Abstract
Inositol polyphosphate 4-phosphatase-II (INPP4B) is a regulator of the phosphoinositide 3-kinase (PI3K) signaling pathway and is implicated as a tumor suppressor in epithelial carcinomas. INPP4B loss of heterozygosity (LOH) is detected in some human breast cancers; however, the expression of INPP4B protein in breast cancer subtypes and the normal breast is unknown. We report here that INPP4B is expressed in nonproliferative estrogen receptor (ER)-positive cells in the normal breast, and in ER-positive, but not negative, breast cancer cell lines. INPP4B knockdown in ER-positive breast cancer cells increased Akt activation, cell proliferation, and xenograft tumor growth. Conversely, reconstitution of INPP4B expression in ER-negative, INPP4B-null human breast cancer cells reduced Akt activation and anchorage-independent growth. INPP4B protein expression was frequently lost in primary human breast carcinomas, associated with high clinical grade and tumor size and loss of hormone receptors and was lost most commonly in aggressive basal-like breast carcinomas. INPP4B protein loss was also frequently observed in phosphatase and tensin homolog (PTEN)-null tumors. These studies provide evidence that INPP4B functions as a tumor suppressor by negatively regulating normal and malignant mammary epithelial cell proliferation through regulation of the PI3K/Akt signaling pathway, and that loss of INPP4B protein is a marker of aggressive basal-like breast carcinomas.
Keywords: phosphatidylinositol 3,4-bisphosphate
The phosphoinositide 3-kinase (PI3K) signaling pathway promotes cell proliferation and survival. In response to extracellular stimuli, activation of PI3K results in the transient production of the phosphoinositides, PtdIns(3,4,5)P3 and PtdIns(3,4)P2, at the plasma membrane. Both phosphoinositides can bind and activate multiple downstream effectors, most importantly the protooncogene, Akt, and both PtdIns(3,4,5)P3 and PtdIns(3,4)P2 are necessary for full Akt activation in vivo (1–3). Deregulated PI3K/Akt activation promotes oncogenesis and has been described in many human cancers (4). Recently, the PI3K signaling pathway has been identified as a putative therapeutic target in a range of human malignancies and there are currently a number of phase I–II clinical trials in progress investigating the efficacy of PI3K pathway inhibitors in the treatment of human cancers (4).
PtdIns(3,4,5)P3 is negatively regulated via dephosphorylation by the 3-phosphatase and tumor suppressor, phosphatase and tensin homolog (PTEN), to form PtdIns(4,5)P2, or by 5-phosphatases, which generate PtdIns(3,4)P2 (1). In turn, PtdIns(3,4)P2 is hydrolyzed by two inositol polyphosphate 4-phosphatases (type I and type II) (INPP4A and B, respectively) (5, 6). INPP4A expression protects neurons from excitotoxic cell death (7, 8) and this enzyme is also a negative regulator of Akt phosphorylation, cell proliferation, and orthotopic tumor formation in mice (9, 10). However, to date analysis of human tumors has revealed little evidence that INPP4A functions as a tumor suppressor. In contrast, the related INPP4B may function as a tumor suppressor in epithelial carcinomas. Short hairpin RNA (shRNA)-targeting of INPP4B leads to cell transformation in human mammary epithelial cells (HMECs) (11) and INPP4B knockdown in HMECs promotes Akt activation and anchorage-independent growth (12). Deletion of the INPP4B chromosome region occurs in some primary human breast cancers (13) and loss of heterozygosity (LOH) of INPP4B is frequently observed in BRCA1 mutant and hormone receptor-negative breast cancers (12). Furthermore, loss of INPP4B protein expression in breast and ovarian cancer is associated with decreased patient survival (12). To date, however, the expression of INPP4B protein in normal breast and in human breast cancer subtypes, relative to clinicopathologic variables, remains to be determined.
Invasive breast carcinomas can be categorized into distinct subtypes, luminal A, luminal B, HER2 positive and basal-like, on the basis of expression of various clinocopathologic markers (14–16). Luminal A and luminal B breast cancer subtypes express estrogen receptor (ER) and/or progesterone receptor (PgR), whereas HER2-positive and basal-like subtypes are hormone receptor negative and are in general more aggressive and confer a worse prognosis compared with luminal-type breast carcinomas (15, 16). The cellular mechanisms underlying these observations, however, are poorly defined and current research is focused on identifying new biomarkers to improve breast cancer classification and treatment.
In this study, we identify INPP4B as a previously undescribed marker of hormone receptor-positive breast cancers that functions to control both normal breast and malignant ER-positive breast cancer cell proliferation through regulation of PI3K signaling. Loss of INPP4B protein expression occurs most frequently in aggressive hormone receptor-negative basal-like breast carcinomas, associated with high tumor grade and size, and is frequently associated with loss of the tumor suppressor PTEN. These studies identify loss of INPP4B protein as a previously undescribed molecular marker for basal-like breast cancers and provide evidence for the cooperative promotion of oncogenesis through the PI3K/Akt signaling pathway.
Results
Expression of INPP4B Protein in Normal Human Breast.
