Abstract
Chlamydiae are obligate intracellular bacteria that frequently cause human disease. Chlamydiae replicate in a membranous vacuole in the cytoplasm termed inclusion but have the ability to transport proteins into the host cell cytosol. Chlamydial replication is associated with numerous changes of host cell functions, and these changes are often linked to proteolytic events. It has been shown earlier that the member of the NF-κB family of inflammation-associated transcription factors, p65/RelA, is cleaved during chlamydial infection, and a chlamydial protease has been implicated. We here provide evidence that the chlamydial protease chlamydial protease-like activity factor (CPAF) is responsible for degradation of p65/RelA during infection. This degradation was seen in human and in mouse cells infected with either Chlamydia trachomatis or Chlamydia pneumoniae where it correlated with the expression of CPAF and CPAF activity. Isolated expression of active C. trachomatis or C. pneumoniae CPAF in human or mouse cells yielded a p65 fragment of indistinguishable size from the one generated during infection. Expression of active CPAF in human cells caused a mild reduction in IκBα phosphorylation but a strong reduction in NF-κB reporter activity in response to interleukin-1β. Infection with C. trachomatis likewise reduced this responsiveness. IL-1β-dependent secretion of IL-8 was further reduced by CPAF expression. Secretion of CPAF is, thus, a mechanism that reduces host cell sensitivity to a proinflammatory stimulus, which may facilitate bacterial growth in vivo.
Keywords: Bacteria, Immunology, Inflammation, NF-κB, Protease, Signal Transduction, Chlamydia, Virulence Factor
Introduction
Chlamydia trachomatis causes eye infections on a large scale especially in the developing world (often leading to blindness) and is the most frequent bacterial agent of sexually transmitted disease (1–3). Chlamydia (Chlamydophila) pneumoniae is an extremely common agent of usually mild respiratory infection and may through infection of arteries play a role in the pathogenesis of atherosclerosis (4). One clinically relevant feature of chlamydial infections is the potential for chronicity. A considerable share of female infertility for instance is the result of chronic pelvic inflammatory disease caused by C. trachomatis (3).
Chlamydiae have a life style that is atypical for bacteria. Chlamydiae can only replicate inside human (or animal) cells where they reside in a membrane-bounded vacuole termed inclusion. From this site the bacteria are able to interact with metabolic and signaling pathways of the host cell. Chlamydial infection causes alterations in gene expression, redirects vesicle transport especially to achieve lipid acquisition by the vacuole, blocks apoptosis, and can induce non-apoptotic cell death in human cells (5–7). These results are probably in many cases linked to the ability of Chlamydia to transfer bacterial proteins from the chlamydial inclusion into the host cell; in many cases this may be achieved by a protein injection apparatus known as type III secretion system (8).
A number of the effects on the host cell are caused by proteolysis of host proteins through bacterial proteases that gain access to the cytosol. A prominent role has been shown here for the protease chlamydial protease-like activity factor (CPAF)2 (9), which cleaves, for instance, the cytoskeleton components vimentin and cytokeratin 8 and the nuclear proteins PARP (poly(ADP-ribose) polymerase) and cyclin B1 (10–12).
It has recently been shown that the transcription factor NF-κB p65/RelA is cleaved at a single site during infection of human but not mouse cells with C. trachomatis or C. pneumoniae (13). Expression of candidate chlamydial proteases showed that the tail-specific protease (Tsp) CT441 has the capacity of cleaving p65 (13, 14).
NF-κB is a family of transcription factors with important gene regulatory functions in inflammation (15), and the inhibition of NF-κB by Chlamydia may be a relevant mechanism by which the bacteria counter inflammation and clearance of the infection by the immune response. Indeed, a second mechanism has been proposed by which Chlamydia blocks NF-κB activation, namely the de-ubiquitination of IκBα (16).
The protease CT441 is not known to be secreted from the vacuole to the cytosol, and although a cytosolic function of the protease is not impossible, CT441 is likely to play a role in the processing of bacterial proteins within the inclusion, as has been proposed for other bacterial Tsp (17). CPAF on the other hand is secreted into the cytosol of infected cells, and its expression and secretion occur around mid-cycle of the chlamydial infection (about 20 h post-infection for C. trachomatis), which coincides with the reported cleavage of p65. We, therefore, undertook this study to test whether CPAF is able to cleave p65 and may be responsible for its cleavage during infection. Our results show that CPAF from both C. trachomatis and C. pneumoniae can cleave both human and mouse p65 and can inhibit the activation of NF-κB that occurs in response to external IL-1β. Analysis of p65 cleavage during infection suggests that the observed cleavage of p65 during infection is mediated by CPAF. In addition to other known effects, CPAF may, therefore, contribute to chlamydial replication by subversion of the NF-κB-mediated host defense.
