Abstract
The arabinogalactan (AG) of slow growing pathogenic Mycobacterium spp. is characterized by the presence of galactosamine (GalN) modifying some of the interior branched arabinosyl residues. The biosynthetic origin of this substituent and its role(s) in the physiology and/or pathogenicity of mycobacteria are not known. We report on the discovery of a polyprenyl-phospho-N-acetylgalactosaminyl synthase (PpgS) and the glycosyltransferase Rv3779 from Mycobacterium tuberculosis required, respectively, for providing and transferring the GalN substrate for the modification of AG. Disruption of either ppgS (Rv3631) or Rv3779 totally abolished the synthesis of the GalN substituent of AG in M. tuberculosis H37Rv. Conversely, expression of ppgS in Mycobacterium smegmatis conferred upon this species otherwise devoid of ppgS ortholog and any detectable polyprenyl-phospho-N-acetylgalactosaminyl synthase activity the ability to synthesize polyprenyl-phospho-N-acetylgalactosamine (polyprenyl-P-GalNAc) from polyprenyl-P and UDP-GalNAc. Interestingly, this catalytic activity was increased 40–50-fold by co-expressing Rv3632, the encoding gene of a small membrane protein apparently co-transcribed with ppgS in M. tuberculosis H37Rv. The discovery of this novel lipid-linked sugar donor and the involvement of a the glycosyltransferase C-type glycosyltransferase in its transfer onto its final acceptor suggest that pathogenic mycobacteria modify AG on the periplasmic side of the plasma membrane. The availability of a ppgS knock-out mutant of M. tuberculosis provides unique opportunities to investigate the physiological function of the GalN substituent and the potential impact it may have on host-pathogen interactions.
Keywords: Bacterial Genetics, Glycosylation, Membrane Enzymes, Membrane Lipids, Polysaccharide, Mycobacterium, Arabinogalactan, Galactosamine, Tuberculosis
Introduction
Galactosamine (d-GalN) was recognized as a minor covalently bound amino sugar component of the cell wall of slow growing mycobacteria (Mycobacterium tuberculosis H37Ra, Mycobacterium lepraemurium, Mycobacterium microti) as early as in the 1970s (1–3). Advances in mass spectrometry later coupled with the use of an arabinanase isolated from Mycobacterium smegmatis allowed the GalN substituent to be mapped to the arabinogalactan (AG)7 component of the cell wall (3), and more specifically to the C2 position of a portion of the internal 3,5-branched d-Araf residues in the AG of M. tuberculosis (4, 5). d-GalN was estimated to occur at the level of about one residue per entire AG molecule (3–5). Interestingly, a similar sugar residue was found to substitute the AG of Mycobacterium avium, Mycobacterium kansasii, Mycobacterium bovis BCG (3) and Mycobacterium leprae,8 but not that of M. smegmatis, M. neoaurum, and M. phlei (3–5), suggesting that fast growing Mycobacterium spp. are devoid of GalN substituent.
The function and biosynthetic origin of this substituent are not known. Non-N-acetylated hexosamine residues, particularly GalN residues, are rather uncommon in prokaryotes. Thus far, they essentially have been described as components of the lipopolysaccharide (LPS) of some Gram-negative bacteria (6–9). In Francisella tularensis and subsp. novicida for instance, GalN covalently modifies the lipid A moiety of LPS neutralizing its negative charge and enhancing the virulence of the bacterium in mice (8, 9). In Gram-positive bacteria, GalN and glucosamine (GlcN) have been reported in the glycosylation motif of a glycoprotein toxin from Bacillus thuringiensis subsp. israelensis and proposed to be important to the biological activities of the toxin (10). GalN was also identified as a possible component of a galactose-containing polysaccharide from Streptococcus mutans (11).
Recent studies have partially elucidated the biosynthetic origin of the d-GalN unit modifying the lipid A of Francisella (8, 9, 12). It was shown to originate in the lipid-linked sugar donor, undecaprenyl phosphate-β-d-N-acetylgalactosamine which, upon deacetylation by a specialized deacetylase, yields undecaprenyl phosphate-β-d-galactosamine. This is then used as the d-GalN donor in a glycosyl transfer reaction onto lipid A catalyzed by the membrane-associated glycosyltransferase FlmK (8, 9, 12). This process is reminiscent of that involved in the addition of aminoarabinose to lipid A in enteric bacteria where an enzyme (termed ArnC in Escherichia coli) catalyzes the synthesis of undecaprenyl-phospho-N-formyl-aminoarabinose from UDP-N-formyl-aminoarabinose, and a glycosyltransferase (ArnT in E. coli) catalyzes the transfer of the deformylated aminoarabinose residue from this lipid donor onto lipid A (13).
Numerous glycosylation events in the biosynthesis of M. tuberculosis cell wall and other glycoconjugates are known to occur on the periplasmic face of the inner membrane, catalyzed by membrane-associated glycosyltransferases of the GT-C superfamily dependent on decaprenyl-phosphate-linked sugar donors rather than nucleotide-sugar donors (for a review, see Refs. 14–16). However, to date, the known polyprenyl-based sugar donors of Mycobacterium spp. only include β-d-glucosyl-1-monophosphoryl-decaprenol, β-d-mannosyl-1-monophosphoryl-decaprenol, β-d-ribosyl-1-monophosphoryl-decaprenol, and β-d-arabinofuranosyl-1-monophosphoryl-decaprenol (17–19). To the best of our knowledge, polyprenyl-linked galactosamine or N-acetylgalactosamine has never been reported in mycobacteria. This study reports on the discovery of the biosynthetic pathway for the synthesis of lipid-linked-d-GalNAc in mycobacteria and subsequent d-GalNAc or d-GalN transfer onto AG.
