Abstract
Naturally occurring CD4+CD25+Foxp3+ regulatory T cells (Tregs) suppress proliferation of CD4+CD25− effector T cells (Teffs) by mechanisms that are not well understood. We have previously demonstrated a novel mechanism of Treg suppression, i.e. interference with extracellular redox remodeling that occurs during activation of T cells by dendritic cells. In this study, we demonstrate that Treg-mediated redox perturbation is antigen-dependent but not antigen-specific, is CTLA-4-dependent, and requires cell-cell contact. Furthermore, we show that Tregs use multiple strategies for extracellular redox remodeling, including diminished GSH synthesis in dendritic cells via decreased expression of γ-glutamylcysteine synthetase, the limiting enzyme for GSH synthesis. Tregs also consume extracellular cysteine and partition it more proficiently to the oxidation product (sulfate), whereas Teffs divert more of the cysteine pool toward protein and GSH synthesis. Tregs appear to block GSH redistribution from the nucleus to the cytoplasm in Teffs, which is abrogated by the addition of exogenous cysteine. Together, these data provide novel insights into modulation of sulfur-based redox metabolism by Tregs, leading to suppression of T cell activation and proliferation.
Keywords: Antioxidant, Glutathione, Immunochemistry, Metabolism, Thiol, T Cell Activation, Dendritic Cells, Glutathione, Regulatory T Cells
Introduction
A fundamental property of the immune system is to distinguish self from non-self. During maturation of T cells in thymus, autoreactive T cells are recognized and eliminated. However, a small fraction of autoimmune T cells escape to the periphery and cause damage to host tissues. By mechanisms that are not well understood, regulatory T cells (Tregs)2 are able to inhibit autoimmune T cells to maintain self-tolerance and immunosuppression (1–3). Tregs also play important roles in antitumor responses as well as transplantation immunity. Dysregulation of Treg function has been shown to be involved in different kinds of immunological diseases ranging from the digestive to the central nervous system (4, 5).
Tregs deploy various strategies to mediate their suppressive activity, including (i) secretion of immunosuppressive cytokines such as TGF-β and IL-10; (ii) cytolysis by granzyme secretion; (iii) metabolic disruption, e.g. by adenosine; (iv) suppression of dendritic cell (DC) function, e.g. via induction of indoleamine 2, 3-dioxygenase (6–8); and (v) perturbing DC-dependent extracellular redox remodeling, leading to restricted extracellular cysteine (Cysex) availability for naïve T (Tn) cells (9). A key role for CTLA-4 (cytotoxic T-lymphocyte antigen 4), a co-receptor expressed preferentially on Tregs, is implicated in the Treg suppression mechanism (1, 8, 10). CTLA-4 interacts with CD80/CD86 (cluster of differentiation) on antigen-presenting cells (APCs) and transduces an intracellular inhibitory signal to APCs. Thus, one strategy for Treg-dependent immunosuppression is via down-regulation of APC function (1, 11).
In addition to the T cell receptor (TCR)-antigen MHC class II interaction, co-stimulatory signals, and cytokines, T cell activation and proliferation also require a reducing microenvironment that is shaped mainly by APCs, especially DCs (9, 12, 13). Upon stimulation by T cells, DCs increase uptake of cystine via the xc− cystine transporter and, by a convoluted metabolic route involving the γ-glutamyl cycle, furnish Cysex, resulting in a relatively more reducing redox potential that is conducive to T cell proliferation (2, 9). Furthermore, cysteine is needed by T cells for synthesis of GSH, which provides reducing power for DNA synthesis (14) and for cell cycle progression from the G1 to S phase (15, 16). Although extracellular cystine is relatively abundant, naïve T cells are inefficient at transporting cystine, the oxidized form of the amino acid cysteine, and depend on DC-derived cysteine to meet their metabolic needs (17). By controlling the Cysex level, DCs are able to affect intracellular GSH levels and subsequent redox signaling pathways in T cells (2).
The physiological relevance of redox remodeling is demonstrated by the dramatic increase in non-protein thiols in lymphoid tissues following immunization (18). Additionally, Peyer's patches from the intestine show very low thiol staining because resident APCs from the lamina propria lack the xc− transporter for cystine. However, under inflammatory conditions as in inflammatory bowel diseases, infiltration of peripheral APCs with high xc− transporter expression allows Cysex accumulation, promoting activation and hyperreactivity of lamina propria T cells (19). We have demonstrated that Tregs suppress Cysex accumulation and that this is correlated with suppression of T cell activation and proliferation (9). However, the mechanism by which Tregs interfere with the redox signaling cross-talk between DCs and effector T cells (Teffs) is unknown.
In this study, we demonstrate that Tregs decrease Cysex levels in an antigen-dependent but antigen-nonspecific and CTLA-4-dependent manner. We show that Tregs use multiple strategies for changing the extracellular redox potential, including modulation of DC and Teff GSH metabolism and competitive uptake of cysteine. This study provides the first mechanistic insights into how Tregs influence redox metabolism in DCs and, consequently, in Teffs.
EXPERIMENTAL PROCEDURES
Animals
DO11.10 TCR transgenic mice were a generous gift from Dr. Nicholas Lukacs (University of Michigan) and were bred at our animal facility. BALB/c mice and CD1 mice (7–10 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME).
Cell Preparation and Cell Culture Conditions
DCs were obtained from bone marrow of DO11.10 mice and induced by recombinant murine GM-CSF and IL-4 (R&D Systems) as described previously (9, 20). Immature bone marrow-derived DCs were harvested at day 7 and used in T cell co-cultures as APCs.