EXPERIMENTAL PROCEDURES
Cell Lines and Cell Culture
The human embryonic kidney cell line T-REx-293, which stably expresses the tetracycline repressor (Invitrogen), was maintained in humidified air at 5% CO2 and 37 °C in Dulbecco modified Eagle's minimal essential medium (DMEM) supplemented with 10% fetal calf serum (FCS, tetracycline negative; PAA Laboratories, Pasching, Austria) and 5 μg/μl blasticidin (PAA Laboratories). T-REx-293 clones stably expressing gyrB-CPAF (T-REx-293 CPAF K6) were generated by electroporation with the pcDNA4/TO/myc-His-gyrB-CPAF construct and antibiotic selection as described before (12). These cells were cultured as above but in the presence of zeocin (InvivoGen, San Diego, CA). Mouse embryonic fibroblasts (MEFs) that had been immortalized by SV40 large T antigen were cultured in DMEM containing 10% FCS and 50 μm 2-mercaptoethanol.
CPAF expression was induced by the addition of 5 ng/μl anhydrotetracycline (AHT; IBA, Göttingen, Germany), and oligomerization was induced by adding 1 μm coumermycin (CM; Sigma). The proteasomal inhibitors epoxomicin (Calbiochem) and clastolactacystin β-lactone (Sigma) were added as indicated.
Chlamydial Infections of Human and Murine Cells
The C. trachomatis strain serovar L2 was obtained from American Type Culture Collection (ATCC). To infect T-REx-293 or MEF cells, the culture medium was replaced with DMEM without FCS and antibiotics before the addition of the bacteria. Chlamydiae were added to a multiplicity of infection (m.o.i.) of three unless otherwise indicated. After 2 h, 10% FCS was added. At indicated time points, cells were harvested and lysed by incubation with radioimmune precipitation assay buffer (1% Triton X-100, 0.5% SDS, 0.5% deoxycholate,1 mm EDTA, 150 mm NaCl, and 50 mm Tris, pH 8.0) supplemented with a protease inhibitor mixture (Roche Applied Science). C. pneumoniae strain CM-1 was obtained from ATCC. Before infection, culture media was removed and changed to infection media consisting of DMEM, C. pneumoniae, and cycloheximide 1 μg/ml. The cells were then centrifuged at 2,000 × g for 35 min at 35 °C.
Transfection of T-REx-293 and MEF Cells
Transient transfections of T-REx-293 and MEF cells with pcDNA4-CPAF and pcDNA3.1myc-HIS A-CT441 (a gift from Dr. Frank Hänel, Leibniz-Institute for Nature Products and Infection Biology, Hans-Knöll-Institute, Jena, Germany) were performed using FuGENE HD (Roche Applied Science) following the manufacturer's instructions. In T-REx-293 cells, CPAF expression was induced by the addition of AHT/CM. Both compounds were added 1 h after transfection. In MEF cells lacking the tet repressor, where induction of the CPAF was not necessary, 1 μm coumermycin was added 1 h after transfection to cluster gyrB-CPAF (12).
Nuclear Fractionation
T-REx-293 CPAF K6 cells (12) were stimulated with 10 ng/ml IL-1β (Peprotech, Hamburg, Germany) for various times. Cells were harvested and washed with PBS. Cells were pelleted (7 min, 1500 rpm, 4 °C) and incubated in ice-cold hypotonic buffer A (10 mm Hepes, 10 mm KCl, 0.1 mm EDTA, 0.1 mm EGTA, 1 mm DTT, pH 7.9) supplemented with a protease inhibitor mixture on ice for 15 min at 4 °C. Cells were lysed by passage through a 26-gauge needle attached to a 1-ml syringe. The nuclei were pelleted by centrifugation for 5 min at 6,800 rpm at 4 °C. The cytosolic fraction was subjected to a second centrifugation step for 15 min at 13,000 rpm at 4 °C, frozen in liquid nitrogen, and stored at −80 °C. The nuclear fraction was washed 8 times in the hypotonic buffer and then incubated in cold buffer B (20 mm Hepes, 0.4 m NaCl, 1 mm EDTA, 1 mm EGTA, 1 mm DTT, pH7.9) supplemented with protease inhibitor mixture and incubated for 45 min at 4 °C and 300 rpm. Finally the nuclear fractions were centrifuged for 15 min at 13,000 rpm, 4 °C. The supernatants were frozen in liquid nitrogen and stored at −80 °C.
Immunoblotting
Cells were harvested after infection or CPAF expression and lysed in radioimmune precipitation assay buffer. Cell extracts were separated using EZ-Run Gels (Fisher), and proteins were transferred onto nitrocellulose membranes. Equivalent amounts of protein were loaded, and equal loading was confirmed by detection of β-actin using a specific antibody (Sigma). Membranes were probed with anti-caspase 8, anti-IκB, anti-phospho-IκB, anti-p42/p44 Erk, anti-phospho-p42/p44 Erk, anti-IKK-α (all from Cell Signaling Technology, Beverly, MA), anti-IKK-β (Upstate Biotechnology, Lake Placid, CA), anti-histone deacetylase 1 (Millipore, Schwalbach, Germany), anti-GAPDH (Chemicon, Temecula, CA), anti-vimentin (Acris, Herford, Germany), anti-p65/RelA (Santa Cruz, Heidelberg, Germany), and anti-CPAF (generated by immunization of rabbits with a peptide from the C-terminal fragment of C. trachomatis CPAF, Pineda, Berlin, Germany) antibodies. Proteins were visualized using peroxidase-conjugated secondary antibodies and a chemiluminescence detection system (GE Healthcare).
