Abstract
Bacterial genomes encode a collection of small peptides that are deleterious to their hosts when overexpressed. The physiological relevance of the majority of these peptides is unknown at present, although many of them have been implicated in regulatory processes important for cell survival and adaptability. One peptide that is of particular interest to us is a 19-amino acid proteic toxin, coined IbsC, whose production is repressed by SibC, an RNA antitoxin. Together, IbsC and SibC constitute a type I toxin-antitoxin (TA) pair. To better understand the function of IbsC and to decipher the sequence determinants for its toxic phenotype, we carried out extensive sequence analyses of the peptide. We generated a series of truncation and single amino acid deletion mutants to determine the minimal sequence required for toxicity. We further probed into functionally relevant amino acids with a comprehensive set of IbsC mutants produced using a systematic sequence randomization strategy. We found that IbsC remained toxic in the presence of multiple deletions and single amino acid substitutions, despite being well-conserved in Escherichia coli and across other Gram-negative bacteria. The toxicity of this peptide was determined to be dependent on a stretch of highly hydrophobic residues near its center. Our results defined sequence-function relationship of IbsC and offered additional insights into properties common to membrane-targeting type I toxins in E. coli and related species.
Keywords: Bacterial Toxins, Membrane Proteins, Peptide Conformation, Protein Sequence, Toxins, Toxin-Antitoxins
Introduction
Small peptides emerging from microbial genomes have been implicated in diverse catalytic and regulatory roles in cells. These functional peptides of only 16–60 amino acids are abundant in the genome of Escherichia coli K-12 where over 50 sequences have been detected in a recent study using a combination of biochemical and computational approaches (1). Additional sequences have also been confirmed to exist in other Gram-positive and Gram-negative species.
With their discovery, the array of functions carried out by these peptides also grows. Of the peptides that have been characterized thus far, a few have been demonstrated to function outside the cell as antimicrobial peptides or as secreted signaling peptides (2). A considerable proportion of peptides that remain in the cell have been found to be toxic to cells when they are overexpressed. Many of these toxic peptides are classified as a part of toxin-antitoxin (TA)3 systems.
Smaller, more hydrophobic peptides are typically found to be a part of type I TA systems, in which the translation of the toxin is suppressed by a noncoding small RNA (sRNA) antitoxin (3). This differs from type II TA systems where the larger and less hydrophobic toxins are antagonized by smaller and more labile proteic antitoxins (3). The most well-characterized type I TA pair identified to date is perhaps the hok-sok system initially discovered on the R1 plasmid of E. coli. Plasmid-encoded toxins contribute to plasmid maintenance in bacteria, as the loss of such plasmids would lead to rapid degradation of the antitoxin and accumulation of the toxin, resulting in post-segregational cell death (4). In addition to plasmids, a number of type I TA pairs are found on the chromosome. However, the exact function of many chromosomally-encoded toxins is obscure. Hypotheses of their physiological roles may be drawn from those proposed for their more well-studied type II counterparts (as reviewed in Refs. 5–7).
Similar to many plasmid-encoded TA pairs, TA pairs present on the chromosome have been implicated in the stabilization of conjugative transposons and phage DNA in the genome (8). Other TA systems have been linked to bacterial stress response. The production of these toxins are elevated in response to specific stress conditions, thereby reversibly suppressing cell growth and preserving energy to counteract these stressors until conditions improve (9, 10). Two type I toxins in E. coli, TisB (11, 12) and SymR (13), have been demonstrated to be induced by the SOS response, allowing for cells to cope with damaged RNA and DNA, respectively. Consistent with this model, some TA pairs have been suggested to promote bacterial persistence, where the production of toxins in a subpopulation of cells causes them to become dormant in response to antibiotic treatments (14, 15). Alternatively, TA systems have been postulated to mediate programmed cell death in a large fraction of cells within a population upon exposure to specific stress signals (16, 17). The irreversible killing of the selected cells releases nutrients and other resources to sustain the surviving population. While the stress response model and the “altruistic killing” models seem contradictory, it is likely that the physiological role of chromosomally encoded TA pairs may not be defined by a single unifying model, but individual TA systems may serve different purposes in the cell. It is also possible that the observed toxicity of these peptides may be an artifact of their overexpression. They may have enzymatic or regulatory functions that are entirely independent of cell killing when expressed at endogenous levels (3). A recent global search for type I TA systems across bacterial species indicated that some TA families are well-conserved (18), implying that such TA systems may be important for bacterial fitness.
