). Inasmuch as the HSV entry pathways are cell line-dependent, the choice as to which route is taken by incoming HSV is determined by the cell itself. The determinants in this choice are not the gD receptors. Specifically, wt-CHO and wt-J cells fail to express the gD receptors and therefore, are intrinsically resistant to HSV infection. When transfected with either nectin1 or HVEM, they become susceptible to HSV (10–12). However, entry into CHO-nectin1 or CHO-HVEM cells is through acidic endosomes, irrespective of which gD receptor is being expressed. In contrast, entry into J-nectin1 or J-HVEM cells is through a neutral compartment (i.e., either the plasma membrane or neutral endosomes, again irrespective of the expressed receptor) (5, 6). Altogether, these data emphasize that routing of HSV to acidic endosomes or a neutral compartment is determined by as yet unknown cellular factors other than the gD receptors.
Integrins are cell surface glycoproteins made of an α- and a β-subunit (16). They trigger several endocytic pathways and contribute to a variety of functions, mainly cell–cell and cell–matrix adhesion and signal transduction (17–20). They serve as receptors for a number of viruses (21), including some herpesviruses, EBV, human cytomegalovirus (HCMV), and Kaposi's sarcoma-associated herpesvirus (KSHV) (22–26). For HSV, our laboratory did not find evidence for interaction of a soluble form of gH/gL with αVβ3-integrin in contrast to a previous report (27, 28).
CHO cells are β3-integrin negative (28). Here, we generated CHO-nectin1 cells expressing αVβ3-integrin and thus, could compare HSV infection in αVβ3-integrin–positive and -negative cells. In addition, we overexpressed αVβ3-integrin in J-nectin1 cells as well as in 293T and HT29 cells. We report that αVβ3-integrin redirects the entry pathway of HSV. In the presence of αVβ3-integrin, entry into CHO-nectin1 cells became sensitive to cholesterol-depleting compounds and factors that interfere with dynamin2. In αVβ3-overexpressng J and 293T cells, the entry was converted to an acidic compartment, and similarly to that in CHO-nectin1-αVβ3 cells, it became sensitive to cholesterol depletion and dynamin2. Thus, αVβ3-integrin, although an accessory factor, routes the pathway of HSV entry.
Results
Generation of CHO-Nectin1 and J-Nectin1 Cells Overexpressing Human αVβ3-Integrin.
CHO cells express endogenous αV- but not β3-integrin (28) and were the reference cells in this study. J cells were included for comparison, because they sustain a neutral HSV entry pathway, in contrast to the acidic pathway in CHO cells (5). Nectin1δ and -α splice variants share the ectodomain (nomenclature according to UniProtB/Swiss-prot Q15223). The cytoplasmic tail of the δ- but not the α-isoform binds afadin PDZ domain and interacts with actin (29).
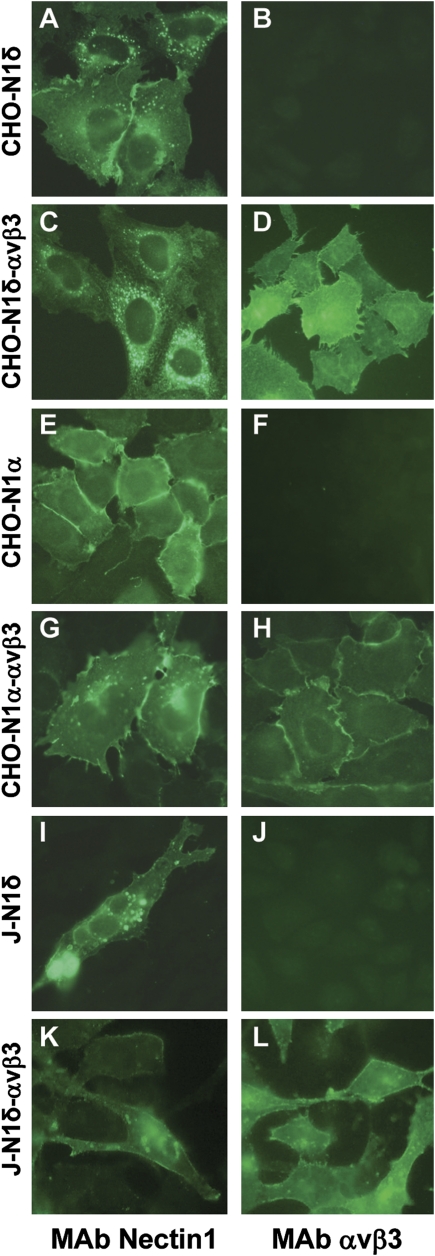
To generate CHO-nectin1δ (CHO-N1δ), CHO-nectin1α (CHO-N1α), and J-nectin1α (J-N1α) cells differing with respect to αVβ3-integrin expression (named CHO-N1δ-αVβ3, CHO-N1α-αVβ3, and J-N1α-αVβ3), cells were cotransfected with nectin1 ± αV- and β3-integrin plasmids, selected for neomycin G418 resistance, and cloned. Fig. 1 shows αVβ3-integrin and nectin1 expression as detected by immunofluorescence assay (IFA).