NF-κB Reporter Assay
Cells stably expressing CPAF were lentivirally transduced with an NF-κB-luciferase reporter construct obtained from Dr. Hans Häcker (St. Jude Children's Research Hospital, Memphis, TN). 2 × 105 cells were seeded in a 96-well plate and either infected with C. trachomatis or treated with AHT/CM to induce and activate CPAF. For the times indicated, 10 ng/ml IL-1β was added. After various periods of time cells were lysed using luciferase cell culture lysis reagent (Promega, Mannheim, Germany), and firefly luciferase activity was assayed with a luminometer (Berthold Detection Systems, Pforzheim, Germany).
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide (MTT) Assay
Cell viability was tested by the MTT (Sigma) assay. After treatment of the cells with either C. trachomatis, AHT/CM, and/or IL-1β, MTT was added to cells at a concentration of 0.5 mg/μl and incubated at 37 °C for 1 h. The formazan crystals generated in viable cells were dissolved in DMSO, and the optical density at a wavelength of 570 nm was measured using a photometer (Tecan, Crailsheim, Germany).
IL-8 ELISA
T-REx-293 CPAF K6 cells were stimulated with IL-1β (10 ng/ml) to activate NF-κB signaling. Simultaneously, AHT/CM was added to induce and activate CPAF. Supernatants were collected after 8 h, and commercially available ELISA (R&D Systems, Wiesbaden-Nordenstedt, Germany, OptEIATM set from BD Biosciences) was used to determine the concentration of secreted IL-8. The assays were performed as instructed by the manufacturer.
RESULTS
Cleavage of p65 during Infection of Human and Mouse Cells with C. trachomatis or C. pneumoniae
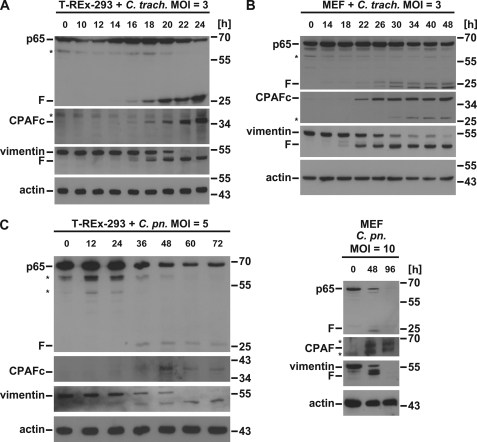
Human 293 cells (we used the variant T-REx-293 for better comparison with CPAF expressing cells; below) or MEFs were infected with C. trachomatis or C. pneumoniae, and p65 levels were followed over time by Western blotting. As reported earlier, p65 fragmentation was observed (13). A fragment of ∼25 kDa appeared in human T-REx-293 cells between 16 and 18 h upon infection with C. trachomatis (Fig. 1A). As for the cleavage of other cellular substrates, the time course of cleavage varied with infectious dose (higher bacterial numbers caused earlier and stronger degradation; not shown). The time course of p65 cleavage correlated well with the appearance of active CPAF and with the cleavage of cellular vimentin, a well known CPAF substrate (Fig. 1A). Similar results were obtained when MEFs were used for infection with the exception that two fragments of similar size appeared (Fig. 1B). This latter finding is in contrast with the earlier report that found cleavage only in human but not mouse cells (13). The discrepancy may be explained by the use of different chlamydial strains or different infectious doses. In our model, there was again a good correlation between the appearance of active CPAF and the degradation of both vimentin and p65 from mouse. Infection of either human or mouse cells with C. pneumoniae caused the appearance of similar fragments (Fig. 1C), suggesting that p65 is cleaved by a chlamydial protease that is conserved between the species.
FIGURE 1.
Infection with C. trachomatis causes cleavage of p65. A, C. trachomatis infection leads to p65 cleavage in T-REx-293 cells. Cells were infected with C. trachomatis with an m.o.i. of 3 for the times indicated. Cell lysates were subjected to Western blot analysis for p65, CPAF, or the CPAF substrate vimentin. Shown is a representative result of five independent experiments. F, cleavage products of p65; *, unspecific background bands. CPAFc, C-terminal autoprocessing product of CPAF. B, in MEF cells C. trachomatis infection leads to p65 cleavage. Mouse embryonic fibroblasts were infected with C. trachomatis with an m.o.i. of 3 as indicated. Radioimmune precipitation assay buffer extracts were prepared and subjected to Western blot analysis for p65, CPAF, or vimentin. Shown is a representative result of four independent experiments. C, C. pneumoniae infection causes p65 cleavage in both in T-REx-293 and MEF cells. Cells were infected with C. pneumoniae (m.o.i. of 5 for T-REx-293 or 10 for MEF) for the time periods indicated. Cell lysates were subjected to Western blot analysis for p65, CPAF, or the CPAF substrate vimentin. Shown is a representative result of three independent experiments.