Tregs (CD4+CD25+) and Tn cells (CD4+CD25−) were isolated from mouse spleen and lymph nodes by magnetic-activated cell sorting using an AutoMACS sorter (Miltenyi Biotec) as described (9, 21). For activation of Teffs, purified Tn cells were cultured in 24-well plates (1 ml) supplemented with 1 μm ovalbumin-(323–329) (OVA323–329) antigen and irradiated splenocyte feeders (1:3) or DCs (2:1). The same conditions were employed for activation of Tregs except that 20 ng/ml IL-2 (R&D Systems) was also added to the medium.
DCs (5 × 105/well) were co-cultured in 24-well plates with Tn cells (1:4) with or without Tregs (1:4:2) for 48 h at 37 °C in a 5% CO2 incubator in RPMI medium 1640 supplemented with 100 μg/ml penicillin and streptomycin, 2 mm l-glutamine, 50 μm 2-mercaptoethanol, and 2.5% heat-inactivated fetal bovine serum in the presence of either 1 μm OVA323–329 antigen or anti-CD3 antibody (1 μg/ml). Alternatively, DCs were treated with LPS (100 ng/ml) in the absence and presence of Tregs and 1 μm OVA323–329 for 48 h. Anti-CTLA-4 antibody (100 μg/ml) was added to the DC/Tn cell/Treg or DC/LPS/Treg co-culture to block the function of CTLA-4. In Transwell experiments, DCs were co-cultured with Tn cells, and Tregs were placed in either direct contact with DCs/Tn cells (at a ratio of 1:4:2) or the upper chamber of the Transwell (Millicell, 0.4-μm pore size; Millipore, Bedford, MA) at the same ratio.
Measurement of Thiols and Disulfides by HPLC
Concentrations of extracellular cystine, cysteine, GSH, and intracellular GSH (GSHin) were measured using an HPLC method as described (9, 22). The GSHin values were normalized to protein concentration determined by the Bradford assay with bovine serum albumin as a standard. The extracellular cysteine/cystine redox potential (Eh) was calculated using the Nernst equation: Eh = E0 + RT/2F ln([cystine]/[cysteine]2), where E0 = −250 mV at pH 7.4.
T Cell Proliferation Assay
Tn cells (4 × 104) were cultured with varying numbers of Tregs for 72 h in round-bottom 96-well plates with DCs (1 × 104) in the presence of either 1 μm OVA323–329 or 1 μg/ml anti-CD3 antibody. When needed, 50 μm cysteine was added every 24 h during the culture. T cell proliferation was assayed by measuring incorporation of [3H]thymidine (1 μCi/ml; PerkinElmer Life Sciences) during the last 16 h of culture.
Western Blot Analysis
DCs from control, DC/LPS-, DC/LPS/Treg-, and DC/LPS/Treg/anti-CTLA-4 antibody-treated cultures were harvested and lysed in lysis buffer on ice. Aliquots of cell lysates (20 μg) were boiled, loaded onto a 10% SDS-polyacrylamide gel, and electroblotted onto a PVDF membrane. Antibodies against γ-glutamyl synthetase (Lab Vision) and β-actin (Sigma) were used to monitor expression of the proteins and detected using the Dura chemiluminescent horseradish peroxidase system (Pierce) following the manufacturer's protocol.
Metabolic Labeling
Activated Teffs and Tregs (2 × 106 each) were cultured in cystine-free RPMI medium 1640 supplemented with 50 μm cysteine, 100 μg/ml penicillin and streptomycin, 2 mm l-glutamine, 50 μm 2-mercaptoethanol, and 2.5% heat-inactivated fetal bovine serum. 1 μCi/ml [35S]cysteine (PerkinElmer Life Sciences) was added to the cell culture medium for 24 h. T cells were collected and suspended in 125 μl of PBS for the following analyses: 1) 25 μl for protein normalization, mixed with 25 μl of lysis buffer; 2) 50 μl for GSH analysis, mixed with 50 μl of metaphosphoric acid solution (16.8 g/liter metaphosphoric acid, 5 m NaCl, and 5 mm EDTA) (intracellular GSH radioactivity was measured and quantified as described previously (9)); and 3) 50 μl for taurine analysis and protein radioactivity measurement, mixed with 50 μl 10% TCA. Proteins were precipitated with 10% TCA and dissolved in 1 m NaOH to measure radioactivity. Supernatants were used for taurine analysis using an HPLC method as described (23). Media samples were collected for the measurement of cysteine and sulfate radioactivity. To measure the radioactivity associated with Cysex, HPLC fractions containing cysteine were collected and measured by scintillation counting. For sulfate measurement, BaCl2 (100 mm final concentration) was added to the medium to precipitate sulfate. The pellet was dissolved in 1 m NaOH, and the radioactivity was analyzed in a scintillation counter. The radioactivity of sulfate in the control medium was subtracted from the final values.
