Abstract
In the fission yeast Schizosaccharomyces pombe, Wee1-dependent inhibitory phosphorylation of the highly conserved Cdc2/Cdk1 kinase determines the mitotic onset when cells have reached a defined size. The receptor of activated C kinase (RACK1) is a scaffolding protein strongly conserved among eukaryotes which binds to other proteins to regulate multiple processes in mammalian cells, including the modulation of cell cycle progression during G1/S transition. We have recently described that Cpc2, the fission yeast ortholog to RACK1, controls from the ribosome the activation of MAPK cascades and the cellular defense against oxidative stress by positively regulating the translation of specific genes whose products participate in the above processes. Intriguingly, mutants lacking Cpc2 display an increased cell size at division, suggesting the existence of a specific cell cycle defect at the G2/M transition. In this work we show that protein levels of Wee1 mitotic inhibitor are increased in cells devoid of Cpc2, whereas the levels of Cdr2, a Wee1 inhibitor, are down-regulated in the above mutant. On the contrary, the kinetics of G1/S transition was virtually identical both in control and Cpc2-less strains. Thus, our results suggest that in fission yeast Cpc2/RACK1 positively regulates from the ribosome the mitotic onset by modulating both the protein levels and the activity of Wee1. This novel mechanism of translational control of cell cycle progression might be conserved in higher eukaryotes.
Keywords: CDK (cyclin-dependent kinase), Cell Cycle, Mitosis, Ribosomes, Yeast Genetics
Introduction
Cell reproduction involves the passing through a series of events collectively known as the cell cycle, which consists of alternative stages involving DNA replication (S phase), chromosome segregation and nuclear division (mitosis), as well as cell division (cytokinesis). Entry into mitosis is induced by the activation of a cyclin B-bound Cdc2/Cdk1 kinase, which is highly conserved among eukaryotic cells (1). In the fission yeast Schizosaccharomyces pombe, the inhibitory phosphorylation at a conserved tyrosine 15 (Tyr15) in Cdc2 regulates its kinase activity that determines the mitotic onset and the start of division when the cells have reached a defined size (2). The kinase Wee1 down-regulates Cdc2 by inhibitory Tyr15 phosphorylation, which is reversed by Cdc25 phosphatase, leading to Cdc2 activation and triggering of mitotic entry (3–6). Consequently, fission yeast cells lacking Wee1 activity enter mitosis before reaching a critical size to produce two small daughter cells (7). On the contrary, mutants in Cdc25 enter mitosis at an increased cell size, indicating that the activities/levels of both Cdc25 and Wee1 must be tightly regulated to provide an accurate control of the mitotic onset. In fission yeast, Wee1 is in turn phosphorylated and its activity negatively regulated by two SAD family kinases, Nim1 (also known as Cdr1 (8–12) and Cdr2 (13, 14). Recently, it has been described that gradients from the cell ends involving the DYRK family kinase Pom1 play a key role in the control of the progression of the cell cycle by coupling cell length to G2/M transition through phosphorylation and negative control of Cdr2 activity (15, 16).
RACK14 (receptor of activated protein C kinase), a member of the large family of proteins with WD repeats, is a 36-kDa protein homologous to the β-subunit of heterotrimeric G proteins and highly conserved in eukaryotic organisms (17). RACK1 was initially described by its ability to interact with distinct protein kinase C isoforms. Further studies have shown its role as a scaffold which mediates cross-talk between various signaling pathways by binding in vivo to other proteins and regulating multiple biological processes like MAPK activation, angiogenesis, tumor growth, neuronal response, apoptosis, chromatin remodeling, and proper function of the circadian clock (17–20). Cpc2, the fission yeast RACK1 ortholog, was identified through its action on protein kinase Ran1/Pat1, which controls the transition from mitosis to meiosis (21). Mutants lacking Cpc2 show several characteristic phenotypes like defective sexual differentiation under nitrogen deprivation and sensitivity to different stresses (21, 22). Interestingly, RACK1/Cpc2 proteins are structural components of the 40 S ribosomal subunit (23–27), located in the proximity of the mRNA exit channel and in close contact with the binding surface of the eIF3 complex (27). In this context, we have recently described that in fission yeast Cpc2 functions from the ribosome by positively regulating the translation of specific mRNAs, like those encoding Pyp1 and Pyp2 tyrosine phosphatases, which control the magnitude of the activation of Pmk1 and Sty1 MAPKs, and the function of transcription factor Atf1, which regulates the global transcriptional response against stress (22). These results support that RACK1/Cpc2 may provide a platform for the translation of specific subsets of mRNAs involved in key cellular processes (22, 25).
Another relevant biological function of RACK1/Cpc2 is related to cell cycle control. For example, in the parasite Trypanosoma brucei, TRACK (RACK1 ortholog) participates in the control of the final stages of mitosis and cytokinesis (28). Also, genetic and biochemical approaches have shown that RACK1 negatively regulates cell cycle progression at the G1/S transition in mammalian cells by suppressing the activity of Src, a nonreceptor protein tyrosine kinase that plays a multitude of roles in cell signaling, and Src-mediated cell cycle regulators in G1, thereby promoting a delay of entry into S phase (29, 30). However, fission yeast mutants lacking Cpc2 display an increased cell size at division, suggesting the existence of a specific cell cycle defect at the G2/M transition (21). In this work we have taken advantage of S. pombe as a suitable model to identify molecular mechanisms related to cell cycle, and we show that Cpc2 is a key element involved in the regulation of the mitotic onset in this organism.
EXPERIMENTAL PROCEDURES
Strains and Growth Conditions
The S. pombe strains (Table 1) were grown with shaking at 28 °C in either YES medium or EMM2 (34) with 2% of glucose, and supplemented with adenine, leucine, histidine, or uracil (100 mg/liter, Sigma) depending on their particular requirements. In experiments performed with cdc25-22 or cdc10-129 thermosensitive mutant strains, the cells were grown in YES medium to an A600 of 0.2 at 25 °C (permissive temperature), shifted to 37 °C for 3.5 h, and released from the growth arrest by transfer back to 25 °C.
TABLE 1.