CPAF Can Cause the Degradation of p65 in the Absence of Infection
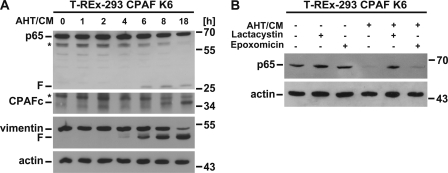
CPAF is in bacteria synthesized as an inactive precursor, and its activation requires its proteolytic autoprocessing. When expressed in human cells, CPAF is inactive, but its activation can be induced by clustering of the precursor (12, 18). We used a system of CPAF activation by induced dimerization in human cells. In this system a fusion protein of bacterial gyrase B and the CPAF precursor is expressed in T-REx-293 cells under the control of a tetracycline-inducible promoter (“tet-on”). Dimerization of the fusion protein is induced by the compound coumermycin, which can bind two gyrase B-molecules and thereby dimerize gyrase B-CPAF; this process leads to autoprocessing and activation of CPAF (12).
We expressed and activated CPAF in this way and again monitored p65 levels by Western blotting. As shown in Fig. 2A, active CPAF (visible as the C-terminal autoprocessing product) was detectable after 6–8 h of CPAF-induction/dimerization. This correlated with the known cleavage of vimentin but also with the appearance of a p65 fragment of the same size as during infection. Disappearance of p65 was blocked by lactacystin (an inhibitor of CPAF and the cellular proteasome) but not by epoxomicin (a specific proteasome inhibitor) (Fig. 2B).
FIGURE 2.
CPAF mediates p65 cleavage. A, p65 is cleaved in cells expressing active CPAF. T-REx-293 CPAF K6 cells (CPAF K6) were induced to express active CPAF with 5 ng/ml AHT and 1 μm CM for the times indicated. Lysates were subjected to Western blotting. Shown is a representative result of three independent experiments. F, CPAF-specific cleavage products; *, unspecific background bands. CPAFc, C-terminal autoprocessing product of CPAF. B, Inhibition of CPAF activity blocks p65 cleavage. T-REx-293 CPAF K6 cells were pretreated with the proteasomal inhibitors lactacystin (25 μm) or epoxomicin (2.5 μm) for 30 min. before CPAF was induced with AHT and CM. After eight h of induction cells were harvested and subjected to Western blotting. Shown is a representative result of six independent experiments.
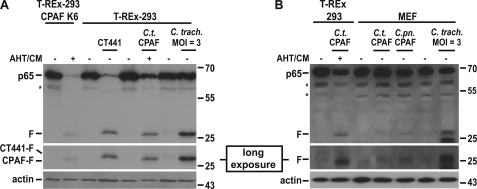
Transient transfection and activation of C. trachomatis CPAF caused the appearance of the same p65 fragment. In these experiments we also transfected CT441 for comparison. As shown in Fig. 3A, CT441 likewise induced degradation of p65 as reported. However, the fragment produced by CT441 was, although similar in size, appreciably bigger than the fragment generated by either CPAF transfection or by infection with C. trachomatis, suggesting that during infection it is CPAF that degrades p65. When transfected into MEFs, C. trachomatis CPAF also caused the appearance of a p65 fragment that ran slightly higher in the SDS-PAGE than the one in human T-REx-293 cells (Fig. 3B). Transfection of CPAF from C. pneumoniae (Cpn CPAF) produced a fragment of indistinguishable size (Fig. 3B). Cleavage of p65, therefore, appears to be conserved between by Ct and Cpn CPAF as well as between human and mouse cells.
FIGURE 3.
p65 cleavage by chlamydial proteases. A, transient transfection of T-REx-293 cells with CPAF or CT 441 is shown. Cells were transfected with either CPAF-1xgyrB-myc or the chlamydial protease CT 441. After 48 h cells were lysed and subjected to Western blotting. As the CPAF-1xgyrB-myc is active in T-REx-293 cells only in the presence of AHT and CM, these were added at the time of transfection. Left two lanes, CPAF-gyrB was induced and activated with AHT/CM in T-REx-293 cells (stably carrying the construct under the control of the tet-responsive promoter). F, CPAF-specific cleavage products; *, unspecific background bands. B, transient transfection of MEF cells with CPAF is shown. MEF cells were transfected for 48 h, lysed, and subjected to Western blotting. As controls, CPAF-1xgyrB-myc transfected T-REx-293 cells (left two lanes) and C. trachomatis infected cells (right lane) were used. Lysates were subjected to Western blot analysis. Shown is a representative result of nine independent experiments.
CPAF Inhibits the Activation of NF-κB by IL-1β
To test for the functional relevance of CPAF-mediated p65-cleavage, we analyzed key steps in the activation of NF-κB in cells expressing active CPAF. T-REx-293-CPAF cells were stimulated with IL-1β, which activates the classical NF-κB-pathway through the IL-1-receptor, its adapter MyD88, and the activation of the IκB kinase IKK-β. Phosphorylated IκB is then degraded, releasing active p65, which translocates to the nucleus and activates NF-κB-dependent promoters, typically together with the NF-κB family member NF-κB1/p50 (15).