EXPERIMENTAL PROCEDURES
Bacterial Strains and Growth Conditions
M. tuberculosis H37Rv ATCC 25618 and H37Rv TMC102 were grown in Sauton's and Middlebrook 7H9 medium. M. smegmatis mc2155 was grown in 7H9 or LB medium. Kanamycin (kan) and hygromycin were added to final concentrations of 20 μg ml−1 and 50 μg ml−1, respectively.
M. tuberculosis Knock-out Mutants
The Ts/sacB method was used to achieve allelic replacement at the Rv3631 (ppgS) locus of M. tuberculosis H37Rv (20). The ppgS gene and flanking regions was PCR-amplified from M. tuberculosis H37Rv genomic DNA with primers Rv3631.5 (5′-ttggtacctctagacccgcaccgtggtcg-3′) and Rv3631.6 (5′-ttgaattccagatcaacgcgtcgccc-3′) and a disrupted allele, ppgS::kan, was obtained by inserting the Tn903 kanamycin resistance cassette at the FseI restriction site of ppgS. ppgS::kan was then cloned into the XbaI-cut and blunt-ended pPR27-xylE (20), yielding pPR27ppgSKX. The Rv3779 knock-out mutant of M. tuberculosis H37Rv TMC102 and complemented mutant strains were described elsewhere (21). The mshB knock-out mutant of M. tuberculosis Erdman (22) was kindly provided by Drs. Fahey and Newton (University of California San Diego), and the mca transposon mutant of M. tuberculosis CDC1551 was obtained from CSU TB Vaccine Testing and Research Materials Contract (National Institutes of Health,NIAID N01-AI-40091).
Expression of ppgS and Rv3632 in M. smegmatis
The entire coding sequences of ppgS and Rv3632 were PCR-amplified from M. tuberculosis H37Rv genomic DNA and cloned into the expression vector pVV16 (23), yielding pVVppgS and pVVRv3632, respectively. pVVppgS-Rv3632 was generated by amplifying the ppgS and Rv3632 ORFs together and cloning the resulting PCR fragment into the same expression plasmid. These constructs allow the constitutive expression of ppgS, Rv3632, or both genes under control of the hsp60 promoter. Primer sequences are available upon request.
Enzyme Assays
Membranes from M. smegmatis mc2155 were prepared as described (24) and resuspended in 50 mm MOPS buffer (pH 7.9) (buffer A). The typical reaction mixture for the production of polyprenyl-P-d-[3H]GalNAc from UDP-α-d-[3H]GalNAc by M. smegmatis membranes contained 0.5 μCi of UDP-α-d-[3H]GalNAc (specific activity 20 Ci mmol−1; American Radiolabeled Chemicals, Inc., St. Louis, MO), 5 mm 2-mercaptoethanol, 10 mm MgCl2, 0.1 mm ATP, 0.1 mm NADH, membrane fraction (0.5 mg of proteins), and buffer A in a final volume of 80 μl; thus, the membranes were the source of the endogenous polyprenyl-P acceptors. After incubation at 37 °C, the reactions were stopped by addition of 1.2 ml of CHCl3/CH3OH/NH4OH (2:1:0.05, v/v). The upper aqueous phase was discarded, and the bottom organic phase was backwashed with CHCl3/CH3OH/H2O (3:47:48). The dried organic phase was dissolved in 50 μl of CHCl3/CH3OH/NH4OH/H2O (65:25:0.5:3.6) prior to TLC analysis on aluminum-backed silica gel 60-precoated plates F254 (Merck, Darmstadt, Germany).
Capillary Electrophoresis
Purified AG (10 μg) was partially degraded by controlled acid hydrolysis (0.1 m HCl for 20 min at 110 °C), and the oligosaccharides liberated were then tagged with the fluorescent probe 8-aminopyrene-1,3,6-trisulfonate (APTS) as described earlier (25). Labeled oligosaccharides were then analyzed by capillary electrophoresis monitored by laser-induced fluorescence (CE-LIF) on a P/ACE 5000 instrument (Beckman Instruments, Inc.). Separations were performed using an uncoated fused-silica capillary column (Sigma) of 50-μm internal diameter and 40-cm effective length (47-cm total length). Analyses were carried out at a temperature of 25 °C, in the reverse mode, with an applied voltage of 20 kV using 1% acetic acid (w/v)-30 mm triethylamine, pH 3.5, as a running electrolyte (25). Picopreparative CE was performed as described previously (26).
MALDI-TOF/MS and MS/MS
Analyses were performed on a 4700 Proteomics Analyzer (with TOF/TOF optics, Applied Biosystems, Voyager DE-STR) in the reflectron mode. The matrix used was 2,5-dihydroxybenzoic acid (10 μg μl−1 in a mixture of ethanol/water (1:1, v/v)). 0.3 μl of the CE-collected oligosaccharides resuspended in 10 μl of water or TLC-extracted lipids resuspended in 10 μl CHCl3/CH3OH (2:1) was mixed with 0.3 μl of the matrix solution. Mass spectra were recorded in the negative mode. The collision-induced dissociation gas type for MS/MS experiments was atmosphere, and the gas pressure was set to medium.