Confocal Microscopy and Fluorescence Analyses
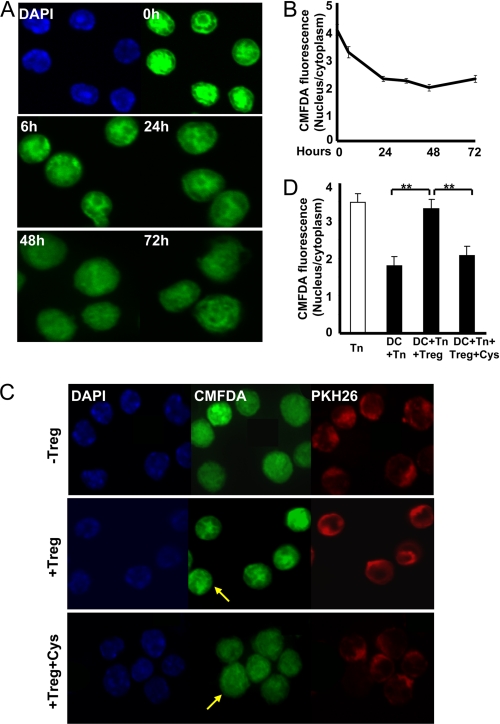
Tn cells were cultured with DCs for 0, 6, 24, 36, 48, and 72 h in the presence of 1 μm OVA323–329. In experiments also containing Tregs, Tn cells were prestained with 2 μm PKH26 (Sigma), a fluorescent dye that labels membranes, to distinguish them from Tregs under co-culture conditions. When used, Tregs were added at a 1:4:2 DC/Tn cell/Treg ratio in the presence of 1 μm OVA323–329 for up to 48 h. Cysteine (50 μm) was added every 24 h during the culture. At the conclusion of the experiment, T cells from different culture conditions were separated from DCs and labeled with 10 μm 5-chloromethylfluorescein diacetate (CMFDA) for 30 min at 37 °C. Following removal of the staining solution, cells were incubated for another 30 min at 37 °C in 1 ml of prewarmed serum-free medium. Hoechst dye (2 μg/ml; Invitrogen) was added during the last 5 min to stain nuclei. Cells were then washed with PBS and fixed with 4% paraformaldehyde. Confocal images were acquired using an Olympus FV500 confocal microscope. The following excitation wavelengths were employed for the individual dyes: 360 nm for Hoechst (emission, 480 nm), 551 nm for PKH26 (emission, 570 nm), and 492 nm for CMFDA (emission, 517 nm).
For quantitative analysis of the GSH fluorescence intensity, the nuclear perimeter was defined by the area of Hoechst staining, and the cell perimeter was defined from the bright field image of the cell seen by light microscopy as described previously (24). The cytoplasmic volume was the difference in the whole cell volume and the nuclear volume. The nuclear/cytoplasmic GSH ratio was represented as the total CMFDA intensity (calculated using ImageJ software) in each compartment.
Statistical Analysis
Comparison between groups was done using Student's two-tailed t test. p values <0.05 were considered to be statistically significant.
RESULTS
Treg-mediated Redox Remodeling Is Antigen-dependent but Antigen-nonspecific
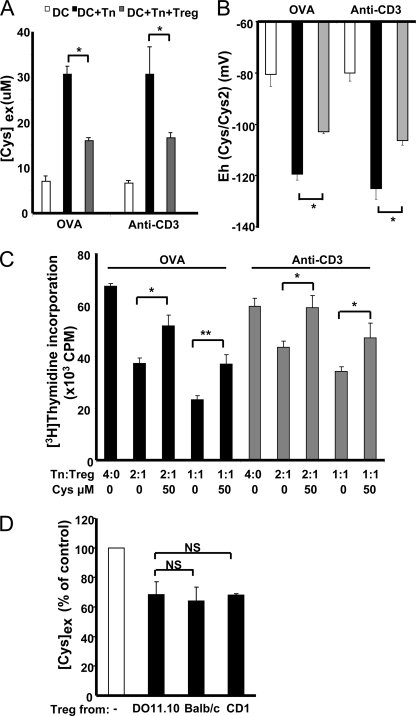
We have previously used BALB/c mice as a source of immune cells and shown that Tregs suppress Cysex accumulation with anti-CD3 antibody stimulation (9). Although this mode of T cell activation is widely used to mimic antigenic stimulation, the absence of antigen activation limits biological interpretation (25). We therefore evaluated the antigen dependence of Treg-mediated redox remodeling using DO11.10 OVA323–329-specific TCR transgenic mice. DCs, Tn cells, and Tregs were purified from DO11.10 mice and co-cultured in the presence of either OVA323–329 antigen or anti-CD3 antibody. As shown in Fig. 1A, the magnitude of Cysex accumulation was similar regardless of whether OVA323–329 antigen or anti-CD3 antibody stimulation was utilized. Furthermore, the magnitude of the decrease in Cysex accumulation by Tregs was also similar under both conditions (Fig. 1A). DCs in culture held the extracellular cysteine/cystine redox potential at approximately −80 mV (Fig. 1B), consistent with the growth arrest/differentiation stage of these cells. Co-culture of DCs with Tn cells in the presence of either OVA323–329 antigen or anti-CD3 antibody stimulation resulted in a reductive shift to approximately −120 mV, consistent with conditions favoring T cell proliferation (2, 26). However, the addition of Tregs to the DC/Tn cell co-culture caused an oxidative shift to approximately −100 mV. We have previously shown that the addition of exogenous cysteine at levels seen under DC/Tn cell co-culture conditions abrogates Treg-mediated suppression of T cell proliferation induced by anti-CD3 antibody activation (9). Similarly, with OVA stimulation, the addition of 50 μm cysteine also alleviated inhibition of T cell proliferation by Tregs (Fig. 1C). In subsequent experiments, DCs, Tn cells, and Tregs from DO11.10 mice were used with OVA323–329 antigen stimulation.
FIGURE 1.