S. pombe strains used in this study
| Strain | Genotype | Source/Reference | |
|---|---|---|---|
| MM1 | h+ | ade6-M216 leu1-32 ura4D-18 | 33 |
| MM2 | h− | ade6-M216 leu1-32 ura4D-18 | 33 |
| AN001 | h+ | ade6-M216 cpc2::KanR leu1-32 ura4D-18 | 22 |
| AN002 | h− | ade6-M216 cpc2::KanR leu1-32 ura4D-18 | 22 |
| PPG148 | h− | cdc25-22 ura4-D18 | P. Pérez laboratory |
| AN-CC18 | h− | cdc25-22 cpc2::KanR ura4-D18 | This work |
| 326 | h− | cdc10-129 | Laboratory stock |
| AN-CC10 | h− | cdc10-129 cpc2::KanR | This work |
| 560 | h− | mcm4-GFP::ura4+leu1-32 ura4− | 35 |
| AN-CC16 | h− | mcm4-GFP::ura4+cpc2::KanR leu1-32 ura4− | This work |
| AN-CC21 | h+ | cdc10-129 mcm4-GFP::ura4+leu1-32 ura4− | This work |
| AN-CC22 | h+ | cdc10-129 mcm4-GFP::ura4+cpc2::KanR leu1-32 ura4− | This work |
| MI709 | h− | wis1DD-12myc::ura4+pmk1-HA6H::ura4+leu 1-32 ura4-D18 | 38 |
| AN600 | h− | wis1DD-12myc::ura4+pmk1-HA6H::ura4+cpc2::KanR leu1-32 ura4-D18 | This work |
| MI204 | h+ | ade−sty1::ura4+pmk1-HA6H::ura4+leu 1-32 ura4-D18 | 38 |
| AN-120 | h+ | ade−sty1::ura4+cpc2::KanR pmk1-HA6H::ura4+leu 1-32 ura4-D18 | This work |
| FY16230 | h− | cdc2–3w cdc25::ura4+leu1-32 ura4-D18 | YGRC |
| AN-CC3 | h− | cdc2–3w cdc25::ura4+cpc2::KanR leu1-32 ura4-D18 | This work |
| FY7108 | h− | ade6-M216 wee1-50 | YGRC |
| AN-CC5 | h− | ade6-M216 wee1-50 cpc2::KanR | This work |
| FY7287 | h− | cdc2-1w leu1-32 | YGRC |
| AN-CC1 | h− | cdc2-1w cpc2::KanR leu1-32 | This work |
| AN071 | h+ | ade6-M216 cpc2::KanR cpc2-GFP::leu1+pmk1-HA6H::ura4+leu1-32 ura4D-18 | This work |
| AN072 | h+ | ade6-M216 cpc2::KanR cpc2(R36D K38E)-GFP::leu1+pmk1-HA6H:ura4+leu1-32 ura4D-18 | This work |
| FM-23 | h− | ade6-M216 cdc25-HA::KanR leu1-32 ura4-D18 | Laboratory stock |
| AN-CC20 | h− | ade6-M216 cdc25-HA::KanR cpc2::KanR leu1-32 ura4-D18 | This work |
| 1081 | h− | wee1-3HA6H ura4-D18 leu1-32 | 31 |
| AN-CC8 | h− | wee1-3HA6H cpc2::KanR leu1-32 ura4-D18 | This work |
| AN-CC45 | h− | wee1-3HA6H cdr2::KanR leu1-32 ura4-D18 | This work |
| AN-CC46 | h− | wee1–3HA6H cdr2::KanR cpc2::KanR leu1–32 ura4-D18 | This work |
| MM-76 | h+ | pom1-GFP::KanR leu1-32 ura4-D18 | Laboratory stock |
| AN-CC35 | h+ | pom1-GFP::KanR cpc2::KanR leu1-32 ura4-D18 | This work |
| LW117 | h− | nim1-2HA6his::ura4+leu1-32 ura4-D18 | P. Russell laboratory |
| AN-CC15 | h− | nim1–2HA6his::ura4+cpc2::KanR leu1-32 ura4-D18 | This work |
| JK2310 | h− | cdr2-2HA6his::ura4+leu1-32 ura4-D18 | P. Russell laboratory |
| AN-CC14 | h− | cdr2-2HA6his::ura4+cpc2::KanR leu1-32 ura4-D18 | This work |
| AN-CC23 | h− | cdr2-2HA6his::ura4+cpc2::KanR cpc2-GFP::leu1+leu1-32 ura4-D18 | This work |
| AN-CC24 | h− | cdr2–2HA6his::ura4+cpc2::KanR cpc2(R36D K38E)-GFP::leu1+leu1-32 ura4-D18 | This work |
| JK2240 | h− | cdr2: ura4+leu1-32 ura4-D18 | P. Russell laboratory |
| AN-CC12 | h− | cdr2::ura4+cpc2::KanR leu1-32 ura4-D18 | This work |
| TS313 | h− | ade6-M216 pom1::KanR pmk1-HA6H::ura4+leu1-32 ura4D-18 | 32 |
| AN-CC27 | h− | ade6-M216 pom1::KanR cpc2::KanR pmk1-HA6H::ura4+leu1–32 ura4D-18 | This work |
Flow Cytometry
Cells (107) were recovered by centrifugation at 2000 × g for 5 min and fixed with 1 ml of 70% cold ethanol. Cells were rehydrated in 50 mm sodium citrate, 0.1 mg/ml RNaseA, incubated at 37 °C for 2 h, and stained with 4 μg/ml propidium iodide. Cells were analyzed using a Becton Dickinson FACSort cytometer equipped with CellQuest software.
In Situ Chromatin Binding Assay for Mcm4
This assay was performed following the protocol described by Kearsey et al. (35), with slight modifications. Briefly, the cells were recovered by centrifugation (3000 × g for 1 min) and resuspended in ZM buffer (50 mm sodium citrate, pH 5.6, 1.2 m sorbitol, 0.5 mm magnesium acetate, and 10 mm DTT) plus 2 mg/ml zymoliase 50T (Seikagaku Corporation), and permeabilized by incubation at 32 °C for 10 min. The cells were then washed twice in Stop buffer (0.1 m MES, pH 6.6, 1.2 m sorbitol, 1 mm EDTA, and 0.5 mm magnesium acetate) and resuspended in EB buffer (20 mm PIPES, pH 6.8, 0.4 m sorbitol, 2 mm magnesium acetate, and 150 mm potassium acetate plus a specific protease inhibitor mixture obtained from Sigma). The cell suspensions were divided into two aliquots; one remained unchanged, and the other was treated with 0.02 volume of Triton X-100 for 5 min at room temperature. Afterward, the cells were recovered by centrifugation, resuspended first in methanol, then in acetone, and finally observed by fluorescence microscopy (see below).
Gene Disruption and Epitope Tagging
The cpc2+-null mutants were obtained by entire deletion of the corresponding coding sequence and its replacement with the KanR cassette by PCR-mediated strategy using plasmid pFA6a-KanR as template (36). Mutant strains were obtained either by transformation (lithium acetate method) (34) or by mating and diploid selection in EMM2 without supplements. Spores were purified by glusulase treatment (37) and allowed to germinate in EMM2 plus the appropriate requirements. Correct construction of strains was verified by PCR and Western blot analyses (see below).