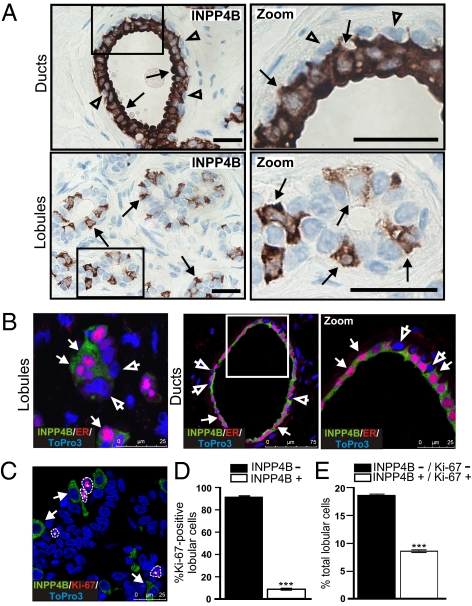
We generated monoclonal and polyclonal antibodies (Abs) to purified 6xHis-tagged human INPP4B, which both detected endogenous INPP4B in ER-positive MCF-7 breast cancer cells and purified recombinant His-INPP4B, but not recombinant INPP4A (Fig. S1). Using these Abs, we assessed INPP4B protein expression in normal human mammary tissue sections (Fig. 1 and Fig. S2). In epithelial cells comprising the mammary ducts, INPP4B expression was restricted to luminal epithelial cells and appeared absent in surrounding myoepithelial cells (Fig. 1A). In control studies, no immunoreactivity was detected using mouse IgG as a negative control (Fig. S2A). Interestingly, in the secretory lobular units, INPP4B was present in only ∼20% of cells, with many cells showing no staining (Fig. 1A). The heterogeneous expression pattern of INPP4B in these structures may indicate a specific role for INPP4B in mammary cell differentiation and function.
Fig. 1.
INPP4B is expressed in nonproliferative ER-positive cells in normal human breast. (A) Normal human breast sections were immunostained using an INPP4B-specific monoclonal antibody (3D5). (Scale bars, 50 μm.) (B) Immunofluorescent images of normal breast sections costained for INPP4B (green), ER (red), and the nuclear marker, ToPro3 iodide (blue) (see also Fig. S2). Coexpression of INPP4B and ER is indicated by arrows. Arrowheads show cells negative for both INPP4B and ER. (Scale bars, 25 μm, 75 μm.) (C) Immunofluorescent costaining of normal human breast lobules for INPP4B (green), Ki-67 (red), and ToPro3 iodide (blue) (Fig. S2). Arrows show INPP4B-positive cells. Outlines and asterisks mark Ki-67–positive nuclei. (Scale bar, 25 μm.) More than 100 Ki-67–positive lobular cells/section were assessed for INPP4B expression, and the mean number of cells that were INPP4B negative (closed bars) or INPP4B positive (open bars) ± SEM from three normal breasts is shown in D. More than 1,000 lobular cells/section were assessed for Ki-67 and INPP4B expression and the mean number of INPP4B-positive lobular cells that were Ki-67 negative (closed bars) or Ki-67 positive (open bars) ± SEM from two normal breasts is shown in E. ***P < 0.002.
In the normal breast, ER is expressed in only 15–30% of epithelial cells and inversely correlates with cell proliferation (17, 18). Costaining normal breast sections with ER and INPP4B Abs revealed that INPP4B was expressed in ER-positive but not ER-negative cells (Fig. 1B and Fig. S2B). Colocalization of INPP4B and the proliferation marker, Ki-67 in breast lobules revealed that ~5–10% of lobular cells were Ki-67 positive and of these the majority (>90%) did not show INPP4B staining (Fig. 1 C–D). Of the INPP4B-positive cells (20% of lobular cells), the majority did not exhibit Ki-67 staining (Fig. 1E). Therefore INPP4B may suppress ER-positive lobular cell proliferation in the normal breast.
Characterization of INPP4B Protein Expression in Human Breast Cancer Cell Lines.
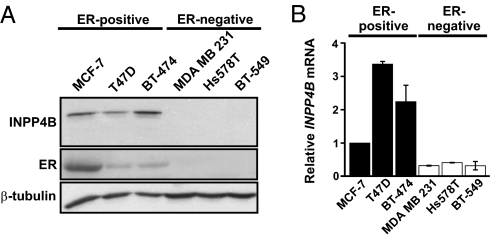
We next assessed INPP4B protein expression in a panel of human breast cancer cell lines. INPP4B protein was expressed in ER-positive (MCF-7, T47D, and BT-474) but not ER-negative (MDA MB 231, Hs578T, and BT-549) cell lines (Fig. 2A). Quantitative real-time reverse-transcription PCR (qRT-PCR) analysis confirmed INPP4B mRNA levels were lower in all ER-negative relative to ER-positive cells, although a low level of INPP4B mRNA was detected in ER-negative cell lines (Fig. 2B). Furthermore, metaanalysis of human breast cancer datasets made publicly available in Oncomine (19, 20) revealed a positive association between INPP4B mRNA and the hormone receptors, ER and PgR, in human breast cancers in 11 and 3 independent studies, respectively (Fig. S3 A and B).
Fig. 2.
INPP4B protein and mRNA expression in a panel of human breast cancer cells lines. (A) Human breast cancer cell line lysates were immunoblotted for INPP4B, ER, and β-tubulin protein expression, revealing INPP4B protein is expressed in ER-positive, but not ER-negative, cell lines. (B) qRT-PCR analysis of INPP4B mRNA, relative to GAPDH, in ER-positive versus ER-negative human breast cancer cell lines. The graph shows the mean INPP4B mRNA expression ± SEM from two independent experiments.
Control studies evaluated whether the related INPP4A is also expressed in breast cancer cell lines using previously characterized INPP4A Abs (21). Endogenous INPP4A was detected in mouse brain lysates but not in breast cancer cell lines (Fig. S3 C and D). Collectively this data indicates INPP4B is the only PtdIns(3,4)P2 4-phosphatase expressed in breast cancer cells and suggests a correlation between INPP4B and hormone receptor status in human breast cancer.