Antigen-dependent but antigen-nonspecific Treg-mediated redox remodeling. DCs were co-cultured with Tn cells (1:4) or with Tn cells and Tregs (1:4:2) in the presence of 1 μm OVA323–329 antigen or 1 μg/ml anti-CD3 antibody. A, Tregs suppressed Cysex accumulation regardless of OVA323–329 antigen or anti-CD3 antibody activation. B, Tregs increased the extracellular cysteine/cystine redox potential in an antigen-dependent manner. C, the addition of exogenous cysteine abrogated Treg suppression of Teff proliferation, which was antigen-dependent. D, Treg suppression of Cysex accumulation was not antigen-specific. DCs were co-cultured with Tn cells (1:4 ratio, both from DO11.10 mice) in the presence of 1 μm OVA323–329 antigen. Tregs from DO11.10, BALB/c, and CD1 mice were added separately to the DC/Tn cell co-culture, and [Cys]ex was measured. [Cys]ex is expressed as a percentage of the concentration in DC/Tn cell co-culture medium. Data represent the mean ± S.D. of four (A and B) and two (C and D) independent experiments, each performed at least in duplicates. *, p < 0.05; **, p < 0.005; NS, not significant (Student's two-tailed t test).
Activation of Tn cells requires antigen presented by MHC class II molecules to be recognized by the TCR. To evaluate whether the Treg effect on Cysex is antigen-specific, DCs and Tn cells from DO11.10 mice and Tregs from different mouse strains were used. Tregs from DO11.10 mice suppressed Cysex levels to ∼70% compared with DC/Tn cell co-cultures, as expected (Fig. 1D). Similarly, Tregs from BALB/c mice and CD1 mice also suppressed Cysex accumulation to a similar extent, indicating that Treg-mediated redox remodeling is antigen-nonspecific.
Treg-mediated Redox Remodeling Is Contact- and CTLA-4-dependent
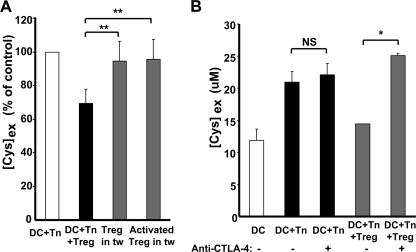
We have previously shown that DC-T cell contact is required for Cysex accumulation (9). However, in principle, Treg suppression of Cysex accumulation could occur simply via competition for cysteine released by DCs. To address this possibility, Tregs were cultured in the upper chamber of a Transwell setup, whereas DCs and Tn cells were in the lower chamber. Under these conditions, Tregs were unable to suppress Cysex accumulation (Fig. 2A). Furthermore, Cysex levels were not reduced when Tregs pre-activated by DCs and IL-2 were employed. These results suggest that cell-cell contact is required for Treg-mediated redox remodeling, consistent with the view that Treg suppression of T cell proliferation in vitro is cell contact-dependent (1, 8).
FIGURE 2.
Contact- and CTLA-4-dependent redox remodeling by Tregs. A, DCs were co-cultured with Tn cells and Tregs (1:4:2) or with Tregs placed in the upper chamber of a Transwell (tw). In another experiment, Tregs were first activated by DCs in the presence of 1 μm OVA323–329 and 20 ng/ml IL-2 and then cultured in the Transwell. [Cys]ex is expressed as a percentage of the concentration in untreated DC/Tn cell co-culture medium. B, DCs were co-cultured with Tn cells (1:4) or with Tn cells and Tregs (1:4:2) with (+) and without (−) anti-CTLA-4 antibody (100 μg/ml). Data represent the mean ± S.D. and are representative of three independent experiments performed in duplicate. *, p < 0.05; **, p < 0.005; NS, not significant (Student's two-tailed t test).
Tregs constitutively express high levels of CTLA-4, which competes with CD28 on Teffs and transmits inhibitory signals to cells (1, 10, 27–29). To investigate the role of CTLA-4 in Treg-mediated redox remodeling, we used anti-CTLA-4 antibody. As shown in Fig. 2B, anti-CTLA-4 antibody abrogated the effect of Tregs on Cysex accumulation. As a control, anti-CTLA-4 antibody added to a DC/Tn cell co-culture did not interfere with Cysex levels. These results demonstrate that diminution of Cysex by Tregs is dependent on co-stimulatory molecules e.g. CTLA-4.
Tregs Interfere with GSH Metabolism in DCs
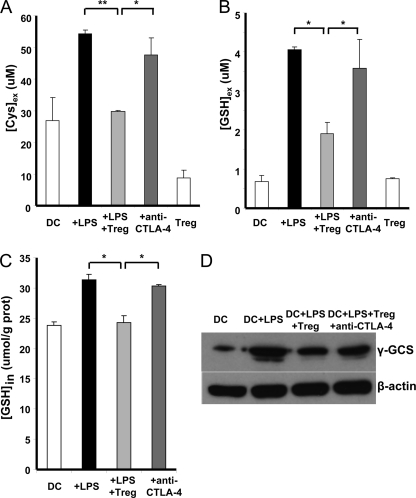
In principle, Tregs can interfere with the redox cross-talk between DCs and Teffs in at least three ways: 1) modulation of DC function that leads to lower cysteine production, 2) competition for the Cysex pool, and 3) modulation of Teff function by limiting cysteine availability. First, we investigated whether Tregs inhibit cysteine secretion by DCs using LPS stimulation to exclude the effects of Teffs on DCs in the experiment. We have demonstrated that, like Teffs, LPS stimulates Cysex accumulation (9), and Tregs suppressed this effect in a CTLA-4-dependent fashion (Fig. 3A). We found that, in addition to decreasing Cysex levels, Tregs also decreased [GSH]ex, a response that was abrogated by anti-CTLA-4 antibody (Fig. 3B). This was paralleled by an increase in [GSH]in in LPS-stimulated DCs, which was inhibited by Tregs in a CTLA-4-dependent manner (Fig. 3C). Intracellular GSH concentrations range from 1 to 10 mm. Hence, an ∼25% reduction in this large pool represents a significant metabolic change that can impact cell function (44). Western blot analysis revealed significant up-regulation of the catalytic subunit of γ-glutamyl synthetase in response to LPS stimulation, attenuation of this response by Tregs, and partial abrogation of the Treg effect by anti-CTLA-4 antibody (Fig. 3D). In contrast, Tregs did not alter the expression of other key proteins involved in mediating Cysex accumulation, viz. the xc− transporter and γ-glutamyl transpeptidase. These results suggest that one mechanism by which Tregs decrease Cysex accumulation is by inhibiting GSH synthesis in DCs.