RESULTS
Disruption of ppgS in M. tuberculosis H37Rv
Rv3631 (thereafter renamed ppgS) is predicted to encode a GT-A-fold glycosyltransferase (GT) (14, 15) and, according to the Carbohydrate-Active enZymes classification of glycosyltransferases (27), to belong to the GT-2 family of inverting GTs dependent on NDP-sugars as donor substrates. Orthologs of ppgS are found in the genomes of slow growing mycobacteria including M. bovis, M. bovis BCG, M. leprae, Mycobacterium ulcerans, Mycobacterium marinum, M. avium, and Mycobacterium paratuberculosis, as well as in Mycobacterium abscessus, but not in the genomes of other rapidly growing Mycobacterium species such as M. smegmatis (supplemental Fig. S1). Interestingly, in all of the genomes analyzed, ppgS and orthologs map in close vicinity to galE1, a gene thought to encode the UDP-glucose 4-epimerase responsible for the conversion of UDP-d-Glcp to UDP-d-Galp in AG synthesis (16). ppgS and orthologs appear to be co-transcribed with a gene potentially encoding a small membrane protein (13 kDa) of unknown function (Rv3632 in M. tuberculosis H37Rv) (supplemental Fig. S1). PpgS shares significant sequence similarities with polyprenyl-monophosphomannose synthases from various prokaryotic sources, including Ppm1 (Rv2051c) from M. tuberculosis H37Rv (24% identity, 39% similarity on a 228-amino acid overlap).
To investigate the putative involvement of this GT in AG synthesis, the ppgS gene from M. tuberculosis H37Rv was disrupted by homologous recombination using the Ts/sacB method (20). Allelic replacement at the ppgS locus was confirmed by Southern hybridization (supplemental Fig. S2A). The mutant grew similarly to its wild-type (WT) H37Rv parent in 7H9-ADC-Tween 80 broth at 37 °C (supplemental Fig. S2B).
Phenotypic Characterization of the ppgS Mutant Strain
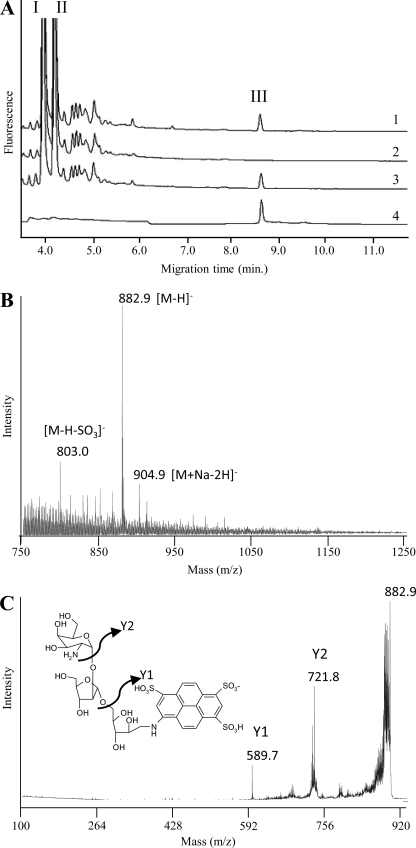
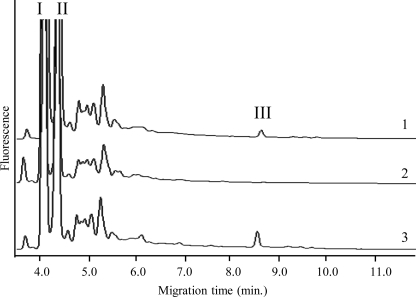
Soluble AG was prepared from the WT and mutant strains as described previously (5), and the Ara/Gal ratios were determined by CE or GC upon total acid hydrolysis. No significant differences between WT and mutant strains were observed, indicating that PpgS was probably not involved in the polymerization of the arabinan or galactan domains. Because the distribution of ppgS within the Mycobacterium genus seemed to correlate with that of d-GalN on AG, we decided to probe the mutant for the presence of this substituent. To this end, a fast and sensitive CE-based methodology was first developed to facilitate the detection of this rare substituent. By analogy to the procedure that we previously developed to analyze the mannose caps of lipoarabinomannan (25, 28), we postulated that a controlled mild acid hydrolysis of AG would cleave the arabinofuranosidic bonds of the arabinan domain while preserving the pyranosidic linkage of d-GalN onto the relevant Araf unit, thereby releasing an oligosaccharide that could be detected by CE-LIF upon tagging with an APTS fluorescent probe. A typical electrophoretogram of M. tuberculosis H37Rv AG resulting from this analysis is shown in Fig. 1A. The monosaccharide region is dominated by two main peaks attributed to d-Araf-APTS (I) and d-Galp-APTS (II) arising from the hydrolysis of the arabinan and galactan domains. A third peak (III) of lower intensity was detected with a migration time matching that of a maltoheptaose-APTS, tentatively attributed to an oligosaccharide-APTS containing d-GalpN and d-Araf units. This assumption was confirmed by collecting peak III in a picopreparative CE step (26) and subjecting it to MALDI/MS analysis (Fig. 1B). The negative MALDI mass spectrum was dominated by a peak at m/z 882.9 attributed to deprotonated molecular ions [M−H]− that could be assigned to an APTS derivative containing two pentosyl and one hexos-aminyl units. The identity of these units as two arabinosyl and one galactosaminyl residues was revealed by CE analysis upon acid hydrolysis and labeling with APTS or fluorescein-succinimidyl ester. The exact trisaccharide sequence, d-GalN-(1→2)-α-d-Araf-(1→5)-α-d-Araf, was revealed by MS/MS analysis (Fig. 1C) showing fragment ions at m/z 721.8 (y2) and m/z 589.7 (y1) arising from the loss of anhydro-d-GalNp and anhydro-d-GalNp-Araf, and based on the described structure of AG (5) (see also Fig. 6). Peak III was thus further used as a reporter peak for the presence of d-GalN on AG.