Fig. 1.
Expression of αVβ3-integrin (B, D, F, H, J, and L) and nectin1 (A, C, E, G, I, and K) in CHO and J cell derivatives as detected by IFA with mAbs LM609 to αVβ3-integrin (Chemicon), R1.302 to nectin1, and fluorescein isothiocyanate anti-mouse IgG (Sigma Aldrich). Cells were paraformaldehyde fixed. Pictures were taken in a Nikon Eclipse €600 microscope equipped with a Nikon digital camera DXM 1200F.
Inhibition of HSV Infection by mAb L230 to αVβ3-Integrin.
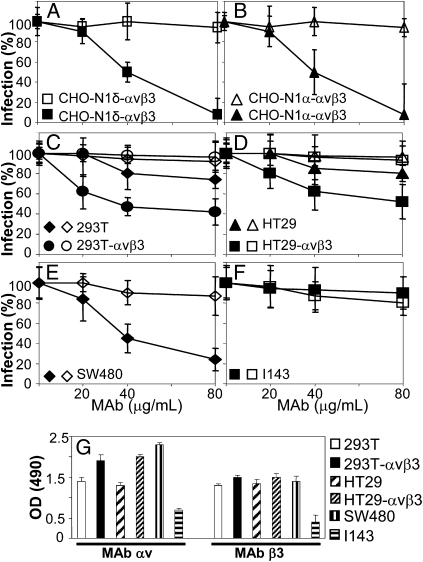
To test whether αVβ3-integrin plays a role in HSV-1 infection, we measured whether mAb L230 inhibits HSV-1 infection. mAb L230 binds αV-integrin, blocks αVβ3-integrin interaction with its natural ligands—generally through their RGD (Arg-Gly-Asp) motif—and therefore, is classified as a function-blocking mAb (30). To quantify HSV infection, we used R8102 recombinant, which carries a lacZ gene under the immediate-early α27 promoter. A large body of evidence indicates that R8102 β-Galactosidase (β-Gal) expression is a quantitative indicator of the extent of HSV entry (11, 12). Cells were exposed to mAb from 1 h before infection until harvesting at 6–8 h after infection. Fig. 2 shows that mAb L230 inhibited HSV infection in the αVβ3-integrin–positive CHO-N1δ (Fig. 2A) and CHO-nectin1α (Fig. 2B) cells in a dose-dependent manner, indicating that the effect was independent of nectin1 interaction with actin. Mouse IgGs had no effect (Fig. 2, open symbols). Importantly, the finding that mAb L230 inhibits infection implies that, in CHO-N1-αVβ3 cells exposed to mAb L230, both the integrin-dependent and -independent pathways functional in CHO-nectin1 cells are precluded.
Fig. 2.
Effect of mAb L230 on R8102 infection (A–F) and αVβ3-integrin cell surface expression (G). (A and B) CHO-N1δ-αVβ3 (A) or CHO-N1α-αVβ3 (B) cells were exposed to indicated concentrations of mAb L230▲ or control IgGsΔ for 1 h, infected with R8102 (3 pfu/cell) in the same medium, and overlaid with mAb-containing medium until harvesting. In A–G, the extent of infection was quantified from the Lac-Z gene engineered in the viral genome under the immediate-early α27 promoter. Cells in 96 wells were fixed at 6–8 h after infection. Extent of β-Gal activity reflects the amount of infection. Each point represents triplicates’ average; 100% infection is the value obtained with no antibody. Bars show SD. (G) Expression of αV- and β3-integrin was measured by cell ELISA (CELISA) with mAbs L230 and AP3, respectively (30, 31). Cells in 96 wells in triplicates were fixed with 4% formaldehyde and reacted with mAbs followed by anti-mouse peroxidase.
Next, we asked whether mAb L230 inhibits HSV infection in human cells. We focused on the cell line HT29 (reported to be αVβ3-integrin–negative but -positive in our investigations) (28), 293T cells (widely used for high-transfection capacity), SW480 cells (highly susceptible to mAb L230; see below), and I143 cells (sustain a neutral pH-dependent pathway of HSV entry). HT29 cells overexpressing αVβ3-integrin (HT29αVβ3) were described (28); 293 cells overexpressing αVβ3-integrin (293αVβ3) were generated by transient transfection. The results in Fig. 2 C–F show that mAb L230 inhibited HSV infection strongly in human SW480 cells (Fig. 2E), but not in I143 cells (Fig. 2F), at intermediate levels in HT29 and 293T cells (Fig. 2 C and D). Inhibition was higher in HT29αVβ3 and 293αVβ3 cells than in their WT counterparts. The extent of αV- and β3-integrin expression in the human cell lines is shown in Fig. 2G. αV-integrin expression was highest in SW480 cells, somewhat lower in HT29αVβ3 and 293αVβ3 cells, intermediate in wt-293T and HT29 cells, and low in I143 cells. In contrast, the extent of β3-integrin expression was relatively uniform, except for the low level in I143 cells. Thus, to a large extent, the extent of inhibition by mAb L230 reflected the level of αV-integrin expression, which likely represented the limiting factor in αVβ3-integrin heterodimer formation. The nonfunction-blocking mAb AP3 (31) to β3-integrin did not significantly modify HSV infection (Fig. S1). Nonimmune IgGs exerted no significant inhibition (Fig. 2, open symbols). The results provide evidence that αv-integrins, particularly αVβ3-integrin, participate in HSV infection.