FIGURE 3.
Tregs interfere with GSH synthesis by DCs. DCs were treated with LPS (100 ng/ml) and cultured with and without Tregs (1:2) and with and without anti-CTLA-4 antibody (100 μg/ml). A and B, [Cys]ex and [GSH]ex, respectively, were measured by HPLC. C, [GSH]in was measured by HPLC and normalized to protein concentration. D, Western blot analysis of the expression of γ-glutamyl synthetase (γ-GCS) in DCs. β-Actin was used as a control for equal loading. Data represent the mean ± S.D. (A–C) and are representative of three independent experiments (A–D). *, p < 0.05; **, p < 0.005 (Student's two-tailed t test).
Cysteine Uptake and Utilization by Tregs and Teffs
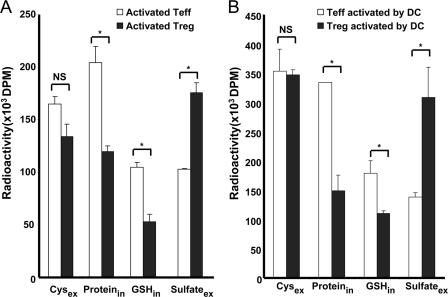
To determine whether competition by Tregs for the Cysex pool represents an additional mechanism of regulating Cysex levels, we used a metabolic labeling approach with [35S]cysteine. After 24 h, the radioactivity associated with the cysteine pool was not significantly different in the Treg versus Teff medium (Fig. 4A). This suggests that cysteine consumption by Tregs might constitute one mechanism for reducing Cysex during activation of T cells.
FIGURE 4.
Fate of cysteine in Tregs and Teffs. A, Tn cells and Tregs were activated by irradiated splenocytes (1:3) in the presence of 1 μm OVA323–329 antigen for 72 h. IL-2 (20 ng/ml) was added for Treg activation. Activated Teffs and Tregs (2 × 106 each) were incubated with [35S]cysteine (1 μCi/ml) in T cell medium containing 50 μm cysteine for 24 h. [35S]Cysteine remaining in the medium and its incorporation into intracellular protein and GSH and extracellular sulfate were determined. B, Tn cells and Tregs were activated by DCs (2:1) in the presence of 1 μm OVA323–329 antigen for 48 h and separated from DCs. [35S]Cysteine remaining in the medium and its incorporation into protein, GSH, and sulfate were determined. Data are representative of three (A) and two (B) independent experiments. *, p < 0.05; NS, not significant (Student's two-tailed t test).
However, for competition to be a plausible mechanism, Tregs, which are non-proliferating, must show net consumption of the imported cysteine to support its continuous influx. To address this issue, we determined the fate of cysteine imported by Teffs and Tregs. The four major intracellular cysteine sinks are protein, GSH, taurine, and sulfate (30), and the incorporation of [35S]cysteine into these pools in activated Tregs and Teffs was assessed. As shown in Fig. 5A, Teffs showed considerably higher incorporation of radioactive cysteine into protein (50%) and GSH (25%) compared with Tregs. This is consistent with activated Teffs being fated to proliferate, whereas Tregs are anergic. In contrast, a greater proportion (50%) of cysteine was oxidized to sulfate by Tregs compared with Teffs (Fig. 4A). Almost no incorporation of radioactivity into taurine was seen in the intra- or extracellular pools in either Teffs or Tregs. A very similar pattern of radioactivity distribution was observed when Teffs and Tregs were activated by DCs instead of irradiated splenocytes (Fig. 4B). Collectively, these results reveal that cysteine is preferentially utilized for protein synthesis by Teffs and is catabolized to sulfate by Tregs.
FIGURE 5.
Tregs block relocalization of nuclear GSH into the cytoplasm in Teffs during T cell activation. A, confocal microscopy images of GSH localization in T cells during 72 h of T cell activation. T cells were co-cultured with DCs for 0, 6, 24, 36, 48, and 72 h and separated from the co-culture by gentle pipetting and centrifugation. Cells were then stained with CMFDA for GSH (green) and with Hoechst 33342 (blue) for nuclei and imaged by confocal microscopy. B, quantification of the nuclear/cytoplasmic GSH ratio from the experiment shown in A (n = 4). The nuclear/cytoplasmic GSH ratio is represented as the total CMFDA intensity in each compartment. C, PKH26-prelabeled Tn cells (red) were co-cultured with DCs in the presence or absence of Tregs (1:4:2) with and without 50 μm cysteine for 24 h. T cells were separated and stained with CMFDA (green) and Hoechst 33342 (blue) to label GSH and nuclei, respectively. Yellow arrows indicate Tregs. D, quantitative analysis of the nuclear/cytoplasmic GSH ratio shown in C (n = 3). **, p < 0.005 (Student's two-tailed t test).