FIGURE 1.
Arabinogalactan composition of the ppgS knock-out mutant of M. tuberculosis H37Rv. A, CE-LIF analysis of AG after mild acid hydrolysis and APTS derivatization. Shown are the partial electrophoretograms obtained with AG prepared from M. tuberculosis H37Rv wild-type (trace 1), the ppgS knock-out mutant (trace 2), and the complemented mutant strain, H37RvΔppgS/pVVppgS (trace 3). An electrophoretogram of CE-collected peak III is shown in trace 4. I, d-Araf-APTS; II, d-Galp-APTS; III, d-GalNp-d-Araf-d-Araf-APTS. B, negative MALDI-TOF mass spectrum of compound III. C, MALDI-MS/MS collision-induced dissociation mass spectrum of the precursor ions 882.9 assigned to the deprotonated molecular ions of compound III.
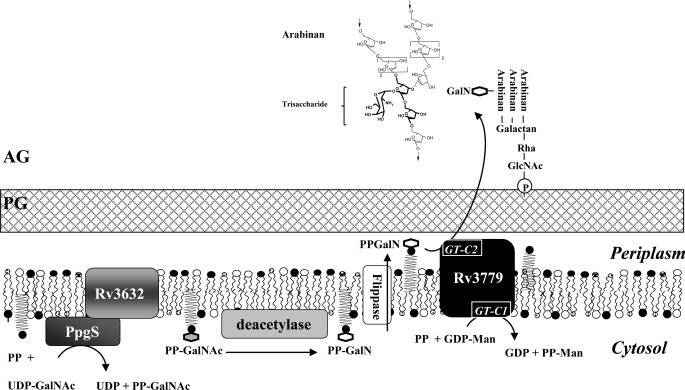
FIGURE 6.
Proposed model for the biosynthesis of the GalN substituent of AG. PG, peptidoglycan; AG, arabinogalactan; PP, polyprenol phosphate; PP-GalNAc, polyprenyl-monophosphoryl-N-acetylgalactosaminyl; PP-GalN, polyprenyl-monophosphoryl-galactosaminyl; PP-Man, polyprenyl-phosphomannose. The detailed structure of a d-GalN residue modifying an arabinan chain of AG is shown. The trisaccharide moiety of compound III is in bold font. Also shown are the two predicted GT-C signature motifs of Rv3779. GT-C1 (DLD at position 86) located on the cytosolic side of the plasma membrane is involved in the transfer of a mannose residue from GDP-α-d-Manp to polyprenyl phosphate (21); GT-C2 (DLD at position 536) is predicted to be in the last extracytoplasmic loop of the protein and to catalyze the transfer of d-GalN(Ac) onto AG.
Suggestive of an involvement of PpgS in the formation of the GalN substituent of AG, peak III was noticeably absent from the electrophoretogram of H37RvΔppgS::kan but restored in the complemented mutant strain H37RvΔppgS::kan/pVVppgS. Corroborating this observation, peak III was also detected in the AG of several ppgS-expressing Mycobacterium species (M. bovis BCG, M. marinum, and M. abscessus) but, as expected, was missing from that of M. smegmatis (supplemental Fig. S3). Although PpgS shares significant sequence similarities with polyprenol-monophosphomannose synthases, no significant differences were found between the mannolipid and lipoglycan (lipomannan and lipoarabinomannan) contents of the WT and mutant strains (supplemental Fig. S4).
Effect of Overexpressing ppgS on the Synthesis of Lipid-linked Sugars and AG in M. smegmatis
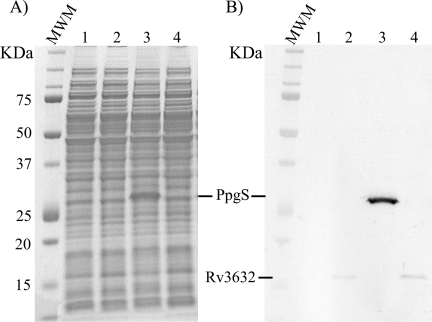
The absence of a ppgS ortholog in M. smegmatis provided a convenient mycobacterial host for the heterologous expression and functional characterization of this gene. Transformation of mc2155 with pVVppgS, the plasmid used for complementation studies in M. tuberculosis, resulted in the production of a C-terminal His6-tagged recombinant protein of the expected size (∼26 kDa) (Fig. 2, A and B). The recombinant protein was almost exclusively found in the membrane and P60 fractions of the bacterium, suggestive of its association with membranes (supplemental Fig. S5). To optimize the chances of producing an active form of PpgS in M. smegmatis, a recombinant strain of M. smegmatis expressing ppgS in combination with Rv3632 and a control strain expressing Rv3632 alone were also constructed. In all cases, recombinant strains grew similarly to the control in liquid broth and the production of recombinant proteins of the expected sizes was confirmed (Fig. 2B).
FIGURE 2.