Involvement of INPP4B in ER-Positive Breast Cancer Cell Signaling, Proliferation, and Tumor Formation.
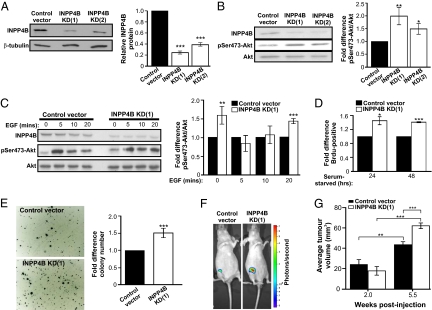
To characterize INPP4B function in human breast cancer cells, shRNA-mediated INPP4B protein knockdown was undertaken in a variant of the ER-positive MCF-7 human breast cancer cell line, MCF-7-luc-F5, which stably expresses luciferase enzyme allowing detection of cells in vivo. MCF-7-luc-F5 cells exhibited similar INPP4B protein levels to the parental line (Fig S4A). In cell populations individually expressing two distinct INPP4B-directed shRNAs (INPP4B KD[1] and [2]) (Fig. S4B), INPP4B protein was decreased by ∼70% and 60% compared with vector controls (Fig. 3A) and similar decreases were observed in INPP4B mRNA (Fig. S4C). The protooncogene Akt is activated by phosphorylation on serine and threonine residues. Significantly, in serum-starved cells, basal phosphorylated Akt (pSer473-Akt) was increased by 1.5- to 2-fold in INPP4B knockdown cells, conditions under which Akt activation is normally minimal (Fig. 3B). Furthermore INPP4B knockdown in MCF-7-luc-F5 cells resulted in increased pSer473-Akt/Akt following 20 min EGF stimulation, relative to vector controls (Fig. 3C).
Fig. 3.
Decreased INPP4B expression enhances Akt phosphorylation and tumorigenic potential. (A) INPP4B protein knockdown in two MCF-7-luc-F5 cell populations expressing unique INPP4B-specific shRNAs (INPP4B KD[1] and [2]) was confirmed by immunoblotting. The mean INPP4B protein levels relative to vector control ± SEM from three independent experiments is shown. (B and C) Immunoblot analysis of INPP4B, pSer473-Akt, and total Akt in cells serum-starved for 24 h (B) or stimulated with EGF (C), demonstrating enhanced pSer473-Akt in INPP4B knockdown cells. The mean fold change in pSer473-Akt/total Akt relative to control ± SEM from six (B) or three (C) independent experiments is shown. (D) INPP4B knockdown and control cells were serum starved for 24 and 48 h and assessed for BrdU incorporation as a marker of proliferation. The mean fold increase in BrdU-positive cells relative to control ± SEM from three independent experiments is shown. (E) INPP4B knockdown or control cells were grown in soft agar. The number of colonies/well was determined and the mean fold increase in colony number relative to control ± SEM from two independent experiments performed in triplicate is shown. (F and G) MCF-7-luc-F5 cells expressing control vector or INPP4B-specific shRNAS were injected into the mammary fat pads of Balbc nu/nu mice in the presence of estrogen and tumor growth was analyzed by bioluminescence and caliper measurement. Bioluminescent imaging of xenograft tumors in vivo (F) and mean tumor volumes at 2 and 5.5 wk postinjection (G) indicate INPP4B knockdown cells form larger tumors in vivo. The mean tumor volumes ± SEM of five animals/group is shown. *P < 0.05, **P < 0.01, ***P < 0.005.
Akt activation promotes cell proliferation. INPP4B knockdown cells exhibited enhanced (1.4-fold) cell proliferation in response to serum-starvation (24–48 h), conditions under which cells normally become quiescent (Fig. 3D). The ability of cells to form colonies in soft agar and tumors in nude mice is a feature of transformed cells. INPP4B knockdown in MCF-7-luc-F5 cells enhanced anchorage-independent cell growth (Fig. 3E). To assess INPP4B regulation of ER-positive breast cancer growth in vivo, INPP4B knockdown or control MCF-7-luc-F5 cells were injected into the abdominal mammary fat pads of athymic nude mice and palpable tumors were measured over 6 wk. Seven of the eight mice injected with INPP4B knockdown cells developed palpable tumors that grew over the course of the experiment, compared with four out of eight mice injected with vector-control cells. Furthermore, INPP4B knockdown tumors exhibited a 3.2-fold increase in tumor size over time, compared with a 1.9-fold increase in vector controls (Fig. 3 F and G).
Effect of INPP4B Reconstitution on ER-Negative Breast Cancer Cell Growth and Akt Phosphorylation.
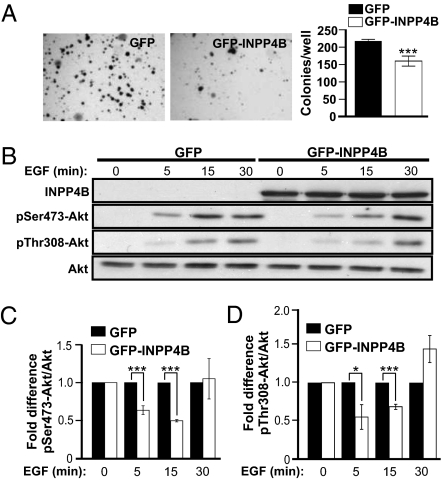
We next evaluated the effects of INPP4B protein reconstitution in INPP4B-null, ER-negative MDA MB 231 breast cancer cells. MDA MB 231 cells are highly proliferative and exhibit anchorage-independent growth in soft agar. Expression of GFP-INPP4B reduced colony formation in soft agar by 35% (Fig. 4A) and suppressed EGF stimulated phosphorylation of Akt at Ser473 (pSer473-Akt) and Thr308 (pThr308-Akt) (Fig. 4 B–D), relative to GFP controls. Therefore in ER-negative breast cancer cell lines, which have lost endogenous INPP4B, reconstitution of this enzyme is sufficient to reduce EGF-stimulated Akt activation and anchorage-independent cell growth.