Assays of Cell Sensitivity for Growth at Different Temperatures
In plate assays, wild-type and mutant strains of S. pombe were grown in YES liquid medium to an A600 of 0.6. Appropriate dilutions were spotted per duplicate on YES solid plates containing 2% (w/v) Bacto Agar (Difco) and incubated at either 25, 30, 32, 34, or 37 °C for 3 days and then photographed.
Detection of Wee1-, Cdc25-, Pom1-, Cdr1-, and Cdr2-tagged Fusions
In all cases 30–40 ml of culture was harvested by centrifugation at 4 °C, the cells washed with cold PBS buffer, and the yeast pellets immediately frozen in liquid nitrogen. Total cell homogenates were prepared under native conditions as described (38), employing chilled acid-washed glass beads and lysis buffer (10% glycerol, 50 mm Tris-HCl, pH 7.5, 150 mm NaCl, 0.1% Nonidet P-40, plus specific protease and phosphatase inhibitor mixtures for fungal and yeast extracts, obtained from Sigma). The lysates were cleared by centrifugation at 20,000 × g for 30 min and resolved in 6–12% SDS-polyacrylamide gels, depending on the relative size of the fusion protein, and transferred to filters. The following antibodies were employed in Western blotting experiments: mouse monoclonal anti-HA antibody (clone 12CA5; Roche Molecular Biochemicals), rabbit polyclonal anti-Cdc2 pY15 antibody (Chemicon International), mouse monoclonal anti-GFP antibody (Roche Applied Science). A rabbit polyclonal anti-Cdk1/Cdc2 (PSTAIR) antibody (Upstate Biotechnology) was used as loading control. Immunoreactive bands were detected employing anti-mouse or anti-rabbit HRP-conjugated secondary antibodies (Sigma) and either the ECL (Amersham Biosciences) or Supersignal (Pierce) systems.
Northern Blot Analysis
Yeast cells were grown in YES medium to an A600 of 0.8, and volumes of 50 ml of the cultures were recovered. Total RNA preparations were obtained as described previously and resolved through 1.5% agarose-formaldehyde gels. Northern (RNA)-hybridization analyses were performed as reported earlier (22). The probes employed were amplified by PCR and included a 0.8-kbp fragment of the wee1+ gene that was amplified with the 5′ oligonucleotide TCTCTCCATTTGCATCGGGC and the 3′ oligonucleotide AGGAGGAGGATCGAACCTCA; a 1.5-kbp fragment of the nim1+ gene amplified with the 5′ oligonucleotide GGGACGTCTATTTTGATTGCC and the 3′ oligonucleotide ATGGTGAAGCGACACAAAAAT; a 1.2-kbp fragment of the cdr2+ gene amplified with the 5′ oligonucleotide GGACGGATTGTCGTTGACGA and the 3′ oligonucleotide AGCAGCATCCAACGGGC; and a 1-kbp fragment of the leu1+ gene amplified with the 5′ oligonucleotide TCGTCGTCTTACCAGGAG and the 3′ oligonucleotide CAACAGCCTTAGTAATAT. For DNA labeling Ready-To-Go DNA labeling beads (GE Healthcare) were used. To establish quantitative conclusions, the level of mRNAs was determined in a PhosphorImager (Molecular Dynamics) and compared with the internal control (leu1+ mRNA).
Fluorescence Microscopy
To determine cell size at division the yeast strains were grown in either EMM2 or YES medium to an A600 of 0.5 and treated with Calcofluor white, which specifically stains cell wall and septum (39). A minimum of 200 septated cells were scored for each mutant. Binucleated cells were estimated after DAPI staining of nuclei as described previously (39). In the Mcm4-GFP chromatin binding assay, the Triton X-100-extracted and nonextracted cells were recovered in acetone, resuspended in TBS buffer with 2 mm CaCl2, and stained with DAPI. The percentage of cells with nuclear Mcm4-GFP in extracted versus nonextracted cells was estimated. A Leica DM 4000B fluorescence microscope equipped with a 100× objective was employed, and the images were captured with a cooled Leica DC 300F camera and IM50 software, and then imported into Adobe PhotoShop CS3 (Adobe Systems).
Reproducibility of Results
All experiments were repeated at least three times with similar results. Representative results are shown.
RESULTS
Cpc2 Positively Regulates G2/M Transition during the Cell Cycle
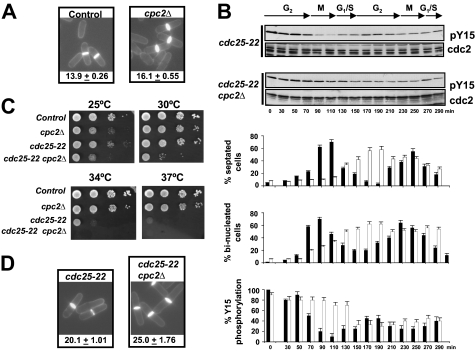
S. pombe mutants lacking Cpc2 increased cell size at division (Fig. 1A) (21, 22). In eukaryotic cells, Cdk1/Cdc2 kinase activity is down-regulated during G2 phase of the cell cycle by phosphorylation at Tyr15 (2). We thus examined the levels of Cdc2 phosphorylation at Tyr15 during the cell cycle in synchronized cultures of strains PPG148 (cdc25-22) and AN-CC18 (cdc25-22 cpc2Δ) after growth at 25 °C to log phase, a shift to 37 °C for 3.5 h to arrest cells in G2, and then incubation back to 25 °C. As indicated in Fig. 1B, the lowest level of Cdc2-Tyr15 phosphorylation in cdc25-22 cpc2Δ cells showed a delayed kinetics compared with control cells (∼150 min in cdc25-22 cpc2Δ cells versus ∼90 min in control cells). The maximum percentage of binucleated (entering mitosis) and septated cells was also retarded in the cdc25-22 cpc2Δ mutant, confirming a cell cycle defect at G2. Interestingly, both the thermosensitive phenotype and the increased cell size at division were enhanced in the cdc25–22 mutant by simultaneous deletion of cpc2+ (Fig. 1, C and D). Taken together, these results suggest that in fission yeast Cpc2 positively regulates G2/M transition during the cell cycle by affecting the phosphorylation status of Cdc2 kinase.
FIGURE 1.