Production of recombinant forms of PpgS and Rv3632 in M. smegmatis mc2155. The production of a recombinant C-terminal His6-tagged form of PpgS from M. tuberculosis was detected by Coomassie Blue staining (A) and Western blot using a monoclonal anti-His tag antibody (B) in the mc2155/pVVppgS strain (lanes 3) but not in the control strain, mc2155/pVV16 (lanes 1). The production of a recombinant C-terminal His6-tagged form of Rv3632 from M. tuberculosis was detected by Western blotting (B) in the mc2155/pVVRv3632 strain (lanes 2) and mc2155/pVVppgS-Rv3632 strain (lanes 4) but not in the control strain, mc2155/pVV16 (lanes 1).
Analysis of the AG of the various recombinant strains as described above failed to reveal the presence of a d-GalNp (or N-acetyl-d-GalNp) substituent on the AG of mc2155/pVV16, mc2155/pVVppgS, mc2155/pVVRv3632, and mc2155/pVVppgS-Rv3632 (supplemental Fig. S6). It thus appeared that the expression of ppgS alone or in combination with Rv3632 was not sufficient for the formation of this substituent in M. smegmatis.
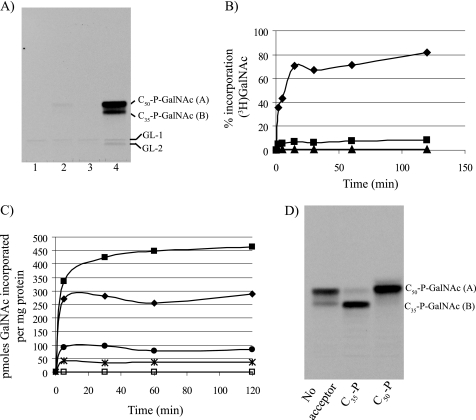
Cell-free assays using membranes from the recombinant M. smegmatis strains were then conducted to determine whether PpgS is involved in some aspects of lipid-linked sugar biosynthesis. TLC autoradiographs clearly revealed the incorporation of d-[3H]GalNAc from UDP-α-d-[3H]GalNAc into two major glycolipids (A and B) by mc2155/pVVppgS membranes that were not detected in the control strain mc2155/pVV16 or in the strain expressing the only Rv3632 gene, mc2155/pVVRv3632 (Fig. 3A). Strikingly, this enzymatic activity was increased 40–50-fold in the strain co-expressing ppgS and Rv3632 (Fig. 3A, lane 4). The accumulated d-[3H]GalNAc- (or d-[3H]GalN)-containing glycolipids were mild alkali-stable and mild acid-labile (supplemental Fig. 7SA) with TLC mobility properties suggestive of lipid-linked sugars of the mycobacterial polyisoprenyl-P class (19, 21, 29). Further assays aimed at testing the effects of time and protein or acceptor substrate concentration on d-[3H]GalNAc transfer by mc2155/pVVppgS-Rv3632 membranes showed clear enzyme and substrate concentration-dependent increases in activity (Fig. 3, B and C). Increases in activity were similar whether decaprenyl (C50)-P or the shorter heptaprenyl (C35)-P were used as acceptor substrates (Fig. 3D) but inexistent when C10-P served as the acceptor (supplemental Fig. S7B) indicative of PpgS preference for longer lipid carriers. Suggestive of PpgS specificity for the sugar transferred, assays performed under similar conditions using other sugar donors including GDP-d-[14C]Man, UDP-d-[14C]GlcNAc, ADP-d-[14C]Glc, and UDP- d-[14C]Gal failed to reveal any effect of overexpressing ppgS on the incorporation of these radiolabeled sugars into glycolipids (data not shown).
FIGURE 3.
Effect of overexpressing ppgS alone and in combination with Rv3632 on the incorporation of d-[3H]GalNAc from UDP-α-d-[3H]GalNAc into lipid-linked sugars by membrane extracts from M. smegmatis. A, TLC analysis of an in vitro cell-free assay using UDP-[3H]-α-d-GalNAc and membranes from mc2155/pVV16 (lane 1), mc2155/pVVppgS (lane 2), mc2155/pVVRv3632 (lane 3), and mc2155/pVVppgS-Rv3632 (lane 4). The synthesized lipid-linked sugars were extracted with CHCl3/CH3OH/NH4OH (2:1:0.05, v/v), and a 10% aliquot of each sample was analyzed by TLC in the solvent system CHCl3/CH3OH/NH4OH/H2O (65:25:0.5:3.6, v/v) followed by autoradiography. The radiolabeled products migrating close to the origin in all assays were identified by co-migration as C50-P-P-d-GlcNAc (GL-1) and C50-P-P-d-GlcNAc-Rha (GL-2) and probably arose from the randomization of UDP-α-d-[3H]GalNAc into UDP-α-d-[3H]GlcNAc which is known to serve as a donor substrate in the initial steps of the biosynthesis of AG (15). B, percentage incorporation of d-[3H]GalNAc into polyprenyl-P-d-GalNAc over time by membranes of mc2155/pVVppgS-Rv3632 as a function of the amount of membrane proteins used in the assay. Triangles, 0.5 μg of protein; squares, 5 μg; diamonds, 50 μg. C, incorporation of d-[3H]GalNAc into polyprenyl-P-d-GalNAc over time by membranes of mc2155/pVV16 (open symbols) and mc2155/pVVppgS-Rv3632 (filled symbols) as a function of the concentration of C50-P present in the assay mixture. Squares, 250 μm C50-P; diamonds, 25 μm C50-P; circles, 2.5 μm C50-P; stars, no added acceptor substrate. D, TLC analysis of in vitro cell-free assays using UDP-α-d-[3H]GalNAc and membranes from mc2155/pVVppgS-Rv3632, in the presence or absence of added acceptor substrates (C35-P and C50-P). Assays were run and analyzed as in A.