Tregs Block GSH Relocalization in Teffs during T Cell Activation
Next, we investigated how restricted cysteine availability in the presence of Tregs affects mobilization of the GSHin pool in activated Teffs. Previous studies have shown that GSH concentrates in the nucleus during early stages of cell proliferation but later redistributes approximately equally between the nuclear and cytoplasmic compartments (24). We imaged changes in GSHin localization during T cell activation and found that GSH co-localized with nuclear DNA during the early stages of T cell activation (Fig. 5A). However, 24 h later, GSH appeared to be more diffusely distributed between the cytoplasmic and nuclear compartments. To quantify this change, we compared the nuclear/cytoplasmic GSH fluorescence ratio observed by confocal microscopy analysis and found that it decreased by ∼2-fold over a 24-h activation period and was unchanged over the next 48 h (Fig. 5B). To examine the effect of Tregs on GSH localization in Teffs, we prestained Teffs with PKH26 (red) to distinguish Teffs from Tregs. In the presence of Tregs, GSH relocalization in Teffs appeared to be inhibited (Fig. 5C). The addition of 50 μm cysteine to the DC/Tn cell/Treg culture restored GSH relocalization. The nuclear/cytoplasmic GSH ratio for Teffs from the DC/Tn cell/Treg co-culture was similar to that for Tn cells (Fig. 5D). Exogenous cysteine abrogated the effect of Tregs on the nuclear/cytoplasmic GSH ratio. Although these data suggest that Tregs stall relocalization of GSHin, we note several limitations with our analysis and therefore interpretation of our results. First, the large nuclear volume of T cells makes accurate determination of the fluorescence intensity in the cytoplasmic compartment difficult. Second, during activation, the volume of Teffs increases, making the comparative estimation of nuclear/cytoplasmic total GSH fluorescence more complicated.
DISCUSSION
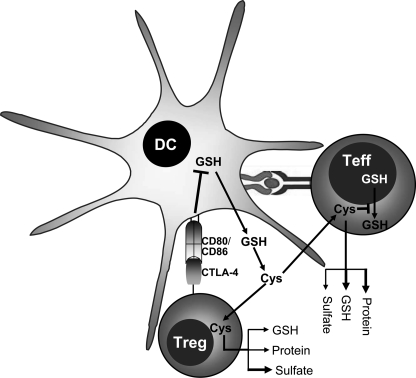
Although the requirement for a reducing microenvironment to support T cell activation and proliferation has been known for quite some time (12, 31), redox modulation has emerged as an immunosuppressive strategy only recently (2, 9). We have previously described this novel suppression mechanism whereby Tregs cause an oxidative shift in the extracellular redox environment during DC-dependent T cell activation (9). In this study, we have demonstrated that Tregs deploy multiple strategies for inhibiting DC-dependent extracellular redox remodeling (Fig. 6).
FIGURE 6.
Model showing the mechanism of Treg-mediated redox remodeling. The interaction of CTLA-4 on Tregs with CD80/CD86 on DCs triggers a signaling response in DCs that inhibits GSH synthesis. Tregs also compete with Teffs for cysteine uptake under in vitro conditions. These two processes decrease the Cysex pool. Limiting the cysteine pool not only decreases [GSH]in in Teffs but also blocks GSH relocalization to the cytoplasm, thus inhibiting T cell activation and proliferation. The fate of cysteine in activated Tregs and Teffs is also shown. More cysteine is incorporated into protein and GSH in Teffs, which is consistent with their proliferative capacity. In contrast, a larger proportion of cysteine is oxidized to sulfate in Tregs, which is consistent with their anergic phenotype. The thickness of the arrows is arbitrarily used to depict the magnitude of cysteine partitioned into competing pathways.
Activation of Teffs requires interaction between the TCR and a specific antigen presented by MHC class II molecules. However, the antigen specificity for the suppressive action of Tregs is controversial. Although several studies suggest that Tregs suppress in an antigen-restricted manner in vitro and in vivo (32–34), others report that Tregs do not require specific TCR-antigen interaction for suppression of Teff proliferation (35, 36). We have demonstrated that although remodeling of the extracellular environment by Tregs is antigen-dependent, it is not antigen-specific. These results are consistent with the observations of Vignali and co-workers (35), who have shown that Tregs can suppress Teffs derived from mouse strains with distinct antigen specificities. Hence, Tregs might harbor constitutive suppressive activity and mediate redox remodeling via a bystander suppression mechanism.
Recent studies by Sakaguchi and co-workers (1, 10) demonstrate an essential role for CTLA-4 in immunosuppression by Tregs. Foxp3, a transcription factor needed for Treg function, regulates CTLA-4 expression. The addition of anti-CTLA-4 antibody or disruption of the CTLA-4 gene in Tregs blocks Treg suppression and causes a variety of autoimmune diseases (10, 37). In this study, we have shown that Treg-mediated redox remodeling is both contact- and CTLA-4-dependent. Administration of anti-CTLA-4 antibody blocked the Treg effect on Cysex, GSHex, GSHin, and γ-glutamyl synthetase expression, suggesting that CTLA-4 plays an important role in several aspects of redox remodeling by Tregs. By interacting with CD80/CD86 on DCs, Treg-derived CTLA-4 triggers several signaling pathways in DCs, including activation of indoleamine 2, 3-dioxygenase expression and induction of the Foxo3 transcription factor (27–29). Tregs are known to restrain the maturation and antigen-presenting capacity of DCs by down-regulating expression of CD80/CD86 co-stimulatory molecules on DCs (38, 39).
We found that GSH synthesis in LPS-stimulated DCs is down-regulated by Tregs, which is consistent with lower GSH extrusion by DCs and, consequently, lower Cysex generation via the γ-glutamyl cycle. The regulatory cross-talk between Tregs and APCs has been extensively investigated. Because Foxo3 is activated via CTLA-4 signaling and Foxo3 is a sensor and regulator of redox signaling (29, 40), a potential link between this transcription factor and the γ-glutamyl cycle merits investigation.