Cpc2 is a positive regulator of G2/M transition during the cell cycle. A, cell morphology and size at division (micrometers ± S.D.) in strains MM2 (control) and AN002 (cpc2Δ), growing in YES medium at 28 °C and stained with Calcofluor white. B, cells from strains PPG148 (cdc25-22) and AN-CC18 (cdc25-22 cpc2Δ) were grown to an A600 of 0.3 at 25 °C, shifted to 37 °C for 3.5 h, and then released from the growth arrest by transfer back to 25 °C. Aliquots were taken at different time intervals, and Cdc2 phosphorylation at Tyr15 or total Cdc2 was detected by immunoblotting with anti-Cdc2 pY15 and anti-Cdk1/Cdc2 (PSTAIR) antibodies, respectively. Lower, corresponding percentages of septated cells, binucleated cells, and Tyr15 phosphorylation for cdc25-22 (filled bars) and cdc25–22 cpc2Δ (open bars) cells (n = 4). C, samples containing 104, 103, 102, or 101 cells of strains MM2 (control), PPG148 (cdc25-22), and AN-CC18 (cdc25-22 cpc2Δ) grown in YES medium were spotted onto Bacto Agar supplemented YES plates and incubated for 3 days at either 25 °C, 28 °C, 34 °C, and 37 °C before being photographed. D, cell morphology and size at division in strains PPG148 (cdc25-22) and AN-CC18 (cdc25-22 cpc2Δ) growing at 28 °C determined as described in A.
Cpc2 Does Not Regulate G1/S Transition in Fission Yeast
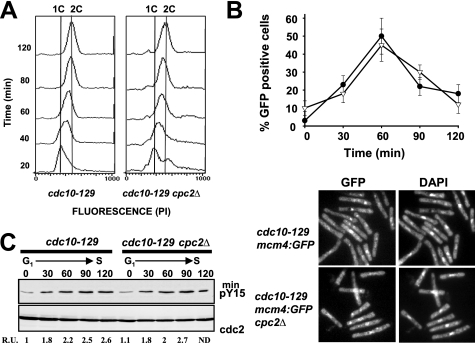
RACK1, the Cpc2 ortholog in higher eukaryotic cells, regulates cell growth by suppressing the activity of Src kinase in G1, therefore delaying entry into S phase (29, 30). Conversely, down-regulation of RACK1 activates Src-mediated signaling and accelerates G1/S progression (29). Although the results shown above suggest that Cpc2 regulates the length of the G2 phase during the cell cycle in fission yeast, it might also be true that it plays a role during G1/S transition. To test this possibility, we performed a set of experiments with control and cpc2Δ cells harboring a thermosensitive allele of Cdc10 (cdc10-129), which induces the transcription of several genes required for progression through G1 into S phase (40). Strains 326 (cdc10-129) and AN-CC10 (cdc10-129 cpc2Δ) were synchronized at early G1 by incubation at 37 °C for 4 h and then cultured again to 25 °C. Flow cytometry analysis of samples taken at subsequent times revealed that the rate of increase in DNA content from 1C to 2C during G1/S transition was similar in both strains (Fig. 2A), supporting that Cpc2 does not participate in the regulation of this phase of the cell cycle. We further analyzed G1/S transition by employing both control and cpc2Δ cdc10ts cells expressing a C-terminal GFP-tagged version of Mcm4, a DNA replication factor that forms part of the prereplicative complex during early S phase (35). Mcm4 is an ideal tool to investigate the kinetics of G1/S transition under different situations because this factor binds stably to chromatin during anaphase B and is displaced as replication proceeds during S phase (35, 41). As shown in Fig. 2B (upper and lower), the presence of chromatin-bound Mcm4-GFP was maximal 60 min after releasing the cells from the restrictive temperature in cdc10tts control (41) and cdc10ts cpc2Δ cultures, decreasing thereafter. Moreover, the increase in Cdc2-Tyr15 phosphorylation during G1/S transition in cdc10ts cells was not affected by the absence of Cpc2 (Fig. 2C). As a whole, these results confirm that Cpc2 does not regulate G1/S transition during the fission yeast cell cycle.
FIGURE 2.
Cpc2 does not regulate G1/S progression during the cell cycle. A, flow cytometry histograms of cells from strains 326 (cdc10-129) and AN-CC10 (cdc10-129 cpc2Δ) that were synchronized by incubation in YES medium at 37 °C for 3.5 h, transferred back to 25 °C, and incubated for the times indicated. B, upper, cells from strains AN-CC21 (cdc10-129 mcm4:GFP) and AN-CC22 (cdc10-129 mcm4:GFP cpc2Δ) synchronized at G1 by incubation in YES medium at 37 °C for 3.5 h and transferred back to 25 °C. Samples were taken at different times, and the percentage of cells with stable chromatin-bound Mcm4 (GFP-positive cells) was estimated by fluorescence microscopy (n = 3). Filled circles, strain AN-CC21; open triangles, strain AN-CC22. B, lower, micrographs of cells from the above strains expressing Mcm4-GFP taken 60 min after release and stained with DAPI. C, synchronized cells from strains 326 (cdc10-129) and AN-CC10 (cdc10-129 cpc2Δ) released at 25 °C. Aliquots were taken at different time intervals, and Cdc2 phosphorylation at Tyr15 or total Cdc2 was detected by immunoblotting with anti-Cdc2 pY15 or anti-Cdk1/Cdc2 (PSTAIR) antibodies, respectively.
Cpc2 Regulation of G2/M Transition Requires Wee1
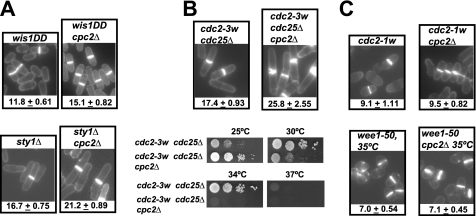
Until now, we have demonstrated that cpc2Δ mutant cells undergo a specific cell cycle delay at G2/M transition. The MAPK Sty1, which is main effector of the SAPK pathway, controls in the fission yeast the mitotic onset in at least two distinct levels: (i) by phosphorylating Plo1 (Polo kinase) at Ser402 and promoting its recruitment in the spindle pole bodies to modulate Cdc25/Wee1 control of Cdc2 activity (42); and (ii) by binding and phosphorylating the MAPKAP kinase-2 related protein Srk1, which controls mitotic entry through direct phosphorylation and inhibition of Cdc25 phosphatase (43). Whereas sty1Δ cells display division at increased size as a result of a G2/M delay, mutant cells expressing a constitutively activated version of MAPK kinase Wis1 (wis1DD), which directly activates Sty1, advance mitotic commitment and divide at reduced cell size (Fig. 3A) (42, 44). However, in both sty1Δ and wis1DD cells we observed that deletion of cpc2+ promoted a clear increase in cell size at division (Fig. 3A), suggesting that the control by Cpc2 of the cell cycle at G2/M is independent of Sty1 function during mitosis.