Fig. 4.
INPP4B protein reconstitution decreases Akt phosphorylation and cell proliferation. (A) INPP4B-null MDA MB 231 cells expressing GFP alone or GFP-tagged INPP4B were suspended in 0.3% agar and colonies allowed to grow for 3 wk. The number of colonies/well was then determined and the mean number of colonies/well ± SEM from two independent experiments performed in triplicate is shown. (B) Immunoblot analysis of INPP4B, pSer473-Akt, pThr308-Akt, and total Akt in GFP or GFP-INPP4B–expressing cells in response to EGF. The mean fold difference in pSer473-Akt (C) and pThr308-Akt (D) normalized to total Akt relative to controls ± SEM from three independent experiments is shown. *P = 0.05, ***P < 0.005.
Expression of INPP4B Protein in Primary Human Breast Carcinoma Subtypes.
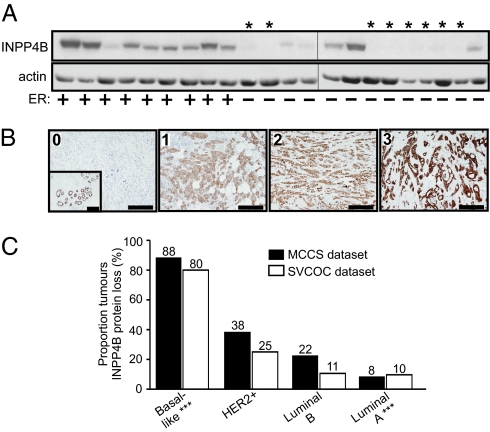
We next assessed the expression of INPP4B protein in clinical human breast carcinomas. In tissue lysates from 22 primary human breast cancers, INPP4B protein was detected in all of the ER-positive tumors, but few of the ER-negative tumors (Fig. 5A). INPP4B protein expression was then evaluated in 107 primary breast carcinoma sections, collected as part of the Melbourne Collaborative Cohort Study (MCCS) (22, 23), by IHC. Notably, INPP4B protein was not detected in 22/107 (21%) of all breast cancers analyzed, but adjacent normal epithelium exhibited INPP4B immunoreactivity in every case (Fig. 5B). In INPP4B-positive tumors, protein expression was exclusively cytoplasmic and ranged from low, moderate, to high (staining score 1–3) (Fig. 5B). Significantly, INPP4B protein deficiency correlated with loss of both ER (P < 0.0001) and PgR expression (P < 0.0001) and positively correlated with the basal marker, CK5/6 (P = 0.0014), and approached significance for epidermal growth factor receptor (EGFR) expression (P = 0.06) (Table 1). Loss of INPP4B was also associated with high tumor grade (P = 0.018). There was no significant correlation between HER2 amplification and INPP4B expression (P = 0.17). Tumors were then classified into breast cancer subtypes as: luminal A (ER+ and/or PgR+, HER2−), luminal B (ER+ and/or PgR+, HER2+), HER2+ (ER/PgR−, HER2+), or basal-like (ER/PgR/HER2−, CK5/6+, and/or EGFR+), as previously described (24–26). Strikingly, loss of INPP4B expression was observed most frequently in the hormone receptor-negative subtypes, basal-like (7/8, i.e., 88%) and HER2+ (3/8, i.e., 38%) and less frequently in the hormone receptor-positive subtypes, luminal B (2/9, i.e., 0.22%) and luminal A (6/72, i.e., 8%) (Fig. 5C).
Fig. 5.
INPP4B protein expression in primary human breast cancers. (A) Immunoblot analysis of INPP4B protein expression in a panel of ER-positive and ER-negative primary human breast cancer lysates. ER-negative tumors were predominantly INPP4B-negative (asterisks). (B) Representative IHC staining of INPP4B in primary human breast carcinomas, scored as: 0, no expression; 1, low; 2, moderate; and 3, strong. Inset image in tumor scored 0 for INPP4B shows INPP4B expression in adjacent normal tissue. (Scale bar, 200 μm.) (C) The frequency of INPP4B protein loss of expression in breast cancer subtypes from two independent human datasets [MCCS (black bars) and SVCOC (open bars)] was determined by IHC. Loss of INPP4B was positively correlated with the basal-like subtype and negatively correlated with the luminal A subtype in both datasets. ***P < 0.0001.
Table 1.