Inhibition of HSV infection by mAb L230 was surprising, because CHO-nectin1 and CHO-HVEM cells, which lack β3-integrin (28), are readily infected by HSV. Collectively, these data indicate that αVβ3-integrin is a nonessential factor in HSV entry; however, when present, it does play a critical role. We hypothesized that αVβ3-integrin serves as a routing factor capable of influencing the path and intracellular route taken by incoming HSV. To test this hypothesis, we measured the effect of validated inhibitors that target different endocytic pathways in CHO-N1 and J-N1 cells, positive or negative, for αVβ3-integrin.
HSV Infection Is Dependent on Cholesterol-Rich Rafts in αVβ3-Integrin–Positive Cells.
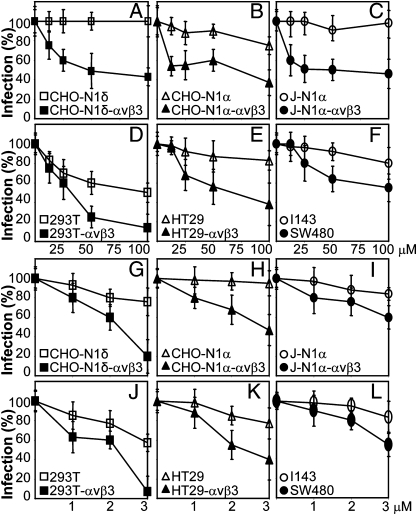
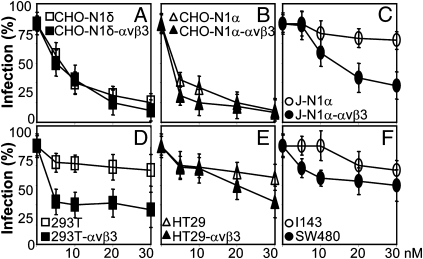
To test whether the αVβ3-integrin–dependent entry requires cholesterol-rich rafts, we exposed cells to the cholesterol-depleting drugs nystatin or filipin. Cells were preincubated with filipin for 30 min, infected in the same medium for 30 min, or preincubated with nystatin for 16 h and infected in medium containing no inhibitor. Inhibitors were absent after infection. HSV infection was inhibited in a dose-dependent manner in αVβ3-integrin–expressing CHO-N1 and J-N1 cells but not in their αVβ3-integrin–negative counterparts (Fig. 3 A–C and G–I). Nystatin or filipin inhibited infection by about 40–50% in the human cell lines SW480 and about 20% in I143 and HT29 cells. αVβ3-integrin overexpression in HT29 and 293T increased the extent of inhibition (Fig. 3 D, E, J, and K). For nystatin and filipin, as well as all inhibitors tested below, we determined cell viability by Alamar Blue staining of replicate specimens at the highest inhibitor concentration. Cell viability was generally around 90% (cumulative results reported in Table S1). The results suggest that (i) the presence of αVβ3-integrin in both CHO-N1 or J-N1 cells rendered HSV infection sensitive to the cholesterol-sequestering compounds and (ii) the pathway of HSV infection into 293T and SW40 cells resembled the pathway into CHO-N1-αVβ3 cells and likely involved cholesterol-rich lipid rafts. This pathway differed from those in CHO-N1, J-N1, and I143 cells, which do not involve lipid rafts.
Fig. 3.
Effect of the cholesterol-sequestering drugs nystatin (A–F) and filipin (G–L) on R8102 infection of indicated cell lines. Nystatin at indicated micromolar concentrations was present for 16 h before infection and removed at the time of R8102 infection (3 pfu/cell). Filipin was present for 30 min before infection and during 30 min of virus attachment (30 pfu/cell). All other details are as in Fig. 2. Cell viability at the highest concentration used is reported in Table S1.
HSV Infection of αVβ3-Integrin–Positive Cells Requires Dynamin2.