The maintenance of GSHin levels requires the availability and transport of cysteine, the amino acid that limits GSH synthesis. Because cysteine is easily oxidized to cystine in the oxidizing extracellular milieu, the concentration of cysteine in the plasma is much lower than of cystine (41). However, the cystine transporter activity in naïve T cells is very low compared with the cysteine transporter activity (42), making them dependent on APCs to meet their cysteine needs (2, 9, 12). DCs express the xc− cystine transporter and import cystine efficiently, stimulating Cysex accumulation during T cell activation (9, 12). This response is inhibited by Tregs at multiple levels, including consumption of cysteine and its preferential oxidation to sulfate. However, the in vivo significance of this competitive mechanism for diminishing Cysex is uncertain because Teffs outnumber Tregs, which represent only ∼5–10% of total CD4+ T cells (3).
GSH plays essential roles in T cell functions, including DNA synthesis (15, 43). Perturbation of GSHin levels and the GSH/GSSG redox status dramatically affects T cell proliferation, DNA synthesis, and cytotoxic T cell activity (44). GSHin levels also substantially influence T cell signal transduction pathways involving tyrosine phosphorylation, AP-1, and NFκB (45). Overexpression of Bcl-2 (B cell leukemia/lymphoma 2) results in increased GSH levels by recruiting GSH to the nucleus to prevent apoptosis (46). GSH is concentrated in the nucleus during the early stage of cell proliferation and is distributed more evenly when cells reach confluency. GSH regulates nuclear protein functions via glutathionylation, which also protects protein thiols from further oxidation (24). Although our analysis of cytoplasmic versus nuclear GSH pools is limited by the technical challenges discussed above, at least qualitatively, the data suggest that GSH is concentrated in the nucleus in naïve T cells but is more diffusely distributed in T cells undergoing activation and proliferation. Progression through the cell cycle is correlated with metabolism-linked redox changes (47), and inhibition of GSH relocalization dynamics by Tregs might represent one mechanism of inhibition of Teff proliferation. Although the significance of GSH relocalization from the nuclear to the cytoplasmic compartment for Teff proliferation is not known, it is interesting that the inhibitory effect of Tregs on GSHin dynamics is alleviated by exogenous cysteine added at a concentration seen under DC/T cell co-culture conditions.
In summary, our study reveals that Tregs deploy multiple strategies for perturbing the extracellular redox environment during T cell activation, affecting both DCs and Tn cells. Elucidation of the signaling pathways that connect Treg engagement and the redox metabolic responses uncovered in this study might allow identification of potentially novel therapeutic targets.
Acknowledgment
We thank Dr. Victor Vitvitsky (University of Michigan) for helpful discussions and for assistance with taurine analysis.
This work was supported, in whole or in part, by National Institutes of Health Grants HL58984 and DK64959. The work performed in the Morphology and Image Analysis Cores of the Michigan Diabetes Research and Training Center was supported by National Institutes of Health Grant 5P60 DK20572 from NIDDK.
- Treg
- regulatory T cell
- DC
- dendritic cell
- Cysex
- extracellular cysteine
- Tn
- naïve T
- APC
- antigen-presenting cell
- TCR
- T cell receptor
- Teff
- effector T cell
- OVA323–329
- ovalbumin-(323–329)
- GSHin
- intracellular GSH
- CMFDA
- 5-chloromethylfluorescein diacetate.
REFERENCES
- 1.Wing K., Sakaguchi S. (2010) Nat. Immunol. 11, 7–13 [DOI] [PubMed] [Google Scholar]
- 2.Yan Z., Banerjee R. (2010) Biochemistry 49, 1059–1066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sakaguchi S. (2004) Annu. Rev. Immunol. 22, 531–562 [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S., Yamaguchi T., Nomura T., Ono M. (2008) Cell 133, 775–787 [DOI] [PubMed] [Google Scholar]
- 5.Randolph D. A., Fathman C. G. (2006) Annu. Rev. Med. 57, 381–402 [DOI] [PubMed] [Google Scholar]
- 6.Vignali D. A., Collison L. W., Workman C. J. (2008) Nat. Rev. Immunol. 8, 523–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Q., Bluestone J. A. (2008) Nat. Immunol. 9, 239–244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyara M., Sakaguchi S. (2007) Trends Mol. Med. 13, 108–116 [DOI] [PubMed] [Google Scholar]
- 9.Yan Z., Garg S. K., Kipnis J., Banerjee R. (2009) Nat. Chem. Biol. 5, 721–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T., Miyara M., Fehervari Z., Nomura T., Sakaguchi S. (2008) Science 322, 271–275 [DOI] [PubMed] [Google Scholar]
- 11.Mahnke K., Bedke T., Enk A. H. (2007) Cell. Immunol. 250, 1–13 [DOI] [PubMed] [Google Scholar]