Association analysis of INPP4B protein loss with clinicopathologic, cell cycle, and PI3K pathway variables in human breast cancers (MCCS and SVCOC datasets)
| MCCS |
SVCOC |
||||||
| Variable | Total (n = 107) | INPP4B loss (n = 22) | P value | Total (n = 267) | INPP4B loss (n = 71) | P value | Nature of association |
| Clinical | |||||||
| Histological grade 3 | 34 | 12 (35%) | 0.018 | 123 | 48 (39%) | <0.0001 | Positive |
| Size (>20mm) | - | - | - | 101 | 36 (33%) | 0.047 | Positive |
| LN status (positive) | - | - | - | 119 | 31 (26%) | 0.89 | Nil |
| Receptors/Markers | |||||||
| ER status (positive) | 68 | 5 (7%) | <0.0001 | 178 | 19 (11%) | <0.0001 | Negative |
| PgR status (positive) | 56 | 3 (5%) | <0.0001 | 150 | 11 (7%) | <0.0001 | Negative |
| HER2 amplification | 26 | 8 (31%) | 0.17 | 47 | 11 (23%) | >0.999 | Nil |
| CK5/6 (positive) | 6 | 5 (83%) | 0.0014 | 62 | 28 (45%) | 0.0003 | Positive |
| EGFR (positive) | 7 | 3 (43%) | 0.06 | - | - | - | Positive* |
| Cell Cycle | |||||||
| Ki-67 (high) | - | - | - | 123 | 40 (33%) | 0.0003 | Positive |
| Cyclin E1 (high) | - | - | - | 66 | 29 (44%) | 0.0001 | Positive |
| p27 (high) | - | - | - | 113 | 18 (16%) | 0.0055 | Negative |
| PI3K pathway | |||||||
| PIK3CA amp/mut | - | - | - | 26 | 8 (31%) | 0.54 | Nil |
| PTEN loss | - | - | - | 73 | 36 (49%) | <0.0001 | Positive |
| pAkt > mean | - | - | - | 62 | 17 (23%) | 0.49 | Nil |
| PTEN loss/pAkt >mean | - | - | - | 17 | 11 (65%) | 0.0002 | Positive |
LN, axillary lymph node involvement; ER, estrogen receptor; PgR, progesterone receptor; CK, cytokeratin; EGFR, epidermal growth factor receptor 1; amp, amplified; mut, mutated; pAkt, phospho-Akt.
*Approaching significance.
These results were independently validated by assessing INPP4B immunostaining in a more extensive cohort of 267 invasive ductal carcinomas drawn from the St. Vincent's Campus Outcome Cohort (SVCOC) (25–27). INPP4B protein expression was lost in 71/267 (27%) of all tumors analyzed from this dataset, associated with loss of both ER and PgR expression (P < 0.0001) and positively correlated with CK5/6 (P = 0.0003), but not HER2 amplification (P > 0.999) (Table 1). Again, INPP4B expression was lost most frequently in basal-like carcinomas (24/30, i.e., 80%, P < 0.0001) and infrequently in luminal A carcinomas (16/158, i.e., 10%, P < 0.0001) (Fig. 5C). The luminal A subtype is the least aggressive tumor type, associated with the best patient prognosis (15, 16, 28, 29), whereas basal-like breast carcinomas are in general aggressive, exhibiting high histologic tumor grade and mitotic index (30). Consistent with this phenotype, loss of INPP4B expression positively correlated with high tumor grade (P < 0.0001) and size >20 mm (P = 0.047) and expression of proliferation markers, Ki-67 (P = 0.003) and cyclin E1 (P = 0.0001), and was inversely associated with the cell cycle inhibitor, p27 (P = 0.0055) (Table 1). Interestingly, there was no correlation between INPP4B loss and axillary lymph node involvement (P = 0.89), indicating that INPP4B may be a regulator of tumor cell proliferation and growth, but not metastasis.
Association of INPP4B Protein Loss with Altered Parameters of the PI3K Pathway.
The incidence of INPP4B loss of expression in breast cancers, as reported here, is comparable to those reported for PIK3CA mutations (7–40%) (26, 31–36) or loss of the tumor suppressor PTEN (13–37%) (26, 37–39). To date, most studies indicate that alterations to PIK3CA and PTEN in human cancers occur in a mutually exclusive manner (39–42). We evaluated whether loss of INPP4B was associated with alterations in other components of the PI3K pathway in 267 tumors from the SVCOC dataset that had been previously assessed for PIK3CA amplification/mutation, Akt hyperactivation, or PTEN loss (26). Notably, loss of INPP4B protein did not correlate with PIK3CA mutation/amplification (P = 0.54), indicating that these events are mutually exclusive in breast cancer (Table 1). In contrast, INPP4B protein expression was lost in 49% (36/73) of PTEN-null tumors, compared with 14% (25/185) of PTEN-expressing tumors, revealing a significant positive correlation between INPP4B deficiency and PTEN loss (P < 0.0001). Whereas high pAkt alone did not significantly correlate with INPP4B loss (P = 0.493), PTEN null tumors that showed high pAkt frequently exhibited INPP4B deficiency (P < 0.0002) (Table 1). Therefore, concomitant loss of both PTEN and INPP4B may contribute to Akt hyperactivation and the development of aggressive forms of breast cancer.
Discussion
This study identifies INPP4B as a previously undescribed regulator of ER-positive normal and breast cancer cell proliferation and tumor growth. In the normal breast INPP4B is expressed predominantly in ER-positive cells that are nonproliferative. INPP4B protein knockdown in ER-positive breast cancer cells enhances baseline and EGF-dependent Akt phosphorylation, cell proliferation, anchorage independent cell growth, and xenograft tumor formation. Conversely, in INPP4B-null, ER-negative MDA MB 231 cells, reintroduction of this PtdIns(3,4)P2 4-phosphatase inhibits EGF-dependent Akt signaling and suppresses cell growth in soft agar.