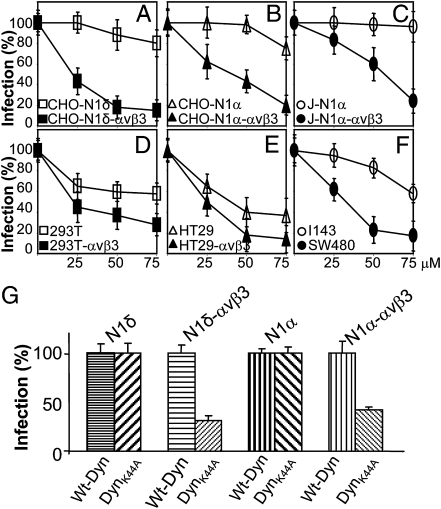
The pinching-off factor dynamin2 is a key component of and differentiates between different endocytic pathways. Dynasore is a specific inhibitor of dynamin1 and dynamin2 (32). It was added to culture medium from 1 h before infection until harvesting. Fig. 4 A–C shows that HSV infection was highly sensitive to this inhibitor in CHO-N1δ, CHO-N1α, and J-N1α cells positive for αVβ3-integrin but not in the αVβ3-integrin–negative counterparts. With respect to human cells (Fig. 4 D–F), dynasore was very effective in SW480, HT29αVβ3, and 293TαVβ3 cells, somewhat less in wt-HT29 and 293T cells, and even less in I143 cells. The results highlight a role for dynamin2 in HSV infection of the αVβ3-integrin–expressing cells CHO-N1 and J-N1 and three human cells under examination (SW40, HT29, and 293T), with lowest sensitivity exhibited by I143 cells.
Fig. 4.
Involvement of dynamin2 in HSV infection of αVβ3-integrin–positive cells. (A–F) Effect of dynasore on R8102 infection. Cells were exposed to micromolar concentrations of dynasore before, during, and after R8102 attachment. All other details are as in Fig. 2. Cell viability is reported in Table S1. (G) Effect of the DN DynK44A dynamin2 mutant on R8102 infection of indicated CHO cells. CHO-N1α or δ ± αVβ3-integrin cells were transfected with wt-dynamin (wt-Dyn) or DynK44A, seeded in 96 wells after 24 h, and infected 24 h later with R8102 (3 pfu/cell). Details are as in Fig. 2; 100% is the β-Gal value in cells transfected with wt-Dyn. Bars represent SD.
To provide genetic evidence for dynamin2 involvement, we tested the effect of the dominant negative (DN) DynK44A-GFP, which was tagged with GFP and impaired in scission activity. CHO derivatives were transfected with DynK44A-GFP or wt-DynGFP and infected with R8102. DynK44A-GFP inhibited infection by about 60–70% relative to DynGFP only in αVβ3-integrin–expressing cells (Fig. 4G). Given that the efficiency of transfection under these conditions is in the range of about 60%, the inhibition figures underestimate the extent of DynK44A-GFP inhibition.
Cumulatively, the results provide evidence that HSV infection of the αVβ3-integrin–positive CHO-N1 and J-N1 cells as well as the human SW480, HT29, and 293T cells is strongly dependent on dynamin2.
HSV Infection Does Not Make Use of Caveolin1.
A major dynamin2-dependent endocytic pathway is caveolin1 (cav1)-dependent. To test the role of cav1, we asked whether infecting virions colocalize with cav1 at the time of virus infection and assayed the effect of Cav1Y14A, a phosphorylation-defective mutant that acts in a DN manner.
CHO-N1δ ± αVβ3-integrin cells were infected with partially purified extracellular virions of K26GFP carrying UL26 capsid protein tagged with GFP (33) and fixed at 1 and 2 h after infection. No colocalization was detected, irrespective of the expression of αVβ3-integrin (Fig. 5 A and B, results shown for 1 h).
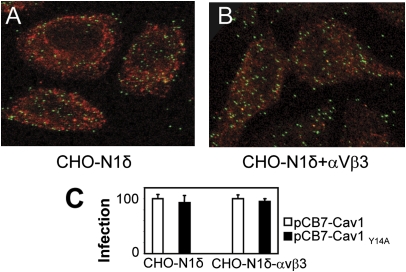
Fig. 5.
Lack of involvement of cav1 in HSV infection. (A and B) Cells were infected with K26-GFP at 4 °C, virus was rinsed off, and cells were shifted to 37 °C for 1 h. Cav1 was detected with PAb 3238 and Dylight 549 anti-rabbit IgGs (red). No colocalization was observed by confocal microscopy. (C) Effect of the DN Cav1Y14A or wt-cav1 on R8102 infection. Cells were transfected with cav1 (wt-cav1) or Cav1Y14A, seeded in 96 wells after 24 h, and infected 24 h later with R8102 (3 pfu/cell). Details are as in Fig. 2; 100% is the β-Gal value in cells transfected with wt-cav1. Bars represent SD.
CHO-N1δ ± αVβ3-integrin cells were transfected with Cav1Y14A or wt-cav1 and infected with R8102. Cav1Y14A failed to inhibit infection, irrespective of αVβ3-integrin expression (Fig. 5C). Thus, HSV infection of CHO-N1 cells is not dependent on cav1, regardless of whether αVβ3-integrin is present.
αVβ3-Integrin Enables Infection Through a Low pH-Dependent Pathway.