FIGURE 3.
Wee1 is a target for Cpc2 during the regulation of G2/M transition. A, cell morphology and size at division (micrometers ± S.D.) in strains MI709 (Wis1DD) and AN600 (Wis1DD cpc2Δ) (upper), MI204 (sty1Δ) and AN-120 (sty1Δ cpc2Δ) (lower), growing in YES medium after staining with Calcofluor white. B, upper, cell morphology and size at division in strains FY16230 (cdc2-3w cdc25Δ) and AN-CC3 (cdc2-3w cdc25Δ cpc2Δ) prepared as described above. B, lower, samples containing 104, 103, 102, or 101 cells of strains FY16230 (cdc2-3w cdc25Δ) and AN-CC3 (cdc2-3w cdc25Δ cpc2Δ) grown in YES medium were spotted onto Bacto Agar-supplemented YES plates and incubated for 3 days at 25 °C, 28 °C, 34 °C, or 37 °C before being photographed. C, cell morphology and size at division in strains FY7287 (cdc2-1w) and AN-CC1 (cdc2-1w cpc2Δ) (upper), FY7108 (wee1-50) and AN-CC5 (wee1-50 cpc2Δ) (lower) prepared as described above.
In fission yeast, the activity of Cdc2 kinase is negatively regulated by phosphorylation at Tyr15 through the antagonistic action of tyrosine phosphatase Cdc25 and Wee1 tyrosine kinase (6). To check whether Cpc2 signaling to Cdc2 is mediated by Cdc25, we used the cdc2-3w cdc25Δ background, in which the cdc2-3w mutation renders S. pombe cells insensitive to the lack of Cdc25 phosphatase (3). As shown in Fig. 3B, cell size at division (upper) and the thermosensitive phenotype (lower) in cdc2-3w cdc25Δ cpc2Δ cells clearly increased compared with cdc2-3w cdc25Δ cells. We thus turned our attention to Wee1 kinase, which maintains Cdc2 in an inactive state (6). Notably, no increase in cell size at division was observed in cdc2-1w cpc2Δ cells compared with the cdc2-1w mutant (Fig. 3C), which displays a wee1 phenotype and is refractile to Wee1 activity (3). Congruent with this observation, cells size at division in a strain expressing a temperature-sensitive mutation in Wee1 (wee1-50) was not affected by simultaneous deletion of cpc2+ (Fig. 3C). These findings support that Wee1 is required for Cpc2-mediated cell cycle control during mitotic commitment.
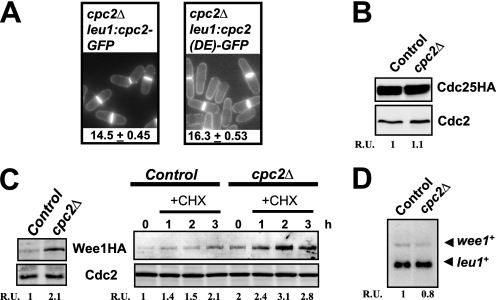
Previous work performed by others and ourselves has demonstrated that in fission yeast Cpc2 is a ribosomal bound protein that regulates the translation of specific mRNAs whose products are involved in various responses, including modulation of MAPK activity and cellular defense against oxidative stress (22, 23). Importantly, by using a strain expressing a mutant version of Cpc2 (R36D/K38E) with reduced ability to associate with ribosomes in vivo (22), we also showed that ribosome binding of Cpc2 is critical for proper control of cell size at the G2/M boundary (Fig. 4A). The above results, together with the fact that Wee1 is known to inhibit mitotic cycle progression in a dose-dependent fashion (3), raised the possibility that the absence of Cpc2 might increase Wee1 protein levels and/or activity. Indeed, although Cdc25 protein levels were not noticeably affected in the presence or absence of Cpc2 (Fig. 4B), growing cultures of cpc2Δ cells showed increased protein levels of Wee1 compared with control cells (expressed as a chromosomally tagged protein fused to the HA6H epitope) (see 0 time, Fig. 4C). Moreover, the increase in Wee1 protein levels observed in cpc2Δ cells did not result from enhanced expression of wee1+ mRNA (Fig. 4D), suggesting that Cpc2 negatively regulates Wee1 expression by repressing the translation of the corresponding mRNA. Alternatively, the cpc2 mutation might be affecting the stability or turnover of Wee1 protein.
FIGURE 4.
Cpc2 negatively regulates Wee1 protein levels. A, ribosome binding of Cpc2 is critical for proper regulation of G2/M transition. Cell morphology and size at division (micrometers ± S.D.) in strains AN071 (cpc2Δ cpc2-GFP) and AN072 (cpc2Δ cpc2 (DE)-GFP) growing in YES medium and stained with Calcofluor white are shown. B, strains FM-23 (cdc25-HA, control) and AN-CC20 (cdc25-HA cpc2Δ) were grown in YES medium to mid log phase. Cdc25 was detected in cell extracts by immunoblotting with anti-HA antibody. Anti-Cdc2 antibody was used for loading control. C, left, strains 1081 (wee1-3HA, control) and AN-CC8 (wee1-3HA cpc2Δ) were grown in YES medium to mid log phase. Total extracts were obtained, and Wee1 was detected by immunoblotting with anti-HA antibody. Anti-Cdc2 antibody was used for loading control. C, right, strains 1081 and AN-CC8 grown in YES medium were treated with 20 μg/ml cycloheximide and aliquots taken at the times indicated. Detection of Wee1 and Cdc2 was performed as described above. D, strains 1081 (control) and AN-CC8 (cpc2Δ) grown in YES medium to early log phase. Total RNA was extracted from each sample and 20 μg resolved in 1.5% agarose-formaldehyde gels. The denatured RNAs were transferred to nylon membranes and hybridized with 32P-labeled probes for wee1+ and leu1+ (loading control).
Convincing evidence has shown that Wee1 is essential for the G2 delay undergone by fission yeast upon partial inhibition of protein synthesis in the presence of cycloheximide and that, under these conditions, Wee1 protein levels are up-regulated (45). Interestingly, cycloheximide-treated cpc2Δ cells further increased Wee1 levels compared with control cells, supporting that Cpc2-mediated regulation of Wee1 operates at a different level (Fig. 4C).
Protein Levels of Cdr2, a Wee1 Inhibitor, Are Down-regulated in cpc2Δ Cells
Wee1 is negatively regulated in fission yeast by two closely related protein kinases, Nim1/Cdr1 and Cdr2 (12, 14). Recently, it has been reported that the DYRK family protein kinase Pom1 phosphorylates and inhibits Cdr2 in a dose-dependent manner to coordinate the time for mitotic entry at the appropriate cell size (15, 16).