Our study is unique in investigating and reporting a correlation between INPP4B protein expression and clinicopathologic parameters in human breast cancer. Gene array studies have predicted a correlation between INPP4B expression and hormone receptor status in human breast cancer (43, 44), and the studies presented here support this association at the protein level. The nature of the relationship between ER and INPP4B, however, remains elusive. Although PI3K can negatively regulate ER protein levels by decreasing ER protein stability (45), we noted that INPP4B protein knockdown in ER-positive MCF-7-luc-F5 cells, or reconstitution in ER/INPP4B-negative MDA MB 231 cells, did not affect ER protein expression (Fig. S5 A and B). Similarly we have demonstrated estrogen stimulation of MCF-7 cells, or treatment with ER antagonists does not affect the protein levels of INPP4B (Fig. S5C), despite gene array studies indicating INPP4B transcription may be enhanced by estrogen stimulation (46). Therefore the nature of the relationship between INPP4B and ER is likely to be complex, possibly involving signaling derived from stromal tissue (47).
We report that INPP4B protein expression is lost in 84% of human basal-like breast carcinomas, which are generally highly aggressive with poor clinical outcome and are frequently associated with breast cancer 1 (BRCA1) gene mutations (30). Basal-like carcinomas are defined as exhibiting basal cytokeratin and EGFR expression and low or absent expression of hormone receptors (30, 48). Our studies indicate that absence of INPP4B protein may be an additional marker of the basal-like carcinoma signature profile. LOH of INPP4B has recently been reported to occur in 55% of human ER/PgR/HER2 triple-negative breast cancers (12); however, this report did not assess expression of basal markers to discriminate between triple-negative and basal-like cancers or investigate INPP4B protein expression in breast cancer subtypes, as reported here. The discrepancy observed between the frequency of INPP4B LOH previously reported (55%) (12) and loss of INPP4B protein expression presented here (84%) in basal-like breast cancers suggests alternative mechanisms for INPP4B protein loss in addition to gene deletion. Interestingly, INPP4B transcription has recently been shown to be influenced by gene hypermethylation (47). Our studies suggest that suppression of INPP4B protein expression specifically in basal-like breast cancers may contribute to the aggressive nature of this breast cancer subtype.
Like INPP4B, expression of the tumor suppressor and 3-phosphatase, PTEN, is also frequently lost in basal-like carcinomas (26, 39). Notably, we report that concomitant loss of both INPP4B and PTEN proteins is commonly observed in human primary breast cancers. Concurrent PTEN and INPP4B absence may result in the accumulation of their respective phosphoinositide substrates, PtdIns(3,4,5)P3 and PtdIns(3,4)P2, which together promote maximal Akt activation. We report that in human breast cancers loss of INPP4B is frequently associated with PTEN-null tumors exhibiting high pAkt, although loss of INPP4B alone did not show such an association with high pAkt. This is of interest as we, and others (12), have noted only a modest increase in Akt activation following INPP4B knockdown in response to cell stimulation (1.2–1.4-fold). Therefore INPP4B may not regulate the amplitude of Akt activation following cell stimulation but rather the duration of the signal. Consistent with this contention we noted elevated pAkt/Akt in serum-starved INPP4B-depleted cells, conditions under which Akt activation is normally minimal. Although recent studies report that knockdown of INPP4B and PTEN together in immortalized normal human mammary epithelial cell lines leads to cellular senescence (12), it is likely that in human cancers loss of INPP4B and PTEN occurs during tumor progression in combination with additional mutations. Indeed, knockdown of the tumor suppressor TP53 in INPP4B/PTEN-deficient HMECs rescues the senescence phenotype (12).
In summary, the studies reported here identify INPP4B as a putative tumor suppressor that functions to regulate PI3K signaling and ER-positive mammary cell proliferation. In addition, we provide evidence that loss of INPP4B is a unique marker of human basal-like carcinomas. This study reports the concurrent loss of two phosphoinositide phosphatases in human breast cancer and provides evidence for the cooperative promotion of oncogenesis through alterations to multiple components of the PI3K signaling pathway. There are currently no directed therapies for the treatment of human basal-like cancers and tumors exhibiting loss of INPP4B protein with or without concurrent PTEN loss may represent ideal candidates for treatment with PI3K pathway inhibitors.
Materials and Methods
Tumorigenicity in Mice.
For xenograft tumor growth assays, all procedures involving mice were approved by the animal ethics committee at the Monash University School of Biomedical Sciences (SOBSB/B/2008/14) and were established and analyzed as described in SI Materials and Methods.
Patients, Breast Carcinoma Samples, and INPP4B Expression Analysis.
Ethics approval was obtained from the Standing Committee on Ethics in Research Involving Humans, Monash University (CF08/1142–2008000564) for all human tissues and cognate clinicopathological data used in this study. Details of the human breast cancer cohorts and analysis of INPP4B are described in SI Materials and Methods.
Further details of materials and methods can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments
This work was supported by grants from the National Health and Medical Research Council (491029 and 504711). We also acknowledge the Cancer Institute New South Wales, the R. T. Hall Trust, and the Petre Foundation.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015245107/-/DCSupplemental.