As mentioned above, HSV infection of CHO-N1δ or -N1α cells is BFLA-sensitive, whereas HSV infection of J-N1α cells is not (3, 5). To provide further evidence that αVβ3-integrin routes HSV to a specific entry pathway, we asked whether J-N1α-αVβ3 cells internalize virus in a neutral or low-pH compartment. BFLA was present from 1 h before infection until harvesting. Results in Fig. 6C show that αVβ3-integrin overexpression rendered J-N1α cell infection sensitive to BFLA. A similar subversion was observed in 293TαVβ3 relative to wt-293T cells (Fig. 6D). As expected, no modification was induced by αVβ3-integrin in infection of CHO-N1δ and -α cells given that the pathway of entry in these cells is already BFLA-sensitive, even in the absence of αVβ3-integrin (Fig. 6 A and B). The results indicate that the pathway of HSV entry into J-N1 and 293T cells occurs through an acidic rather than neutral compartment when cells overexpress αVβ3-integrin, and consequently, αVβ3-integrin routes HSV to an acidic endocytic compartment.
Fig. 6.
Effect of BFLA on R8102 infection of indicated cell lines. (A–F) Cells were exposed to nanomolar concentrations of BFLA from 1 h before R8102 (3 pfu/cell) infection until harvesting. All other details are as in Fig. 2. Cell viability is reported in Table S1.
Discussion
To infect cells, a macromolecule on the surface of virus particles must react with a receptor on the cell surface. The actual pathway of entry varies on both a virus- and cell-type basis. Moreover, a given virus may enter different cell types by different pathways. For example, HSV-1 can enter cells by fusion of the envelope with the plasma membrane or after endocytosis through neutral or acidic compartments; in some cells, it may enter by macropinocytosis/phagocytosis. In all cases, HSV entry is mediated by four glycoproteins on the virion envelope (gD, gH/gL, and gB) and the specific interaction of the virion gD with either nectin1 or HVEM receptors. The central question that we have posed in this report is what cellular factors determine the pathway of HSV-1 entry into cells. To answer this question, we focused on several cell lines but in particular, on cells that lack on their surface αVβ3-integrin. In the example that we selected, HSV-1 enters CHO cells expressing nectin1 through an acidic compartment, independently of cholesterol-rich rafts and dynamin2. In CHO-nectin1 cells overexpressing αVβ3-integrin, HSV-1 enters through an acidic cholesterol-rich rafts- and dynamin2-dependent pathway (for a summary of inhibitors' results, see Table S2). HSV enters J-nectin1 and 293T cells through a neutral compartment, likely the plasma membrane, independently of cholesterol-rich rafts and dynamin2. Overexpression of αVβ3-integrin reroutes HSV to an acidic, cholesterol-rich rafts- and dynamin2-dependent compartment. Moreover, in the presence of αVβ3-integrin, the pathway of entry into CHO-nectin1 cells becomes irreversible. Thus, a number of treatments (inhibition of αVβ3-integrin signal transduction by a specific monoclonal antibody, cholesterol depletion, block of dynamin2, or of endosomal acidification) prevent not only the αVβ3-integrin–dependent pathway, but also the entry functional in the αVβ3-integrin–negative CHO-nectin1 cells, characterized for being integrin-, cholesterol-raft–, and dynamin2-independent. Other cells lines, including some human cell lines, behave in a similar fashion. In particular, cells rich in αVβ3-integrin (SW480) recapitulate the entry pathway seen in αVβ3-integrin–overexpressing CHO-nectin1 cells. Conversely, cells poor in αVβ3-integrin (I143) recapitulate the integrin-independent pathway. The salient conclusion of the studies reported here is that cell surface components direct the pathway of HSV entry. In particular, αVβ3-integrin routes HSV to a cholesterol-rich rafts– and dynamin2–dependent acidic compartment. The results presented here raise several questions that impact our understanding of the biology of HSV and its entry into susceptible cells.
The foremost question relates to the mechanism by which αVβ3-integrin routes HSV to a different pathway of entry. For heuristic reasons, it is convenient to consider two nonexclusive hypotheses. The first hypothesis is that αVβ3-integrin binds HSV-1, and this triggers endocytosis of the virus. An alternative hypothesis is that αVβ3-integrin modifies the cell surface to preclude virus entry through the cholesterol-rich raft– and dynamin2–independent compartment and for some cells (exemplified here by J-nectin1 and 293T cells), through a nonacidic cholesterol-rich raft– and dynamin2–independent compartment. There is no evidence currently that any component of the virus directly interacts with αVβ3-integrin (28). The second hypothesis predicts that αVβ3-integrin does not interact with HSV-1 virions and that the remodeling of the cell surface or signaling activities is a function of αVβ3-integrin that ultimately suppresses the pathway independent of cholesterol-rich rafts and dynamin2.
The second issue concerns the large number of pathways of HSV entry into cells and more specifically, the selective pressures that generated these pathways. Numerous studies have shown that entry of HSV into cells activates host innate immunity even in the absence of viral gene expression (e.g., UV-inactivated virus) (34, 35). One relevant example is that virus particles lacking gD and thus unable to proceed to an infectious entry are endocytosed and degraded (36). It is noteworthy that many Toll-like receptors reside in lipid rafts. Uptake of virions through cholesterol-rich rafts may be a screening mechanism to identify and signal the presence of potential pathogens. In this role, αVβ3-integrin would become a sensor and a first line of defense against HSV infection.
Materials and Methods
Cells and Viruses.