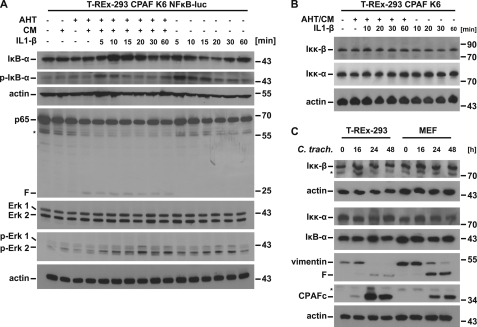
In cells expressing active CPAF, phosphorylation and consecutive degradation of IκBα upon stimulation with IL-1β were reduced although still clearly detectable, whereas the IL-1β-dependent phosphorylation of the mitogen-activated protein kinases Erk1/2 was normal (Fig. 4A). This may reflect a destabilization of the IκBα-p65 complex due to the detectable degradation of p65 (Fig. 4A). There was no reduction in the levels of the IκB kinases IKKα/β either upon expression of active CPAF or during infection with C. trachomatis (Fig. 4, B and C). This suggests that the upstream pathway of MyD88-dependent signaling to the activation of the IKK-complex is unaffected. This is in accordance with the interpretation that it is specifically p65 and p65-dependent transcriptional activity that is targeted by CPAF during infection.
FIGURE 4.
NF-κB signaling upstream of p65/RelA remains functional during expression of active CPAF. A, phosphorylation of IκB-α upon stimulation with IL-1β is shown. T-REx-293 CPAF K6 NFκB-luc cells were treated with IL-1β (10 ng/ml) for the times indicated. In some aliquots of cells, active CPAF had been expressed by stimulation with AHT/CM for 7 h before the addition of IL-1β. Cells were lysed and subjected to Western blotting for IκB-α, phospho-IκB-α, Erk1/2 and phospho-Erk1/2. F, CPAF-specific cleavage products; *, unspecific background bands. Shown is a representative result of six independent experiments. B, IKK protein levels remain unchanged during expression of active CPAF. CPAF expressing cells (7 h after addition of AHT/CM) were treated with IL-1β (10 ng/ml) for the times indicated. Cell lysates were subjected to Western blotting. Shown is a representative result of five independent experiments. C, IKK protein levels remain unchanged after chlamydial infection. T-REx-293 cells or MEF cells were infected with C. trachomatis (m.o.i. = 3) for the times indicated. Cell extracts were analyzed by Western blotting. Shown is a representative result of three independent experiments.
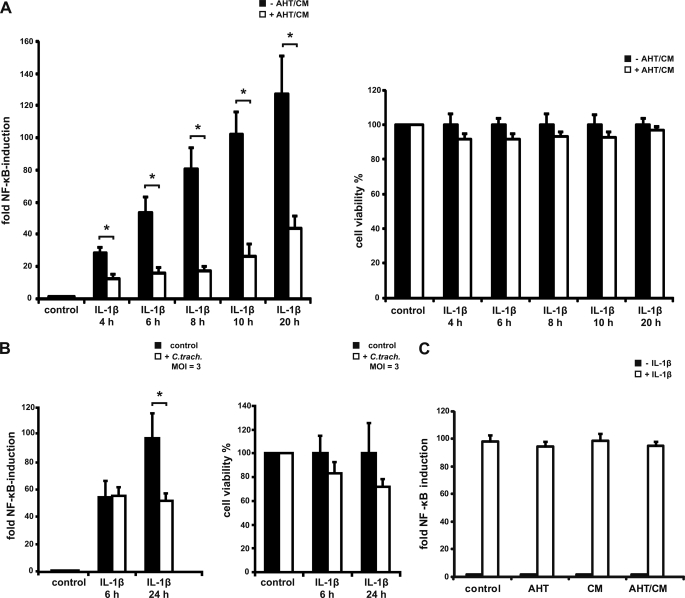
To test for this activity directly, we used T-REx-293-CPAF cells that had been transduced with a lentivirus carrying an NF-κB-luciferase reported plasmid. In these cells the expression of active CPAF was induced as above for 8 h, and IL-1β was added for various periods of time. As shown in Fig. 5A, in the absence of CPAF, IL-1β induced a strong NF-κB-response as expected. When active CPAF was present, IL-1β-dependent induction was substantially reduced. As prolonged expression of active CPAF can induce cell death under these conditions, we controlled viability of the cells in parallel and found only very minor effects on viability under these conditions (Fig. 5A, right panel). A similar reduction in IL-1β-dependent NF-κB activation was detectable at later time points (i.e. upon prolonged stimulation with IL-1β) (Fig. 5A). Treatment of T-REx-293 NF-κB-luciferase cells which do not carry the CPAF construct, with AHT and CM had no effect on reporter activity (Fig. 5C).
FIGURE 5.