- 12.Angelini G., Gardella S., Ardy M., Ciriolo M. R., Filomeni G., Di Trapani G., Clarke F., Sitia R., Rubartelli A. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 1491–1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sido B., Braunstein J., Breitkreutz R., Herfarth C., Meuer S. C. (2000) J. Exp. Med. 192, 907–912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Avval F. Z., Holmgren A. (2009) J. Biol. Chem. 284, 8233–8240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Suthanthiran M., Anderson M. E., Sharma V. K., Meister A. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 3343–3347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messina J. P., Lawrence D. A. (1989) J. Immunol. 143, 1974–1981 [PubMed] [Google Scholar]
- 17.Garg S. K., Yan Z., Vitvitsky V., Banerjee R. (2010) Antioxid. Redox Signal., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Castellani P., Angelini G., Delfino L., Matucci A., Rubartelli A. (2008) Eur. J. Immunol. 38, 2419–2425 [DOI] [PubMed] [Google Scholar]
- 19.Sido B., Lasitschka F., Giese T., Gassler N., Funke B., Schröder-Braunstein J., Brunnemer U., Meuer S. C., Autschbach F. (2008) Gastroenterology 134, 179–191 [DOI] [PubMed] [Google Scholar]
- 20.Lutz M. B., Kukutsch N., Ogilvie A. L., Rössner S., Koch F., Romani N., Schuler G. (1999) J. Immunol. Methods 223, 77–92 [DOI] [PubMed] [Google Scholar]
- 21.Kipnis J., Avidan H., Caspi R. R., Schwartz M. (2004) Proc. Natl. Acad. Sci. U.S.A. 101, 14663–14669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Garg S. K., Banerjee R., Kipnis J. (2008) J. Immunol. 180, 3866–3873 [DOI] [PubMed] [Google Scholar]
- 23.Foster D. J., Vitvitsky V. M., Banerjee R., Heacock A. M., Fisher S. K. (2009) J. Neurochem. 108, 437–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Markovic J., Borrás C., Ortega A., Sastre J., Viña J., Pallardó F. V. (2007) J. Biol. Chem. 282, 20416–20424 [DOI] [PubMed] [Google Scholar]
- 25.Gajewski T. F., Schell S. R., Nau G., Fitch F. W. (1989) Immunol. Rev. 111, 79–110 [DOI] [PubMed] [Google Scholar]
- 26.Moriarty-Craige S. E., Jones D. P. (2004) Annu. Rev. Nutr. 24, 481–509 [DOI] [PubMed] [Google Scholar]
- 27.Grohmann U., Orabona C., Fallarino F., Vacca C., Calcinaro F., Falorni A., Candeloro P., Belladonna M. L., Bianchi R., Fioretti M. C., Puccetti P. (2002) Nat. Immunol. 3, 1097–1101 [DOI] [PubMed] [Google Scholar]
- 28.Fallarino F., Grohmann U., Hwang K. W., Orabona C., Vacca C., Bianchi R., Belladonna M. L., Fioretti M. C., Alegre M. L., Puccetti P. (2003) Nat. Immunol. 4, 1206–1212 [DOI] [PubMed] [Google Scholar]
- 29.Dejean A. S., Beisner D. R., Ch'en I. L., Kerdiles Y. M., Babour A., Arden K. C., Castrillon D. H., DePinho R. A., Hedrick S. M. (2009) Nat. Immunol. 10, 504–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stipanuk M. H., Coloso R. M., Garcia R. A., Banks M. F. (1992) J. Nutr. 122, 420–427 [DOI] [PubMed] [Google Scholar]
- 31.Ishii T., Hishinuma I., Bannai S., Sugita Y. (1981) J. Cell. Physiol. 107, 283–293 [DOI] [PubMed] [Google Scholar]
- 32.Corthay A. (2009) Scand J. Immunol. 70, 326–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yu P., Gregg R. K., Bell J. J., Ellis J. S., Divekar R., Lee H. H., Jain R., Waldner H., Hardaway J. C., Collins M., Kuchroo V. K., Zaghouani H. (2005) J. Immunol. 174, 6772–6780 [DOI] [PubMed] [Google Scholar]
- 34.Tanchot C., Vasseur F., Pontoux C., Garcia C., Sarukhan A. (2004) J. Immunol. 172, 4285–4291 [DOI] [PubMed] [Google Scholar]
- 35.Szymczak-Workman A. L., Workman C. J., Vignali D. A. (2009) J. Immunol. 182, 5188–5192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Homann D., Holz A., Bot A., Coon B., Wolfe T., Petersen J., Dyrberg T. P., Grusby M. J., von Herrath M. G. (1999) Immunity 11, 463–472 [DOI] [PubMed] [Google Scholar]
- 37.Sakaguchi S., Wing K., Onishi Y., Prieto-Martin P., Yamaguchi T. (2009) Int. Immunol. 21, 1105–1111 [DOI] [PubMed] [Google Scholar]
- 38.Cederbom L., Hall H., Ivars F. (2000) Eur. J. Immunol. 30, 1538–1543 [DOI] [PubMed] [Google Scholar]
- 39.Misra N., Bayry J., Lacroix-Desmazes S., Kazatchkine M. D., Kaveri S. V. (2004) J. Immunol. 172, 4676–4680 [DOI] [PubMed] [Google Scholar]
- 40.Kops G. J., Dansen T. B., Polderman P. E., Saarloos I., Wirtz K. W., Coffer P. J., Huang T. T., Bos J. L., Medema R. H., Burgering B. M. (2002) Nature 419, 316–321 [DOI] [PubMed] [Google Scholar]
- 41.Eck H. P., Gmünder H., Hartmann M., Petzoldt D., Daniel V., Dröge W. (1989) Biol. Chem. Hoppe-Seyler 370, 101–108 [DOI] [PubMed] [Google Scholar]
- 42.Ishii T., Sugita Y., Bannai S. (1987) J. Cell. Physiol. 133, 330–336 [DOI] [PubMed] [Google Scholar]
- 43.Pallardó F. V., Markovic J., García J. L., Viña J. (2009) Mol. Aspects Med. 30, 77–85 [DOI] [PubMed] [Google Scholar]
- 44.Grimble R. F. (2006) J. Nutr. 136, 1660S–1665S [DOI] [PubMed] [Google Scholar]
- 45.Staal F. J. (1998) Eur. J. Clin. Invest. 28, 194–196 [DOI] [PubMed] [Google Scholar]
- 46.Voehringer D. W., McConkey D. J., McDonnell T. J., Brisbay S., Meyn R. E. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 2956–2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Menon S. G., Goswami P. C. (2007) Oncogene 26, 1101–1109 [DOI] [PubMed] [Google Scholar]