Structural Characterization of Glycolipids A and B
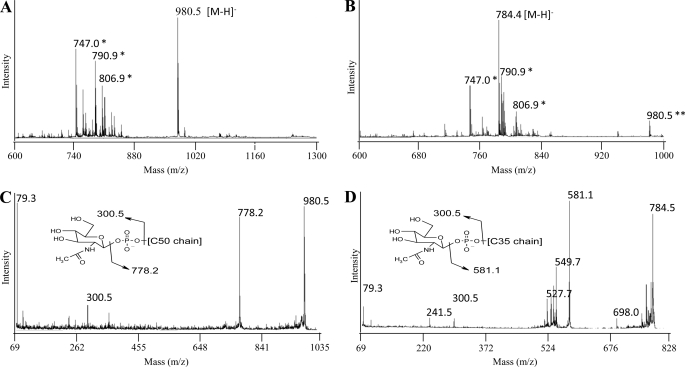
To establish the nature of the products formed in the assay using mc2155/pVVppgS-Rv3632 membranes and UDP-α-d-[3H]GalNAc, a nonradioactive assay was up-scaled, and the products of the reaction, purified by preparative TLC, were submitted to MALDI/MS analyses. The negative MALDI-TOF mass spectra of purified products A and B were dominated by peaks at m/z 980.5 and 784.4, respectively, attributed to deprotonated molecular ions [M−H]− that could be assigned to C50-P-d-GalNAc and C35-P-d-GalNAc (Fig. 4, A and B). This assignment was confirmed by MS/MS analyses showing for compound A fragment ions at m/z 300.5 and 778.2, arising from the loss of the decaprenyl and N-acetylgalactosaminyl moieties, respectively (Fig. 4C). The negative MALDI-TOF/TOF mass spectrum of purified compound B exhibited the same reporter fragment ions at m/z 300.5 and 581.1 (Fig. 4D). Altogether, our results thus indicate that ppgS encodes a polyprenyl-phospho-N-acetylgalactosaminyl synthase required for the synthesis of the GalN substituent of AG.
FIGURE 4.
Structural characterization of products A and B by MALDI/MS. A and B, negative MALDI-TOF mass spectra of product A (A) and product B (B). C and D, negative MALDI-TOF/TOF mass spectra of product A (C) and product B (D). *, matrix ions; **, [M−H]− ions from contaminating C50-P-d-GalNAc.
Identification of the Glycosyltransferase Responsible for GalN Transfer onto AG
Because the involvement of a lipid-linked d-GalN(Ac) donor suggested that the transfer of the amino sugar onto AG occurred on the periplasmic side of the plasma membrane, we thought to search for the responsible GT among the membrane-associated GT-C enzymes of M. tuberculosis (14). Of the 17 proposed GT-Cs of strain H37Rv, only 2 are missing an ortholog in M. smegmatis mc2155 but have orthologs in M. leprae, M. abscessus, and other species harboring a d-GalNp substituent on AG, namely Rv1635c and Rv3779. Rv1635c was demonstrated earlier to catalyze the addition of the first mannose residue in the Man-capping of mannosylated lipoarabinomannan (30). Rv3779 clusters on the chromosome of M. tuberculosis with genes involved in the biosynthesis of the cell wall core and was recently found to participate in the synthesis of polyprenylphosphomannose (21). Analysis of the AG of mutants deficient in the expression of each of these genes (21, 30) revealed that although the M. tuberculosis CDC1551 Rv1635c knock-out strain still carried the d-GalNp substituent (supplemental Fig. S8A), the AG of M. tuberculosis H37RvΔRv3779 was totally devoid of it (Fig. 5). Production of galactosaminylated AG was restored in the Rv3779 mutant upon complementation with a WT copy of Rv3779 expressed from a replicative plasmid (Fig. 5).
FIGURE 5.
Arabinogalactan composition of a Rv3779 knock-out mutant of M. tuberculosis H37Rv. CE-LIF analysis of the AG prepared from M. tuberculosis H37Rv wild-type (trace 1), the Rv3779 knock-out mutant (trace 2), and the complemented mutant strain, H37RvΔRv3779/pVVRv3779 (trace 3). I, d-Araf-APTS; II, d-Galp-APTS; III, d-GalNp-d-Araf-d-Araf-APTS.
DISCUSSION
The presence of a covalently bound GalN residue typifies the AG of slow growing mycobacteria. We have identified the key components of the biosynthetic machinery required for the synthesis and transfer of this amino sugar in M. tuberculosis and provided evidence that a previously unknown mycobacterial lipid-linked sugar, polyprenyl-P-(N-acetyl)-galactosamine served as the sugar donor in this process. PpgS, a sugar nucleotide-utilizing GT, catalyzes the formation of polyprenyl (C35/C50)-P-d-GalNAc from UDP-α-d-GalNAc and polyprenyl-P on the cytosolic side of the plasma membrane. This sugar donor is then presumably deacylated by an as yet unknown deacetylase before or after being translocated to the outer leaflet of the plasma membrane where the membrane-associated GT-C enzyme Rv3779 transfers the d-GalNp (or d-GalNAc) residue from polyprenyl-P-d-GalN(Ac) to the C2 position of a portion of the internal 3,5-branched d-Araf residues of AG (Fig. 6). The identity of the enzymes involved and deduced compartmentalization of this biosynthetic pathway are in line with what is currently known of the biogenesis of AG, whose early biosynthetic steps take place on the cytosolic side of the plasma membrane whereas later stages leading to the elongation and branching of the arabinan domain occur extracytoplasmically (16). The distribution of the ppgS, Rv3632, and Rv3779 genes within the Mycobacterium genus matches the reported presence or absence of a d-GalNp substituent on the AG of the mycobacterial species analyzed to date.