References
- 1.Ma K, Cheung SM, Marshall AJ, Duronio V. PI(3,4,5)P3 and PI(3,4)P2 levels correlate with PKB/akt phosphorylation at Thr308 and Ser473, respectively; PI(3,4)P2 levels determine PKB activity. Cell Signal. 2008;20:684–694. doi: 10.1016/j.cellsig.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Franke TF, Kaplan DR, Cantley LC, Toker A. Direct regulation of the Akt proto-oncogene product by phosphatidylinositol-3,4-bisphosphate. Science. 1997;275:665–668. doi: 10.1126/science.275.5300.665. [DOI] [PubMed] [Google Scholar]
- 3.Scheid MP, et al. Phosphatidylinositol (3,4,5)P3 is essential but not sufficient for protein kinase B (PKB) activation; phosphatidylinositol (3,4)P2 is required for PKB phosphorylation at Ser-473: Studies using cells from SH2-containing inositol-5-phosphatase knockout mice. J Biol Chem. 2002;277:9027–9035. doi: 10.1074/jbc.M106755200. [DOI] [PubMed] [Google Scholar]
- 4.Liu P, Cheng H, Roberts TM, Zhao JJ. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat Rev Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Norris FA, Majerus PW. Hydrolysis of phosphatidylinositol 3,4-bisphosphate by inositol polyphosphate 4-phosphatase isolated by affinity elution chromatography. J Biol Chem. 1994;269:8716–8720. [PubMed] [Google Scholar]
- 6.Norris FA, Atkins RC, Majerus PW. The cDNA cloning and characterization of inositol polyphosphate 4-phosphatase type II. Evidence for conserved alternative splicing in the 4-phosphatase family. J Biol Chem. 1997;272:23859–23864. doi: 10.1074/jbc.272.38.23859. [DOI] [PubMed] [Google Scholar]
- 7.Sasaki J, et al. The PtdIns(3,4)P(2) phosphatase INPP4A is a suppressor of excitotoxic neuronal death. Nature. 2010;465:497–501. doi: 10.1038/nature09023. [DOI] [PubMed] [Google Scholar]
- 8.Sachs AJ, David SA, Haider NB, Nystuen AM. Patterned neuroprotection in the Inpp4a(wbl) mutant mouse cerebellum correlates with the expression of Eaat4. PLoS ONE. 2009;4:e8270. doi: 10.1371/journal.pone.0008270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas P, Norris FA, Joseph R, Majerus PW, Orkin SH. Inositol polyphosphate 4-phosphatase type I regulates cell growth downstream of transcription factor GATA-1. Proc Natl Acad Sci USA. 2000;97:13696–13701. doi: 10.1073/pnas.250476397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ivetac I, et al. Regulation of PI(3)K/Akt signalling and cellular transformation by inositol polyphosphate 4-phosphatase-1. EMBO Rep. 2009;10:487–493. doi: 10.1038/embor.2009.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Westbrook TF, et al. A genetic screen for candidate tumor suppressors identifies REST. Cell. 2005;121:837–848. doi: 10.1016/j.cell.2005.03.033. [DOI] [PubMed] [Google Scholar]
- 12.Gewinner C, et al. Evidence that inositol polyphosphate 4-phosphatase type II is a tumor suppressor that inhibits PI3K signaling. Cancer Cell. 2009;16:115–125. doi: 10.1016/j.ccr.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Naylor TL, et al. High resolution genomic analysis of sporadic breast cancer using array-based comparative genomic hybridization. Breast Cancer Res. 2005;7:R1186–R1198. doi: 10.1186/bcr1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bertucci F, et al. Gene expression profiling identifies molecular subtypes of inflammatory breast cancer. Cancer Res. 2005;65:2170–2178. doi: 10.1158/0008-5472.CAN-04-4115. [DOI] [PubMed] [Google Scholar]
- 15.Sørlie T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98:10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sorlie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci USA. 2003;100:8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Russo J, Ao X, Grill C, Russo IH. Pattern of distribution of cells positive for estrogen receptor α and progesterone receptor in relation to proliferating cells in the mammary gland. Breast Cancer Res Treat. 1999;53:217–227. doi: 10.1023/a:1006186719322. [DOI] [PubMed] [Google Scholar]
- 18.Capuco AV, Ellis S, Wood DL, Akers RM, Garrett W. Postnatal mammary ductal growth: three-dimensional imaging of cell proliferation, effects of estrogen treatment, and expression of steroid receptors in prepubertal calves. Tissue Cell. 2002;34:143–154. doi: 10.1016/s0040-8166(02)00024-1. [DOI] [PubMed] [Google Scholar]
- 19.Rhodes DR, et al. Oncomine 3.0: Genes, pathways, and networks in a collection of 18,000 cancer gene expression profiles. Neoplasia. 2007;9:166–180. doi: 10.1593/neo.07112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rhodes DR, et al. ONCOMINE: A cancer microarray database and integrated data-mining platform. Neoplasia. 2004;6:1–6. doi: 10.1016/s1476-5586(04)80047-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Munday AD, et al. The inositol polyphosphate 4-phosphatase forms a complex with phosphatidylinositol 3-kinase in human platelet cytosol. Proc Natl Acad Sci USA. 1999;96:3640–3645. doi: 10.1073/pnas.96.7.3640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giles GG, English DR. The Melbourne Collaborative Cohort Study. IARC Sci Publ. 2002;156:69–70. [PubMed] [Google Scholar]
- 23.Blows FM, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: A collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7:e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cheang MC, et al. Basal-like breast cancer defined by five biomarkers has superior prognostic value than triple-negative phenotype. Clin Cancer Res. 2008;14:1368–1376. doi: 10.1158/1078-0432.CCR-07-1658. [DOI] [PubMed] [Google Scholar]