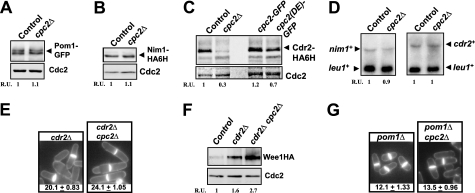
Our observation that Wee1 is up-regulated in cpc2Δ cells led us to explore the possibility that protein levels of Pom1, Cdr1, or Cdr2 might be altered in this mutant. As shown in Fig. 5, A and B, neither Pom1 nor Cdr1 levels (expressed as genomic versions fused to GFP and HA6H epitopes, respectively) were significantly affected by cpc2+ deletion. However, compared with control cells, cpc2Δ cells presented a clear reduction in the protein levels of Cdr2 kinase, expressed as a genomic version fused to a HA6H epitope (Fig. 5C). This decreased amount of Cdr2 was also evident in cells expressing a mutant Cpc2 (DE) protein that does not bind to the ribosome (Fig. 5C). Again, the lower Cdr2 protein levels did not result from a decreased expression of cdr2+ mRNA (Fig. 5D). Surprisingly, both the cell size at division and Wee1 protein levels in cells from the cdr2Δ cpc2Δ mutant were still higher than in cdr2Δ cells (Fig. 5, E and F). Moreover, cpc2+ deletion also increased cell size at division in pom1Δ cells (Fig. 5G), which divide at a short size due to lack of Cdr2 inhibition (15, 16). As a whole, our findings suggest that both Cdr2-dependent and -independent mechanisms are responsible for the increased Wee1 levels and the G2/M defect in cpc2Δ cells.
FIGURE 5.
Protein level of the Wee1 inhibitor Cdr2 is positively regulated by Cpc2. A, strains MM-76 (pom1-GFP, control) and AN-CC35 (pom1-GFP cpc2Δ) were grown in YES medium to mid log phase, total cell extracts were obtained, and Pom1 was detected by immunoblotting with anti-GFP antibody. Anti-Cdc2 antibody was used for loading control. B, strains LW117 (nim1-HA6H, control) and AN-CC15 (nim1-HA6H cpc2Δ) were grown as above described, and Nim1 was detected by immunoblotting with anti-HA antibody. C, strains JK2310 (cdr2-HA6H, control), AN-CC14 (cdr2-HA6H cpc2Δ), AN-CC23 (cdr2-HA6H cpc2-GFP), and AN-CC24 (cdr2-HA6H cpc2(DE)-GFP) were grown in YES medium as above, and Cdr2 was detected by immunoblotting with anti-HA antibody. D, strains LW117 (control), AN-CC15 (cpc2Δ), JK2310 (control), and AN-CC14 (cdr2-HA6H cpc2Δ) were grown in YES medium to early log phase. Total RNA was extracted from each sample and 20 μg resolved in 1.5% agarose-formaldehyde gels. The denatured RNAs were transferred to nylon membranes and hybridized with 32P-labeled probes for nim1+ (strains LW117 and AN-CC15), cdr2+ (strains JK2310 and AN-CC14), and leu1+ (loading control). E, cell morphology and size at division (micrometers ± S.D.) in strains JK2240 (cdr2Δ) and AN-CC12 (cdr2Δ cpc2Δ) growing in YES medium and stained with Calcofluor white are shown. F, strains 1081 (wee1-3HA, control), AN-CC45 (wee1-3HA cdr2Δ), and AN-CC46 (wee1-3HA cdr2Δ cpc2Δ) were grown in YES medium to mid log phase. Total extracts were obtained, and Wee1 was detected by immunoblotting with anti-HA antibody. Anti-Cdc2 antibody was used for loading control. G, cell morphology and size at division (micrometers ± S.D.) in strains TS313 (pom1Δ) and AN-CC27 (pom1Δ cpc2Δ) were determined as described in A.
DISCUSSION
The results of our work provide strong biochemical and genetic evidence to indicate that Cpc2, the RACK1 ortholog in fission yeast, positively regulates from the ribosome the cell cycle progression at G2/M transition by modulating the protein levels of Wee1 kinase. This kinase, in turn, directly phosphorylates and inhibits the activity of Cdc2, which is the master regulator of mitosis onset in eukaryotic organisms. The activities of Wee1 kinases are well known as dose-dependent inhibitors of mitotic commitment in eukaryotes (3), and thus their cellular levels and functions must be tightly regulated during cell cycle progression. Indeed, Wee1 has been shown to be destabilized during G2 and M phases of cell cycle in diverse organisms ranging from yeast to humans (46). In fission yeast, whereas wee1+ mRNA remains constant during the cell cycle, Wee1 protein levels oscillate throughout the cell cycle, decreasing during M and G1 phases (47). In this work we have shown that S. pombe mutants devoid of Cpc2 undergo a G2/M delay that is dependent on Wee1 because the increased cell size at division in Cpc2 mutants suppressed in the absence of Wee1 function. Contrariwise, either abrogation of Cdc25 activity or down-regulation of the Sty1 MAPK pathway, which modulates Cdc25 activity via Srk1 MAPKAPK (43), aggravates the cell cycle defect in the absence of Cpc2. Importantly, Wee1 protein levels, but not those of Cdc25, are up-regulated in Cpc2-null mutants, suggesting that they are responsible, at least in part, for the defect in cell cycle progression in these cells as discussed below.
It has been reported that cycloheximide-mediated partial inhibition of protein synthesis increases in S. pombe the levels of Wee1 protein and promotes cell cycle arrest at G2 (45). We have confirmed this result and furthermore found that Cpc2 deletion provokes an additive increase of Wee1 levels in cycloheximide-treated cells. This result makes sense when considering that, whereas Cpc2 associates with the 40 S ribosomal subunit, cycloheximide specifically binds the 60 S ribosomal subunit to block the translocation step in elongation during protein synthesis (48). Considering the versatility of Cpc2/RACK1 as a platform to bind different proteins, it appears likely that one or several Cpc2-binding partners might be involved in this process. Recently it has been described that Cpc2 associates with Moc2/Ded1, an essential RNA helicase involved in sexual differentiation which acts as a general translational regulator, and also with Moc1/Sds23, an inducer of sexual development which participates in stress resistance (49). The Cpc2-Moc-mediated complex appears to act as a translational regulator involved in controlling sexual differentiation in fission yeast through Ste11 transcription factor (49). Because both Moc2/Ded1 and Moc1/Sds23 are also known to play important roles in the regulation of the mitotic cell cycle (50, 51), they might be considered strong candidates to form part of the Cpc2-dependent translational mechanism controlling Wee1.