The identification of the PPM synthase Rv3779 (21) as the galactosaminyltransferase (or N-acetylgalactosaminyltransferase) of the system indicates that this GT-C enzyme is endowed with at least two distinct enzymatic activities and highlights the complexity of the GT-C enzymes responsible for much of the biosynthesis of mycobacterial cell wall polysaccharides. Consistent with this finding, in addition to the cytoplasmic GT-C signature motif (DLD at position 86) which we proposed earlier to be involved in the transfer of a mannose residue from GDP-α-d-Manp to polyprenyl phosphate (14, 21), a second putative GT-C motif predicted to be located in the last extracytoplasmic loop of the protein (TMHMM 2.0) and susceptible of catalyzing the transfer of d-GalN(Ac) was identified (DLD at position 536). This motif and associated downstream acidic, aromatic, and proline residues (YDYP at position 578) (14) are conserved among the Mycobacterium species reported to display a GalN-substituted AG. The predicted spatial distribution of each GT-C motif, which is in line with that of their respective donor and acceptor substrates (cytosolic GDP-Man and periplasmic arabinan chains of AG in particular), would thus govern the dual catalytic activities of Rv3779 (Fig. 6). Further experiments are under way to validate this hypothesis.
Missing biosynthetic components required for producing the GalN substituent of AG include the deacetylase responsible for deacetylating polyprenyl-P-d-GalNAc to the corresponding d-GalN derivative and the translocase required for translocating polyprenyl-P-d-GalN/polyprenyl-P-d-GalNAc from the inner to the outer leaflet of the plasma membrane (Fig. 6). A search for the missing deacetylase of the system in the genome of M. tuberculosis H37Rv led to our interest in Rv1170 (mshB) and Rv1082 (mca). MshB participates in the deacetylation of 1-d-myo-inosityl-2-acetamido-2-deoxy-α-d-glucopyranoside in the biosynthesis of mycothiols and, in vitro, displays deacetylase activity on various other acetylated substrates. The mycothiol S-conjugate amidase Mca shares with MshB sequence similarities and deacetylase activity in vitro, although its primary function in the bacterium is the cleavage of various S-conjugates of mycothiol (22, 31, 32). Analysis of AG in the M. tuberculosis mshB and mca knock-out strains, however, revealed WT profiles in both mutants (supplemental Fig. S8B), suggesting either that these enzymes are not involved in the formation of the d-GalNp substituent or that compensatory enzymatic activities exist in the cell.
With regard to the translocase of the system, studies are in progress in our laboratories to investigate whether the small integral membrane protein Rv3632 may assume this function, thereby explaining its tight functional association with PpgS. Alternatively, the role of Rv3632 may be to target PpgS to the plasma membrane as shown for GTs involved in O-polysaccharide synthesis in Gram-negative bacteria (33) or to stabilize PpgS in the membrane as suggested for M. smegmatis polyprenyl phosphomannose synthase Ppm1 and associated transmembrane protein Ppm2 (fused as one single polypeptide, Rv2051c, in M. tuberculosis H37Rv) (34).
The availability of a ppgS knock-out mutant of M. tuberculosis now provides a unique opportunity to elucidate the function(s) of the d-GalNp substituent of AG. Preliminary studies indicate that the mutant does not display any defects in growth or susceptibility to drugs under optimal laboratory growth conditions. Thus, it is unlikely that d-GalNp serves a critical role in stabilizing the structure of the envelope as suggested earlier (3). Instead, the relative restriction of this substituent to pathogenic slow growing mycobacterial species (and emerging nosocomial pathogen, M. abscessus) together with the reported role of the d-GalN substituent of lipid A in the pathogenicity of F. tularensis (8) make it tempting to speculate that the d-GalNp substituent of AG serves a function during host infection. In vivo studies have been undertaken to verify this hypothesis.
Supplementary Material
Acknowledgments
We thank A. Noguera (IPBS) for technical assistance and Dr. M. McNeil (Colorado State University) for helpful discussions.
This work was supported, in whole or in part, by National Institutes of Health Grants AI064798 and AI018357 through the NIAID. This work was also supported by AIDS-Fogarty International Research Collaboration Award Grant TW 006487 and its parent National Institutes of Health Grant R37 AI18357 through the NIAID.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S8.
S. Bhamidi, unpublished observation.
- AG
- arabinogalactan
- APTS
- 8-aminopyrene-1,3,6-trisulfonate
- CE
- capillary electrophoresis
- CE-LIF
- CE monitored by laser-induced fluorescence
- GT
- glycosyltransferase
- kan
- kanamycin
- PpgS
- polyprenyl-phospho-N-acetylgalactosaminyl synthase.