CPAF expression impairs NF-κB signaling. A, reduced NF-κB activation in T-REx-293 CPAF K6 NFκB-luc cells is shown. CPAF K6 cells were stably transduced with an NF-κB luciferase reporter gene construct using a lentiviral delivery system. Cells were stimulated with 10 ng/ml IL-1β for the times indicated. This treatment was carried out in the presence (induced for a period of 8 h with AHT/CM; AHT/CM were added either 4 h before (labeled 4 h), 2 h before (6 h), concurrently with IL-1β stimulation (8 h), and after 2 and 12 h of IL-1β stimulation (10 h, 20 h)) or in the absence of active CPAF. Cells were lysed, and luciferase activity was measured. Relative induction of NF-κB activity is shown (the base line is untreated cells). As a viability control, MTT assays were performed in cells treated the same way. Relative cell viability was calculated (untreated cells served as the base line). Data are the means/S.E. of five independent experiments. *, p value < 0.05 (Student's t test). B, reduced NF-κB activation in C. trachomatis-infected cells is shown. NF-κB reporter cells were infected with C. trachomatis with an m.o.i. = 3 for the times indicated. Aliquots were simultaneously stimulated with IL-1β (10 ng/ml). Both luciferase assay and MTT assay were performed as above. Data are the means/S.E. of three independent experiments. *, p value < 0.05 with Student's t test. C, NF-κB signaling is not affected by AHT or CM. AHT and CM have no effect on NF-κB signaling in the absence of CPAF. T-REx-293 cells were stably transduced with an NF-κB-luciferase reporter gene construct using a lentiviral delivery system. Cells were stimulated with 10 ng/ml IL-1β for 8 h. This treatment was carried out in the presence of AHT (5 ng/ml), CM (1 μm), or a combination of both. Cells were lysed, and luciferase activity was measured. Relative induction of NF-κB activity is shown (the base line was untreated cells). Data are the means/S.E. of three independent experiments.
When the T-REx-293-CPAF NF-κB-luciferase cells were infected with C. trachomatis simultaneously to the addition of IL-1β, there was no effect of Chlamydia at 6 h post-infection (Fig. 5B). However, when luciferase activity was measured 24 h after infection, the activation of NF-κB was again reduced (active CPAF is detectable after 16–18 h under these conditions; Fig. 1). Although it is difficult to gauge the possible effect of bacterial factors other than CPAF during infection (especially as the viability of the cells is also affected), the magnitude of the reduction during infection was not higher than during activation of CPAF in uninfected cells (compare Fig. 5, A and B). This is in accordance with the interpretation that CPAF is the main factor that Chlamydia uses to down-regulate the response to NF-κB-inducing stimuli.
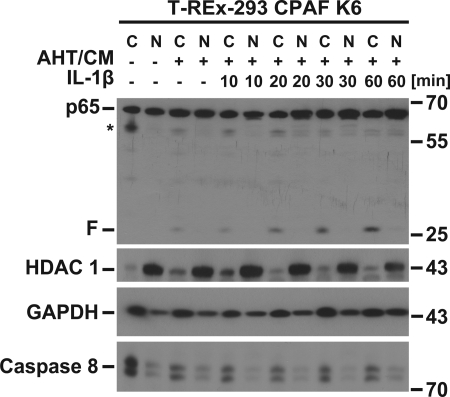
The p65 fragment generated by active CPAF (the antibody detects the C-terminal fragment) remained exclusively cytosolic during stimulation with IL-1β (Fig. 6). This fragment contains the NF-κB-activating domain but lacks the nuclear localization domain (19). The observed effects (no nuclear translocation and lack of reporter activity) are in accordance with these structural features.
FIGURE 6.
The CPAF-dependent fragment of p65 remains cytosolic. The p65 protein fragment does not translocate into the nucleus upon IL-1β stimulation. CPAF was induced and activated in T-REx-293 CPAF K6 cells for 7 h before cells were treated with IL-1β (10 ng/ml) for the times indicated. Cytosolic and nuclear extracts were prepared and subjected to Western blot analysis. C, cytosol; N, nucleus. Histone deacetylase 1 (HDAC 1) was used as a nuclear marker; GAPDH and caspase-8 were used as cytosolic markers. Shown is a representative result of four independent experiments.
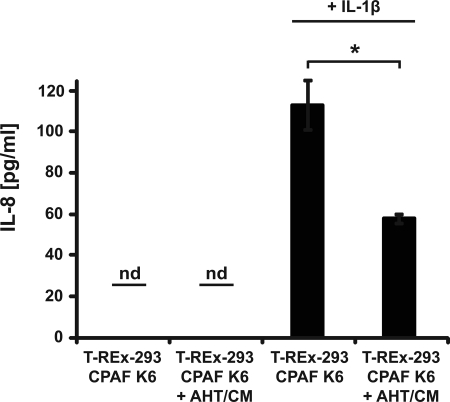
As a functional test of the relevance of this reduction in NF-κB activation by CPAF, we determined its effect on the NF-κB-dependent secretion of IL-8 upon IL-1β stimulation (20). We treated T-REx-293-CPAF cells with IL-1β for 8 h and induced and activated CPAF at the same time. Supernatants were analyzed for secreted IL-8. Activation of CPAF led to a clear reduction in the amounts of secreted IL-8 (Fig. 7). Taken together, these data suggest that the CPAF-dependent degradation of p65 and the consecutive reduction in NF-κB activity during infection with human or mouse cells with Chlamydia inhibits a proinflammatory response of infected host cells.
FIGURE 7.
IL-1β-dependent IL-8 secretion is diminished upon CPAF activation. CPAF expression and activation leads to a reduction of IL-1β-dependent IL-8-secretion. T-REx-293 CPAF K6 NF-κB cells were stimulated with 10 ng/ml IL-1 β. Some aliquots of cells were at the same time stimulated with AHT/CM to express active CPAF. Secretion of IL-8 was determined by ELISA after 8 h. Data are the means ± S.E. of three independent experiments. nd, not detected. *, p value < 0.05 (Student's t test.).