Kinase-dependent hyperphosphorylation is a well conserved mechanism for Wee1 inactivation during M phase (46). In fission yeast this task is performed at least by two SAD kinases, Nim1/Cdr1 and Cdr2, which phosphorylate and inactivate Wee1 (8–14). As a result, deletion of either nim1+/cdr1+ or cdr2+ causes a G2/M delay due to lack of Wee1 down-regulation (8–14). Importantly, we have found that protein levels of Cdr2 are down-regulated in the absence of Cpc2, supporting that Cpc2 exerts its function as a positive translational regulator of the mRNA encoding Cdr2. Moreover, this control is specific for cdr2+ because the protein levels of Cdr1 remained unchanged in the absence of Cpc2. However, the possibility that Cpc2 is positively affecting Cdr2 turnover cannot be ruled out.
As a whole, our findings strongly suggest that in fission yeast Cpc2 targets Wee1 by at least two different mechanisms: down-regulation of the Wee1 protein levels and positive regulation of the translation of Wee1 inhibitor Cdr2. The increase in both cell cycle defect and Wee1 levels in cdr2Δ cpc2Δ cells compared with cdr2Δ or cpc2Δ single mutants, together with the partial suppression of short size in pom1Δ cells (lacking Cdr2 inhibition) by simultaneous deletion of cpc2+, favor the existence of these two alternative regulatory mechanisms. In this context, it has been recently described that in human cells RACK1 binds to hypoxia-inducible factor-1 and promotes its degradation through the E3 ubiquitin ligase complex which is associated with Elongin C (52). Conversely, RACK1 loss of function by RNA interference increases hypoxia-inducible factor-1 levels, enhancing its transcriptional activity (52). Interestingly, the 22-amino acid sequence in the WD-40 repeat 6 (WD6) of RACK1 which is responsible for its binding to hypoxia-inducible factor-1 and Elongin C (52) is conserved in Cpc2, strongly suggesting that that in fission yeast Cpc2 might regulate Wee1 stability and/or turnover by a similar mechanism.
In mouse NIH3T3 cells RACK1 binds and suppresses the activity of Src1 at G1, delaying entry of cells into S phase (29). Disruption of the RACK1-Src complex results in increased activities of cyclin-dependent kinases CDK2 and CDK4, the induction of cyclins A, E, and D1, inhibited expression of cyclin-dependent kinase inhibitors p16, p15, and p27, and an increased phosphorylation of pRB (retinoblastoma protein), all of which are key modulators of G1/S transition (30). On the contrary, our results suggest that in fission yeast Cpc2 does not perform a similar role because the kinetics of G1/S transition estimated by flow cytometry analysis, prereplication complex formation, and Cdc2 phosphorylation at Tyr15, was virtually identical both in control and cpc2Δ cells. Thus, it appears likely that the function of RACK1 proteins as modulators of cell cycle progression has somehow diverged through evolution. In this context, it has been shown that expression of rat RACK1 in fission yeast can structurally and functionally complement cpc2Δ cells, including the cell cycle defect at G2/M (21). Because RACK1 also resides on the 40 S ribosomal subunit, it seems reasonable that in higher eukaryotes RACK1 may also control from the ribosome the G2/M transition of the cell cycle in a Wee1-dependent manner, similarly to Cpc2. S. pombe cells spend most of their time (about three-quarters of the cycle) in G2, whereas G1 is typically the longest phase of the cell cycle in mammalian cells (6). This situation might somehow hide the existence of the different subtle control by RACK1 and Cpc2. In any case, the results presented here reveal a pivotal role for Cpc2 at the G2/M transition in fission yeast and define the existence of a novel control at the translational level during the mitotic onset in this model organism.
Acknowledgments
We thank R. R. Daga (University Pablo de Olavide, Spain) for critical reading of the manuscript; T. Kato (ERATO, Japan), S. E. Kearsey (University of Oxford, United Kingdom), P. Nurse (Rockefeller University, New York, NY), J. B. Millar (University of Warwick, United Kingdom), P. Pérez (CSIC/University of Salamanca, Spain), P. Russell (The Scripps Research Institute, La Jolla, CA), and the Yeast Genetic Resource Center (YGRC, Japan) for supplying yeast strains; and P. Pérez for help with flow cytometry analysis.
This work was supported by MICINN Grants BFU2008-01653 and Fundación Séneca (Región de Murcia), Spain, Grant 08725/PI/08 (to J. C.).
- RACK1
- receptor of activated protein C 1
- EMM2
- Edinburgh minimal medium 2
- HA6H
- epitope comprising hemagglutinin antigen plus six histidine residues
- YES
- yeast extract plus supplements.