REFERENCES
- 1.Acharya P. V., Goldman D. S. (1970) J. Bacteriol. 102, 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Draper P. (1971) J. Gen. Microbiol. 69, 313–324 [DOI] [PubMed] [Google Scholar]
- 3.Draper P., Khoo K. H., Chatterjee D., Dell A., Morris H. R. (1997) Biochem. J. 327, 519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee A., Wu S. W., Scherman M. S., Torrelles J. B., Chatterjee D., McNeil M. R., Khoo K. H. (2006) Biochemistry 45, 15817–15828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bhamidi S., Scherman M. S., Rithner C. D., Prenni J. E., Chatterjee D., Khoo K. H., McNeil M. R. (2008) J. Biol. Chem. 283, 12992–13000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beer W., Adam M., Seltmann G. (1986) J. Basic Microbiol. 26, 201–204 [DOI] [PubMed] [Google Scholar]
- 7.Sonesson A., Jantzen E., Tangen T., Zähringer U. (1994) Microbiology 140, 2663–2671 [DOI] [PubMed] [Google Scholar]
- 8.Kanistanon D., Hajjar A. M., Pelletier M. R., Gallagher L. A., Kalhorn T., Shaffer S. A., Goodlett D. R., Rohmer L., Brittnacher M. J., Skerrett S. J., Ernst R. K. (2008) PLoS Pathog. 4, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Ribeiro A. A., Guan Z., Raetz C. R. (2009) Biochemistry 48, 1162–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pfannenstiel M. A., Muthukumar G., Couche G. A., Nickerson K. W. (1987) J. Bacteriol. 169, 796–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu T. H. (1988) Biochim. Biophys. Acta 963, 359–366 [DOI] [PubMed] [Google Scholar]
- 12.Song F., Guan Z., Raetz C. R. H. (2009) Biochemistry 48, 1173–1182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Raetz C. R., Reynolds C. M., Trent M. S., Bishop R. E. (2007) Annu. Rev. Biochem. 76, 295–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berg S., Kaur D., Jackson M., Brennan P. J. (2007) Glycobiology 17, 35R–56R [DOI] [PubMed] [Google Scholar]
- 15.Lairson L. L., Henrissat B., Davies G. J., Withers S. G. (2008) Annu. Rev. Biochem. 77, 521–555 [DOI] [PubMed] [Google Scholar]
- 16.Kaur D., Guerin M., Škovierová H., Brennan P. J., Jackson M. (2009) Adv. Appl. Microbiol. 69, 23–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schultz J., Elbein A. D. (1974) Arch. Biochem. Biophys. 160, 311–322 [DOI] [PubMed] [Google Scholar]
- 18.Yokoyama K., Ballou C. E. (1989) J. Biol. Chem. 264, 21621–21628 [PubMed] [Google Scholar]
- 19.Wolucka B. A., McNeil M. R., de Hoffmann E., Chojnacki T., Brennan P. J. (1994) J. Biol. Chem. 269, 23328–23335 [PubMed] [Google Scholar]
- 20.Pelicic V., Jackson M., Reyrat J. M., Jacobs W. R., Jr., Gicquel B., Guilhot C. (1997) Proc. Natl. Acad. Sci. U.S.A. 94, 10955–10960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Scherman H., Kaur D., Pham H., Škovierová H., Jackson M., Brennan P. J. (2009) J. Bacteriol. 191, 6769–6772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buchmeier N. A., Newton G. L., Koledin T., Fahey R. C. (2003) Mol. Microbiol. 47, 1723–1732 [DOI] [PubMed] [Google Scholar]
- 23.Korduláková J., Gilleron M., Mikušová K., Puzo G., Brennan P. J., Gicquel B., Jackson M. (2002) J. Biol. Chem. 277, 31335–31344 [DOI] [PubMed] [Google Scholar]
- 24.Mikušová K., Yagi T., Stern R., McNeil M. R., Besra G. S., Crick D. C., Brennan P. J. (2000) J. Biol. Chem. 275, 33890–33897 [DOI] [PubMed] [Google Scholar]
- 25.Nigou J., Vercellone A., Puzo G. (2000) J. Mol. Biol. 299, 1353–1362 [DOI] [PubMed] [Google Scholar]
- 26.Ludwiczak P., Brando T., Monsarrat B., Puzo G. (2001) Anal. Chem. 73, 2323–2330 [DOI] [PubMed] [Google Scholar]
- 27.Cantarel B. L., Coutinho P. M., Rancurel C., Bernard T., Lombard V., Henrissat B. (2009) Nucleic Acids Res. 37, D233–D238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nigou J., Gilleron M., Cahuzac B., Bounéry J. D., Herold M., Thurnher M., Puzo G. (1997) J. Biol. Chem. 272, 23094–23103 [DOI] [PubMed] [Google Scholar]
- 29.Gurcha S. S., Baulard A. R., Kremer L., Locht C., Moody D. B., Muhlecker W., Costello C. E., Crick D. C., Brennan P. J., Besra G. S. (2002) Biochem. J. 365, 441–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dinadayala P., Kaur D., Berg S., Amin A. G., Vissa V. D., Chatterjee D., Brennan P. J., Crick D. C. (2006) J. Biol. Chem. 281, 20027–20035 [DOI] [PubMed] [Google Scholar]
- 31.Newton G. L., Av-Gay Y., Fahey R. C. (2000) J. Bacteriol. 182, 6958–6963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Newton G. L., Ko M., Ta P., Av-Gay Y., Fahey R. C. (2006) Protein Expr. Purif. 47, 542–550 [DOI] [PubMed] [Google Scholar]
- 33.Clarke B. R., Greenfield L. K., Bouwman C., Whitfield C. (2009) J. Biol. Chem. 284, 30662–30672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baulard A. R., Gurcha S. S., Engohang-Ndong J., Gouffi K., Locht C., Besra G. S. (2003) J. Biol. Chem. 278, 2242–2248 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.