DISCUSSION
Many bacteria have the ability to interfere with host signaling pathways. Chlamydia is well known for its capacity to affect gene transcription in the host; it has a type III secretion system, which it can use to inject bacterial proteins into the host cell cytoplasm, and proteolysis of host cell proteins is a recognized way by which Chlamydia exerts effects on the host cell (21). The majority of these proteolytic events are mediated by CPAF, which translocates from the inclusion into the cytosol by an unknown mechanism (9). It has been demonstrated that p65 is cleaved during chlamydial infection and that p65 can be cleaved by the Tsp CT441. Expression of CT441 inhibited TNF-induced activation of NF-κB (13, 14).
Our data show that both C. trachomatis and C. pneumoniae CPAF can cleave p65 and suggest that CPAF is the protease that is responsible for this cleavage during infection. Although CPAF was tested in the earlier study and was found not to cleave p65 (13), this was done by transient expression of CPAF. It is now clear that CPAF is not active when expressed in that way but requires intermolecular clustering for its activation (12, 18, 22). This peculiarity explains the lack of activity of CPAF in those experiments. CT441 was further shown not to cleave mouse p65 (13, 14), whereas CPAF did (this study). However, although the previous studies also failed to detect p65 cleavage during C. trachomatis infection of mouse cells, we did detect this cleavage. Infection and growth of Chlamydia in mammalian cells can vary strongly between host cells, and it is plausible that the lack of detectable p65 cleavage was the result of less efficient growth of C. trachomatis in the NIH 3T3 cells used (13). We find clear, albeit somewhat weaker cleavage of mouse p65 compared with human p65; because recombinant CT441 can cleave human but not mouse p65, this profile, therefore, also argues for CPAF as the protease responsible. Furthermore, the p65 cleavage product generated during infection is indistinguishable on SDS-PAGE from the product generated by CPAF cleavage but was slightly smaller than the product generated by expression of CT441. Last, although CPAF is clearly secreted into the cytosol, there is no reported evidence that CT441 is released from the vacuole into the host cell. Together, we believe that this is strong evidence that the cleavage of p65 during chlamydial infection is mediated by CPAF.
It is perhaps surprising that two chlamydial proteases, CPAF and CT441, have the ability to cleave at least human p65. However, both proteases have structural features of Tsp (although only CT441 has the typical PDZ-domain), and this may explain this related cleavage specificity. Tsp are a relatively little characterized group of bacterial proteases (23, 24) and are usually involved in processing steps of bacterial proteins (25, 26). CT441 may, therefore, have important functions in growth and development of Chlamydia in the inclusion. Although this may also be the case for CPAF, a number of host cell substrates have been identified that are cleaved by active CPAF and that are cleaved during infection (21). The precise role of CPAF for chlamydial development is not clear. Effects of CPAF activity include impairment in antigen presentation (cleavage of transcription factors (9) and the major histocompatibility complex-like molecule CD1d (27)) and the cleavage of cytoskeletal proteins (vimentin, cytokeratin-8 (10, 11)). Any of these events may contribute to the success of Chlamydia in surviving and replicating in human cells.
However, the phylum Chlamydiae does not only include the human-pathogenic chlamydiae such as C. trachomatis and C. pneumoniae but also a large group of bacteria that have been found as symbionts in free-living amoebae (28). Notably, these “environmental chlamydiae” can also contain genes coding for CPAF (29). It may, therefore, be speculated that CPAF is an enzyme that has already been required for replication of Chlamydia-like organisms (the evolutionary precursor of today's bacteria) in single-celled hosts. Some core CPAF functions may be conserved to all these Chlamydia-like organisms, and some may have been acquired for survival in metazoan hosts, and p65 cleavage would be an example of the latter category.
NF-κB is an important transcription factor family in the immune response, and it is likely that impaired NF-κB signaling will be of benefit to Chlamydia. During the infection and the accompanying inflammation, a broad array of inflammatory mediators is produced that also signal to the infected cells. As in these cells, NF-κB activation will be impaired; this inhibition can be expected to limit the defense response of the infected cell and to enhance replication of Chlamydia. CPAF may, therefore, have adopted a new function in human pathogenic chlamydiae, which adds to the ability of this group of bacteria to survive for a sufficient time to replicate despite a functional immune system. This novel function may even contribute to the ability of the bacteria to survive the host response and to proceed into the chronic state of the infection.
Acknowledgments
We thank Dr. Frank Hänel, Jena, Germany for providing the CT441-expression plasmid and Dr. Hans Häcker, Memphis, TN for help with the generation of the NF-κB-luciferase reporter cell lines.
This work was supported by the Else-Krömer-Fresenius-Stiftung and the Deutsche Forschungsgemeinschaft (to G. H.).
- CPAF
- chlamydial protease-like activity factor
- NF-κB
- nuclear factor κ light chain enhancer of activated B cells
- IκBα
- nuclear factor κ light polypeptide gene enhancer in B cells inhibitor α
- IKK
- IκB kinase
- Tsp
- tail-specific protease
- AHT
- anhydrotetracycline
- CM
- coumermycin
- m.o.i.