HT29, 293T, I143, and J, a BHK-TK− (baby hamster kidney-thymidine kinase−) derivative lacking HSV receptors) (12) cells were grown in DMEM containing 5–10% FBS. CHO and colon carcinoma SW480 were grown in F12 and L15 media, respectively; 293T cells transiently expressing αVβ3-integrin (293TαVβ3) were transfected with αV- and β3-integrin plasmids (37) or epidermal growth factor receptor 2 (HER-2) as negative control. CHO-nectin1δ-αvβ3 (CHO-N1δ-αVβ3), CHO-nectin1δ (CHO-N1δ), CHO-nectin1α (CHO-N1α), CHO-nectin1α-αVβ3 (CHO-N1α-αVβ3), J-nectin1α (J-N1α), and J-nectin1α-vβ3 (J-N1α-αVβ3) were generated by cotransfection of appropriate plasmids, neomycin G418 (400–800 μg/mL) selection for 5 d, and limiting dilution cloning.
HSV R8102 recombinant expresses lacZ under the α27 promoter (12). The K26GFP carries the UL26 capsid protein fused to GFP (33). The WT HSV-1(F) was described (38).
Plasmids.
Plasmids encoding nectin1δ (11), nectin1α (12), human αV-integrin, human β3-integrin (39), and HER-2 (40) were described. DynGFP and DynK44A-GFP (41) encode dynamin2 fused to GFP in the WT or DN version (K44A substitution). pCB7-Cav1 and pCB7-Cav1Y14A (42) encode cav1 in the WT or DN version.
Inhibition of Infection by mAbs to Integrin.
Cells in 96 wells were preincubated with indicated amounts of mAbs L230 (αv-integrin), AP3 (β3-integrin), or mouse IgGs for 60 min at 37 °C. R8102 (3 pfu/cell) in 5 μL was added for another 90 min. Viral inoculum was removed, and cells were overlaid with DMEM containing mAbs. β-Gal activity was determined 6–8 h later by o-nitrophenyl-β-d-galactopyranoside and optical density reading or in situ staining with 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-gal) (12). Each point represents triplicates’ average. The value with untreated infected cells was taken as 100%.
Inhibition of Infection by Pharmacological Inhibitors.
Cells were pretreated with inhibitors at the amounts indicated in Figs. 1–6 for 60 min at 37 °C and were infected with R8102 (3 pfu/cell) for 90 min at 37 °C in the same medium. Inoculum was removed, and cells were overlaid with DMEM containing appropriate inhibitor for another 6–8 h. For filipin, cells were preincubated with the compound at 37 °C for 30 min and infected for 30 min (30 pfu/cell) in the same medium. For nystatin, cells were preincubated with nystatin for 16 h, the inhibitor was removed, and cells were washed and infected for 60 min at 37 °C (3 pfu/cell) in the absence of nystatin. With both inhibitors, infected cells were overlaid without inhibitor. In all assays, each point represent triplicates’ average.
Effect of DN Dynamin2 or Cav1 on Infection.
CHO derivatives in T25 flasks were transfected with 4 μg plasmid DNA encoding DynGFP, DynK44A-GFP, pCB7-Cav1, pCB7-Cav1-Y14A, or HER-2 by Arrest-in (Euroclone). After 24 h, cells were seeded into 96 or 24 wells on glass coverslips and were infected 24 h later with R8102 (3 pfu/cell) for 1 h at 37 °C in triplicate. β-Gal activity was measured as above 16 h later. Cells in glass coverslips were used to monitor transgene expression.
Confocal Microscopy.
Partially purified extracellular virions of K26-GFP (50 pfu/cell) were allowed to attach to CHO-N1δ or CHO-N1δ-αVβ3 cells for 2 h at 4 °C. Unabsorbed virus was removed, and cells were rinsed two times, shifted to 37 °C for 0, 1, or 2 h, and paraformaldehyde-fixed and permeabilized with 0.1% Triton ×100. Cav1 was detected with polyclonal Ab 3238 (Cell Signaling) and Dylight 549 anti-rabbit IgGs (Jackson Immunoresearch). Cells were observed with a Leica TCS-SL confocal microscope. Images were collected with a 63× Leica oil immersion objective (numerical aperture = 1.62); confocal slices were 1.0- to 1.5-μm thick.
Supplementary Material
Acknowledgments
We thank S. Blystone (State University of New York Upstate Medical University, Syracuse, NY), M. Sargiacomo (Istituto Superiore di Sanità, Rome, Italy), S. L. Schimd (Scripps Research Institute, La Jolla, CA), E. Spisni (University of Bologna, Bologna, Italy), and B. Roizman (University of Chicago, Chicago, IL) for generous gifts of reagents, and Luciana Dipietrangelo (Department of Biology, University of Bologna) for invaluable help with confocal microscopy. This work was supported by grants from Associazione Italiana per la Ricerca sul Cancro, Fondi Roberto and Cornelia Pallotti from our department, and University of Bologna Ricerca Fondamentale Orientata.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1014923108/-/DCSupplemental.