- 25.Millar EK, et al. Prediction of local recurrence, distant metastases, and death after breast-conserving therapy in early-stage invasive breast cancer using a five-biomarker panel. J Clin Oncol. 2009;27:4701–4708. doi: 10.1200/JCO.2008.21.7075. [DOI] [PubMed] [Google Scholar]
- 26.López-Knowles E, et al. PI3K pathway activation in breast cancer is associated with the basal-like phenotype and cancer-specific mortality. Int J Cancer. 2010;126:1121–1131. doi: 10.1002/ijc.24831. [DOI] [PubMed] [Google Scholar]
- 27.Millar EK, et al. BAG-1 predicts patient outcome and tamoxifen responsiveness in ER-positive invasive ductal carcinoma of the breast. Br J Cancer. 2009;100:123–133. doi: 10.1038/sj.bjc.6604809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh DS, et al. Estrogen-regulated genes predict survival in hormone receptor-positive breast cancers. J Clin Oncol. 2006;24:1656–1664. doi: 10.1200/JCO.2005.03.2755. [DOI] [PubMed] [Google Scholar]
- 29.Sotiriou C, et al. Breast cancer classification and prognosis based on gene expression profiles from a population-based study. Proc Natl Acad Sci USA. 2003;100:10393–10398. doi: 10.1073/pnas.1732912100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rakha EA, Reis-Filho JS, Ellis IO. Basal-like breast cancer: A critical review. J Clin Oncol. 2008;26:2568–2581. doi: 10.1200/JCO.2007.13.1748. [DOI] [PubMed] [Google Scholar]
- 31.Bachman KE, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–775. doi: 10.4161/cbt.3.8.994. [DOI] [PubMed] [Google Scholar]
- 32.Barbareschi M, et al. Different prognostic roles of mutations in the helical and kinase domains of the PIK3CA gene in breast carcinomas. Clin Cancer Res. 2007;13:6064–6069. doi: 10.1158/1078-0432.CCR-07-0266. [DOI] [PubMed] [Google Scholar]
- 33.Li SY, Rong M, Grieu F, Iacopetta B. PIK3CA mutations in breast cancer are associated with poor outcome. Breast Cancer Res Treat. 2006;96:91–95. doi: 10.1007/s10549-005-9048-0. [DOI] [PubMed] [Google Scholar]
- 34.Maruyama N, et al. Clinicopathologic analysis of breast cancers with PIK3CA mutations in Japanese women. Clin Cancer Res. 2007;13:408–414. doi: 10.1158/1078-0432.CCR-06-0267. [DOI] [PubMed] [Google Scholar]
- 35.Wu G, et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005;7:R609–R616. doi: 10.1186/bcr1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Campbell IG, et al. Mutation of the PIK3CA gene in ovarian and breast cancer. Cancer Res. 2004;64:7678–7681. doi: 10.1158/0008-5472.CAN-04-2933. [DOI] [PubMed] [Google Scholar]
- 37.Bose S, Chandran S, Mirocha JM, Bose N. The Akt pathway in human breast cancer: A tissue-array-based analysis. Mod Pathol. 2006;19:238–245. doi: 10.1038/modpathol.3800525. [DOI] [PubMed] [Google Scholar]
- 38.Pérez-Tenorio G, et al. PIK3CA mutations and PTEN loss correlate with similar prognostic factors and are not mutually exclusive in breast cancer. Clin Cancer Res. 2007;13:3577–3584. doi: 10.1158/1078-0432.CCR-06-1609. [DOI] [PubMed] [Google Scholar]
- 39.Saal LH, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 40.Stemke-Hale K, et al. An integrative genomic and proteomic analysis of PIK3CA, PTEN, and AKT mutations in breast cancer. Cancer Res. 2008;68:6084–6091. doi: 10.1158/0008-5472.CAN-07-6854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Abubaker J, et al. PIK3CA mutations are mutually exclusive with PTEN loss in diffuse large B-cell lymphoma. Leukemia. 2007;21:2368–2370. doi: 10.1038/sj.leu.2404873. [DOI] [PubMed] [Google Scholar]
- 42.Wang Y, et al. High prevalence and mutual exclusivity of genetic alterations in the phosphatidylinositol-3-kinase/akt pathway in thyroid tumors. J Clin Endocrinol Metab. 2007;92:2387–2390. doi: 10.1210/jc.2006-2019. [DOI] [PubMed] [Google Scholar]
- 43.West M, et al. Predicting the clinical status of human breast cancer by using gene expression profiles. Proc Natl Acad Sci USA. 2001;98:11462–11467. doi: 10.1073/pnas.201162998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang F, et al. Laser microdissection and microarray analysis of breast tumors reveal ER-alpha related genes and pathways. Oncogene. 2006;25:1413–1419. doi: 10.1038/sj.onc.1209165. [DOI] [PubMed] [Google Scholar]
- 45.Bhat-Nakshatri P, et al. Tumour necrosis factor and PI3-kinase control oestrogen receptor alpha protein level and its transrepression function. Br J Cancer. 2004;90:853–859. doi: 10.1038/sj.bjc.6601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harvell DME, Richer JK, Allred DC, Sartorius CA, Horwitz KB. Estradiol regulates different genes in human breast tumor xenografts compared with the identical cells in culture. Endocrinology. 2006;147:700–713. doi: 10.1210/en.2005-0617. [DOI] [PubMed] [Google Scholar]
- 47.Lin H-JL, et al. Breast cancer-associated fibroblasts confer AKT1-mediated epigenetic silencing of Cystatin M in epithelial cells. Cancer Res. 2008;68:10257–10266. doi: 10.1158/0008-5472.CAN-08-0288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nielsen TO, et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin Cancer Res. 2004;10:5367–5374. doi: 10.1158/1078-0432.CCR-04-0220. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.