- multiplicity of infection
- MTT
- 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
- MEF
- mouse embryonic fibroblast.
REFERENCES
- 1.Gerbase A. C., Rowley J. T., Mertens T. E. (1998) Lancet 351, 2–4 [DOI] [PubMed] [Google Scholar]
- 2.Miller W. C., Ford C. A., Morris M., Handcock M. S., Schmitz J. L., Hobbs M. M., Cohen M. S., Harris K. M., Udry J. R. (2004) JAMA 291, 2229–2236 [DOI] [PubMed] [Google Scholar]
- 3.Belland R., Ojcius D. M., Byrne G. I. (2004) Nat. Rev. Microbiol. 2, 530–531 [DOI] [PubMed] [Google Scholar]
- 4.Belland R. J., Ouellette S. P., Gieffers J., Byrne G. I. (2004) Cell. Microbiol. 6, 117–127 [DOI] [PubMed] [Google Scholar]
- 5.Moulder J. W. (1991) Microbiol. Rev. 55, 143–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyrick P. B. (2000) Cell. Microbiol. 2, 275–282 [DOI] [PubMed] [Google Scholar]
- 7.Byrne G. I., Ojcius D. M. (2004) Nat. Rev. Microbiol. 2, 802–808 [DOI] [PubMed] [Google Scholar]
- 8.Peters J., Wilson D. P., Myers G., Timms P., Bavoil P. M. (2007) Trends Microbiol. 15, 241–251 [DOI] [PubMed] [Google Scholar]
- 9.Zhong G., Fan P., Ji H., Dong F., Huang Y. (2001) J. Exp. Med. 193, 935–942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong F., Su H., Huang Y., Zhong Y., Zhong G. (2004) Infect. Immun. 72, 3863–3868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar Y., Valdivia R. H. (2008) Cell Host Microbe 4, 159–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paschen S. A., Christian J. G., Vier J., Schmidt F., Walch A., Ojcius D. M., Häcker G. (2008) J. Cell Biol. 182, 117–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lad S. P., Li J., da Silva Correia J., Pan Q., Gadwal S., Ulevitch R. J., Li E. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 2933–2938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lad S. P., Yang G., Scott D. A., Wang G., Nair P., Mathison J., Reddy V. S., Li E. (2007) J. Bacteriol. 189, 6619–6625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Häcker H., Karin M. (2006) Sci. STKE 2006, re13. [DOI] [PubMed] [Google Scholar]
- 16.Le Negrate G., Krieg A., Faustin B., Loeffler M., Godzik A., Krajewski S., Reed J. C. (2008) Cell. Microbiol. 10, 1879–1892 [DOI] [PubMed] [Google Scholar]
- 17.Silber K. R., Keiler K. C., Sauer R. T. (1992) Proc. Natl. Acad. Sci. U.S.A. 89, 295–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang Z., Feng Y., Chen D., Wu X., Huang S., Wang X., Xiao X., Li W., Huang N., Gu L., Zhong G., Chai J. (2008) Cell Host Microbe 4, 529–542 [DOI] [PubMed] [Google Scholar]
- 19.Ghosh S., May M. J., Kopp E. B. (1998) Annu. Rev. Immunol. 16, 225–260 [DOI] [PubMed] [Google Scholar]
- 20.Mukaida N., Mahe Y., Matsushima K. (1990) J. Biol. Chem. 265, 21128–21133 [PubMed] [Google Scholar]
- 21.Zhong G. (2009) Trends Microbiol. 17, 467–474 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong F., Pirbhai M., Zhong Y., Zhong G. (2004) Mol. Microbiol. 52, 1487–1494 [DOI] [PubMed] [Google Scholar]
- 23.Paetzel M., Dalbey R. E. (1997) Trends Biochem. Sci. 22, 28–31 [DOI] [PubMed] [Google Scholar]
- 24.Liao D. I., Qian J., Chisholm D. A., Jordan D. B., Diner B. A. (2000) Nat. Struct. Biol. 7, 749–753 [DOI] [PubMed] [Google Scholar]
- 25.Hara H., Yamamoto Y., Higashitani A., Suzuki H., Nishimura Y. (1991) J. Bacteriol. 173, 4799–4813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ostberg Y., Carroll J. A., Pinne M., Krum J. G., Rosa P., Bergström S. (2004) J. Bacteriol. 186, 2074–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kawana K., Quayle A. J., Ficarra M., Ibana J. A., Shen L., Kawana Y., Yang H., Marrero L., Yavagal S., Greene S. J., Zhang Y. X., Pyles R. B., Blumberg R. S., Schust D. J. (2007) J. Biol. Chem. 282, 7368–7375 [DOI] [PubMed] [Google Scholar]
- 28.Horn M. (2008) Annu. Rev. Microbiol. 62, 113–131 [DOI] [PubMed] [Google Scholar]
- 29.Horn M., Collingro A., Schmitz-Esser S., Beier C. L., Purkhold U., Fartmann B., Brandt P., Nyakatura G. J., Droege M., Frishman D., Rattei T., Mewes H. W., Wagner M. (2004) Science 304, 728–730 [DOI] [PubMed] [Google Scholar]