References
- 1.Mercer J, Schelhaas M, Helenius A. Virus entry by endocytosis. Annu Rev Biochem. 2010;79:803–833. doi: 10.1146/annurev-biochem-060208-104626. [DOI] [PubMed] [Google Scholar]
- 2.Menotti L, et al. Inhibition of human tumor growth in mice by an oncolytic herpes simplex virus designed to target solely HER-2-positive cells. Proc Natl Acad Sci USA. 2009;106:9039–9044. doi: 10.1073/pnas.0812268106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nicola AV, Straus SE. Cellular and viral requirements for rapid endocytic entry of herpes simplex virus. J Virol. 2004;78:7508–7517. doi: 10.1128/JVI.78.14.7508-7517.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Milne RS, Nicola AV, Whitbeck JC, Eisenberg RJ, Cohen GH. Glycoprotein D receptor-dependent, low-pH-independent endocytic entry of herpes simplex virus type 1. J Virol. 2005;79:6655–6663. doi: 10.1128/JVI.79.11.6655-6663.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gianni T, Campadelli-Fiume G, Menotti L. Entry of herpes simplex virus mediated by chimeric forms of nectin1 retargeted to endosomes or to lipid rafts occurs through acidic endosomes. J Virol. 2004;78:12268–12276. doi: 10.1128/JVI.78.22.12268-12276.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nicola AV, McEvoy AM, Straus SE. Roles for endocytosis and low pH in herpes simplex virus entry into HeLa and Chinese hamster ovary cells. J Virol. 2003;77:5324–5332. doi: 10.1128/JVI.77.9.5324-5332.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clement C, et al. A novel role for phagocytosis-like uptake in herpes simplex virus entry. J Cell Biol. 2006;174:1009–1021. doi: 10.1083/jcb.200509155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nicola AV, Hou J, Major EO, Straus SE. Herpes simplex virus type 1 enters human epidermal keratinocytes, but not neurons, via a pH-dependent endocytic pathway. J Virol. 2005;79:7609–7616. doi: 10.1128/JVI.79.12.7609-7616.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campadelli-Fiume G, et al. The multipartite system that mediates entry of herpes simplex virus into the cell. Rev Med Virol. 2007;17:313–326. doi: 10.1002/rmv.546. [DOI] [PubMed] [Google Scholar]
- 10.Montgomery RI, Warner MS, Lum BJ, Spear PG. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell. 1996;87:427–436. doi: 10.1016/s0092-8674(00)81363-x. [DOI] [PubMed] [Google Scholar]
- 11.Geraghty RJ, Krummenacher C, Cohen GH, Eisenberg RJ, Spear PG. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science. 1998;280:1618–1620. doi: 10.1126/science.280.5369.1618. [DOI] [PubMed] [Google Scholar]
- 12.Cocchi F, Menotti L, Mirandola P, Lopez M, Campadelli-Fiume G. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J Virol. 1998;72:9992–10002. doi: 10.1128/jvi.72.12.9992-10002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cocchi F, et al. The soluble ectodomain of herpes simplex virus gD contains a membrane-proximal pro-fusion domain and suffices to mediate virus entry. Proc Natl Acad Sci USA. 2004;101:7445–7450. doi: 10.1073/pnas.0401883101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fusco D, Forghieri C, Campadelli-Fiume G. The pro-fusion domain of herpes simplex virus glycoprotein D (gD) interacts with the gD N terminus and is displaced by soluble forms of viral receptors. Proc Natl Acad Sci USA. 2005;102:9323–9328. doi: 10.1073/pnas.0503907102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heldwein EE, et al. Crystal structure of glycoprotein B from herpes simplex virus 1. Science. 2006;313:217–220. doi: 10.1126/science.1126548. [DOI] [PubMed] [Google Scholar]
- 16.Hynes RO. Integrins: Bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 17.ffrench-Constant C, Colognato H. Integrins: Versatile integrators of extracellular signals. Trends Cell Biol. 2004;14:678–686. doi: 10.1016/j.tcb.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 18.Gahmberg CG, et al. Regulation of integrin activity and signalling. Biochim Biophys Acta. 2009;1790:431–444. doi: 10.1016/j.bbagen.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Polo S, Di Fiore PP. Endocytosis conducts the cell signaling orchestra. Cell. 2006;124:897–900. doi: 10.1016/j.cell.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 20.Caswell PT, Vadrevu S, Norman JC. Integrins: Masters and slaves of endocytic transport. Nat Rev Mol Cell Biol. 2009;10:843–853. doi: 10.1038/nrm2799. [DOI] [PubMed] [Google Scholar]