REFERENCES
- 1.Nurse P. (1990) Nature 344, 503–508 [DOI] [PubMed] [Google Scholar]
- 2.Rupes I. (2002) Trends Genet. 18, 479–485 [DOI] [PubMed] [Google Scholar]
- 3.Russell P., Nurse P. (1987) Cell 49, 559–567 [DOI] [PubMed] [Google Scholar]
- 4.Gould K. L., Nurse P. (1989) Nature 342, 39–45 [DOI] [PubMed] [Google Scholar]
- 5.Featherstone C., Russell P. (1991) Nature 349, 808–811 [DOI] [PubMed] [Google Scholar]
- 6.MacNeil S. A., Nurse P. (1997) in The Molecular and Cellular Biology of the Yeast Saccharomyces (Pringle J. R., Broach J. R., Jones E. W. eds) Vol. 3, pp. 697–763, Cell cycle and Cell Biology, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 7.Nurse P. (1975) Nature 256, 547–551 [DOI] [PubMed] [Google Scholar]
- 8.Russell P., Nurse P. (1987) Cell 49, 569–576 [DOI] [PubMed] [Google Scholar]
- 9.Feilotter H., Nurse P., Young P. G. (1991) Genetics 127, 309–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coleman T. R., Tang Z., Dunphy W. G. (1993) Cell 72, 919–929 [DOI] [PubMed] [Google Scholar]
- 11.Parker L. L., Walter S. A., Young P. G., Piwnica-Worms H. (1993) Nature 363, 736–738 [DOI] [PubMed] [Google Scholar]
- 12.Wu L., Russell P. (1993) Nature 363, 738–741 [DOI] [PubMed] [Google Scholar]
- 13.Breeding C. S., Hudson J., Balasubramanian M. K., Hemmingsen S. M., Young P. G., Gould K. L. (1998) Mol. Biol. Cell 9, 3399–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kanoh J., Russell P. (1998) Mol. Biol. Cell 9, 3321–3334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin S. G., Berthelot-Grosjean M. (2009) Nature 459, 852–856 [DOI] [PubMed] [Google Scholar]
- 16.Moseley J. B., Mayeux A., Paoletti A., Nurse P. (2009) Nature 459, 857–860 [DOI] [PubMed] [Google Scholar]
- 17.McCahill A., Warwicker J., Bolger G. B., Houslay M. D., Yarwood S. J. (2002) Mol. Pharmacol. 62, 1261–1273 [DOI] [PubMed] [Google Scholar]
- 18.López-Bergami P., Habelhah H., Bhoumik A., Zhang W., Wang L. H., Ronai Z. (2005) Mol. Cell 19, 309–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.He D. Y., Neasta J., Ron D. (2010) J. Biol. Chem. 285, 19043–19050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robles M. S., Boyault C., Knutti D., Padmanabhan K., Weitz C. J. (2010) Science 327, 463–466 [DOI] [PubMed] [Google Scholar]
- 21.McLeod M., Shor B., Caporaso A., Wang W., Chen H., Hu L. (2000) Mol. Cell. Biol. 20, 4016–4027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Núñez A., Franco A., Madrid M., Soto T., Vicente J., Gacto M., Cansado J. (2009) Mol. Biol. Cell 20, 3996–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shor B., Calaycay J., Rushbrook J., McLeod M. (2003) J. Biol. Chem. 278, 49119–49128 [DOI] [PubMed] [Google Scholar]
- 24.Baum S., Bittins M., Frey S., Seedorf M. (2004) Biochem. J. 380, 823–830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nilsson J., Sengupta J., Frank J., Nissen P. (2004) EMBO Rep. 5, 1137–1141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sengupta J., Nilsson J., Gursky R., Spahn C. M., Nissen P., Frank J. (2004) Nat. Struct. Mol. Biol. 11, 957–962 [DOI] [PubMed] [Google Scholar]
- 27.Coyle S. M., Gilbert W. V., Doudna J. A. (2009) Mol. Cell. Biol. 29, 1626–1634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rothberg K. G., Burdette D. L., Pfannstiel J., Jetton N., Singh R., Ruben L. (2006) J. Biol. Chem. 281, 9781–9790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mamidipudi V., Zhang J., Lee K. C., Cartwright C. A. (2004) Mol. Cell. Biol. 24, 6788–6798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mamidipudi V., Miller L. D., Mochly-Rosen D., Cartwright C. A. (2007) Biochem. Biophys. Res. Commun. 352, 423–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O'Connell M. J., Raleigh J. M., Verkade H. M., Nurse P. (1997) EMBO J. 16, 545–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Soto T., Villar-Tajadura M. A., Madrid M., Vicente J., Gacto M., Pérez P., Cansado J. (2010) J. Biol. Chem. 285, 11516–11525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Madrid M., Soto T., Franco A., Paredes V., Vicente J., Hidalgo E., Gacto M., Cansado J. (2004) J. Biol. Chem. 279, 41594–41602 [DOI] [PubMed] [Google Scholar]
- 34.Moreno S., Klar A., Nurse P. (1991) Methods Enzymol. 194, 795–823 [DOI] [PubMed] [Google Scholar]
- 35.Kearsey S. E., Montgomery S., Labib K., Lindner K. (2000) EMBO J. 19, 1681–1690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bähler J., Wu J. Q., Longtine M. S., Shah N. G., McKenzie A., 3rd, Steever A. B., Wach A., Philippsen P., Pringle J. R. (1998) Yeast 14, 943–951 [DOI] [PubMed] [Google Scholar]
- 37.Soto T., Beltrán F. F., Paredes V., Madrid M., Millar J. B. A., Vicente-Soler J., Cansado J., Gacto M. (2002) Eur. J. Biochem. 269, 5056–5065 [DOI] [PubMed] [Google Scholar]
- 38.Madrid M., Núñez A., Soto T., Vicente-Soler J., Gacto M., Cansado J. (2007) Mol. Biol. Cell 18, 4405–4419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alfa C., Fantes P., Hyams J., Mcleod M., Warbrick E. (1993) Experiments with Fission Yeast, Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 40.McInerny C. J., Kersey P. J., Creanor J., Fantes P. A. (1995) Nucleic Acids Res. 23, 4761–4768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tvegård T., Soltani H., Skjølberg H. C., Krohn M., Nilssen E. A., Kearsey S. E., Grallert B., Boye E. (2007) Genes Dev. 21, 649–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Petersen J. (2009) Biochem. Soc. Trans. 37, 273–277 [DOI] [PubMed] [Google Scholar]
- 43.López-Avilés S., Grande M., González M., Helgesen A. L., Alemany V., Sánchez-Piris M., Bachs O., Millar J. B., Aligue R. (2005) Mol. Cell 17, 49–59 [DOI] [PubMed] [Google Scholar]
- 44.Shiozaki K., Russell P. (1995) Nature 378, 739–743 [DOI] [PubMed] [Google Scholar]
- 45.Suda M., Yamada S., Toda T., Miyakawa T., Hirata D. (2000) FEBS Lett. 486, 305–309 [DOI] [PubMed] [Google Scholar]
- 46.Kellogg D. R. (2003) J. Cell. Sci. 116, 4883–4890 [DOI] [PubMed] [Google Scholar]
- 47.Aligue R., Wu L., Russell P. (1997) J. Biol. Chem. 272, 13320–13325 [DOI] [PubMed] [Google Scholar]
- 48.Schneider-Poetsch T., Ju J., Eyler D. E., Dang Y., Bhat S., Merrick W. C., Green R., Shen B., Liu J. O. (2010) Nat. Chem. Biol. 6, 209–217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Paul S. K., Oowatari Y., Kawamukai M. (2009) FEBS J. 276, 5076–5093 [DOI] [PubMed] [Google Scholar]
- 50.Ishii K., Kumada K., Toda T., Yanagida M. (1996) EMBO J. 15, 6629–6640 [PMC free article] [PubMed] [Google Scholar]
- 51.Grallert B., Kearsey S. E., Lenhard M., Carlson C. R., Nurse P., Boye E., Labib K. (2000) J. Cell Sci. 113, 1447–1458 [DOI] [PubMed] [Google Scholar]
- 52.Liu Y. V., Baek J. H., Zhang H., Diez R., Cole R. N., Semenza G. L. (2007) Mol. Cell 25, 207–217 [DOI] [PMC free article] [PubMed] [Google Scholar]