- 21.Stewart PL, Nemerow GR. Cell integrins: Commonly used receptors for diverse viral pathogens. Trends Microbiol. 2007;15:500–507. doi: 10.1016/j.tim.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Shenk T. Human cytomegalovirus virion protein complex required for epithelial and endothelial cell tropism. Proc Natl Acad Sci USA. 2005;102:18153–18158. doi: 10.1073/pnas.0509201102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akula SM, Pramod NP, Wang FZ, Chandran B. Integrin alpha3beta1 (CD 49c/29) is a cellular receptor for Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) entry into the target cells. Cell. 2002;108:407–419. doi: 10.1016/s0092-8674(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 24.Chesnokova LS, Nishimura SL, Hutt-Fletcher LM. Fusion of epithelial cells by Epstein-Barr virus proteins is triggered by binding of viral glycoproteins gHgL to integrins alphavbeta6 or alphavbeta8. Proc Natl Acad Sci USA. 2009;106:20464–20469. doi: 10.1073/pnas.0907508106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Feire AL, Koss H, Compton T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc Natl Acad Sci USA. 2004;101:15470–15475. doi: 10.1073/pnas.0406821101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chandran B. Early events in Kaposi's sarcoma-associated herpesvirus infection of target cells. J Virol. 2010;84:2188–2199. doi: 10.1128/JVI.01334-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parry C, Bell S, Minson T, Browne H. Herpes simplex virus type 1 glycoprotein H binds to alphavbeta3 integrins. J Gen Virol. 2005;86:7–10. doi: 10.1099/vir.0.80567-0. [DOI] [PubMed] [Google Scholar]
- 28.Gianni T, et al. Herpes simplex virus glycoproteins H/L bind to cells independently of alphaVbeta3 integrin and inhibit virus entry, and their constitutive expression restricts infection. J Virol. 2010;84:4013–4025. doi: 10.1128/JVI.02502-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takai Y, Miyoshi J, Ikeda W, Ogita H. Nectins and nectin-like molecules: Roles in contact inhibition of cell movement and proliferation. Nat Rev Mol Cell Biol. 2008;9:603–615. doi: 10.1038/nrm2457. [DOI] [PubMed] [Google Scholar]
- 30.Bazzoni G, Shih DT, Buck CA, Hemler ME. Monoclonal antibody 9EG7 defines a novel beta 1 integrin epitope induced by soluble ligand and manganese, but inhibited by calcium. J Biol Chem. 1995;270:25570–25577. doi: 10.1074/jbc.270.43.25570. [DOI] [PubMed] [Google Scholar]
- 31.Newman PJ, Allen RW, Kahn RA, Kunicki TJ. Quantitation of membrane glycoprotein IIIa on intact human platelets using the monoclonal antibody, AP-3. Blood. 1985;65:227–232. [PubMed] [Google Scholar]
- 32.Macia E, et al. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Desai P, Person S. Incorporation of the green fluorescent protein into the herpes simplex virus type 1 capsid. J Virol. 1998;72:7563–7568. doi: 10.1128/jvi.72.9.7563-7568.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sciortino MT, et al. Involvement of HVEM receptor in activation of nuclear factor kappaB by herpes simplex virus 1 glycoprotein D. Cell Microbiol. 2008;10:2297–2311. doi: 10.1111/j.1462-5822.2008.01212.x. [DOI] [PubMed] [Google Scholar]
- 35.MacLeod IJ, Minson T. Binding of herpes simplex virus type-1 virions leads to the induction of intracellular signalling in the absence of virus entry. PLoS One. 2010;5:e9560. doi: 10.1371/journal.pone.0009560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou G, Galvan V, Campadelli-Fiume G, Roizman B. Glycoprotein D or J delivered in trans blocks apoptosis in SK-N-SH cells induced by a herpes simplex virus 1 mutant lacking intact genes expressing both glycoproteins. J Virol. 2000;74:11782–11791. doi: 10.1128/jvi.74.24.11782-11791.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gianni T, Amasio M, Campadelli-Fiume G. Herpes simplex virus gD forms distinct complexes with fusion executors gB and gH/gL in part through the C-terminal profusion domain. J Biol Chem. 2009;284:17370–17382. doi: 10.1074/jbc.M109.005728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ejercito PM, Kieff ED, Roizman B. Characterization of herpes simplex virus strains differing in their effects on social behaviour of infected cells. J Gen Virol. 1968;2:357–364. doi: 10.1099/0022-1317-2-3-357. [DOI] [PubMed] [Google Scholar]
- 39.Blystone SD, Graham IL, Lindberg FP, Brown EJ. Integrin alpha v beta 3 differentially regulates adhesive and phagocytic functions of the fibronectin receptor alpha 5 beta 1. J Cell Biol. 1994;127:1129–1137. doi: 10.1083/jcb.127.4.1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rovero S, et al. DNA vaccination against rat her-2/Neu p185 more effectively inhibits carcinogenesis than transplantable carcinomas in transgenic BALB/c mice. J Immunol. 2000;165:5133–5142. doi: 10.4049/jimmunol.165.9.5133. [DOI] [PubMed] [Google Scholar]
- 41.Llorente A, Rapak A, Schmid SL, van Deurs B, Sandvig K. Expression of mutant dynamin inhibits toxicity and transport of endocytosed ricin to the Golgi apparatus. J Cell Biol. 1998;140:553–563. doi: 10.1083/jcb.140.3.553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Felicetti F, et al. Caveolin-1 tumor-promoting role in human melanoma. Int J Cancer. 2009;125:1514–1522. doi: 10.1002/ijc.24451. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.