Among the six main families of type I TA pairs identified in E. coli K-12 is the Ibs/Sib family. Five Ibs/Sib homologs have been found in the E. coli K-12 genome, and they have been coined Ibs/SibA-E respectively (19). The ibs and sib genes are arranged on opposite strands, with the antitoxin encoding sequences completely overlapping the toxin open reading frames (ORFs). These five TA repeats are distributed at three loci in the genome: ibs/sibA and B are found in tandem at the same intergenic region (IGR), while ibs/sibD and E are arranged in the same manner at another IGR. Here, we are primarily interested in ibs/sibC, the sole pair detected in the IGR between ygfA and serA. We initially came across ibs/sibC in a screen for antisense sequences that can knock down the expression of sRNAs and elicit growth defects in E. coli (20). Through our screen, we observed growth suppression upon the induction of the reverse complement of the SibC sRNA. Our subsequent analyses demonstrated that the toxic phenotype was not attributed to sRNA interference. Rather, it was caused by the overproduction of a 19-amino acid peptide encoded in the antisense sequence. Our observations corroborate with the results reported in an earlier study published by the laboratory of Gisela Storz, who coined this peptide IbsC (for induction brings stasis) (19).
It has been postulated that IbsC and other members of the Ibs/Sib family localize to the inner membrane following overexpression, and their accumulation contributes to membrane depolarization (19). Like many type I TA pairs, the biological relevance and the exact mechanism of action of IbsC is currently unknown. It is also uncertain why this potentially deleterious element is maintained in the genome of various E. coli strains and in other proteobacterial species, including those from the Enterobacteriaceae, Pasteurellaceaes, and Helicobacteraceae families (18, 21). Herein, we have established a comprehensive set of IbsC mutants to be used to address questions pertaining to the sequence conservation and functionality of this enigmatic peptide.
We carried out extensive sequence truncation studies on IbsC to deduce the minimal sequence for toxicity. A sequence randomization strategy was implemented to systematically introduce mutations in IbsC, allowing us to deduce the amino acid requirements for toxicity. IbsC was found to tolerate high frequencies of amino acid substitutions, considering that a large proportion of mutants retained their toxicity and membrane depolarization capabilities. Our mutagenesis data suggest that the sequence space for toxic peptides is quite large. In general, amino acids with hydrophobic side chains were strongly preferred at multiple positions, and mutations disrupting consecutive hydrophobic residues near the core of the peptide gave rise to inactive mutants. Using IbsC as a model, we defined additional sequence requirements for toxic peptides and refined current parameters used to guide searches for these elements across genomes in vivo and in silico.
EXPERIMENTAL PROCEDURES
Oligonucleotides
Sequences encoding IbsC and its derivatives were generated by PCR or by extending oligonucleotides with 15–20-nt complementary regions. The sequences of these oligonucleotides are presented in supplemental Table S1. Oligonucleotides were chemically synthesized by Integrated DNA Technologies (Coralville, IA). Oligonucleotides used for the sequence randomization studies were synthesized such that each nucleotide within the codon(s) of interest was prepared using a mixture of 25% adenine, cytosine, thymine, and guanine phosphoramidites. Oligonucleotides that were longer than 30 nt were purified by 10% (8 m urea) denaturing PAGE prior to usage.
Peptide Synthesis
Peptides, including wild-type IbsC and mutants IbsC (6–19), IbsC (L8G, L11L, S15A) and IbsC (L11R), were chemically synthesized by Genscript (Piscataway, NJ).
Strains and Plasmids
Information pertaining to the E. coli strain and plasmid used in this study are presented in supplemental Tables S2 and S3. The E. coli strain used in this study was DH5αZ1, which was previously described in Ref. 22. IbsC and its derivatives were cloned into pNYL-MCS11, a derivative of pZE21-MCS1 (courtesy of H. Bujard), downstream of the tetracycline-inducible promoter. Modifications were made to the parent vector, as previously described in Ref. 20. Briefly, we removed the ribosome binding site (RBS) present on pZE21-MCS1 and restored the multiple cloning sites that were lost during the RBS excision.
Molecular cloning procedures, including primer extension, restriction digestion, and ligation, were carried out following supplier-provided protocols. High Fidelity PCR Enzyme Mix, Klenow fragment (exo−) and T4 DNA ligase were purchased from Fermentas (Burlington, ON, Canada). EcoRI and BamHI restriction enzymes were purchased from New England Biolabs (Pickering, ON, Canada). Ligation products were transformed into DH5αZ1 cells by electroporation. With the exception of the mutants from the random sequence libraries, cloned constructs were confirmed by DNA sequencing at Mobix Lab (McMaster University).
Growth Media
Bacteria were cultured in Luria-Burtani (LB) medium at 37 °C in a shaking incubator. Growth media were routinely supplemented with 50 μg ml−1 of kanamycin and spectinomycin (both purchased from Sigma-Aldrich). To induce the expression of ibsC and its derivatives, 200 ng ml−1 of anhydrotetracycline (Atc; Sigma-Aldrich) was added to the medium.
Growth Curves and Lethality Screens
Growth curves were used to assess the toxicity of IbsC truncation and deletion mutants. Cells carrying pNYL-MCS11 (the negative plasmid control) or plasmid with ibsC (the positive control) and its derivatives were cultured overnight. The cells were then subcultured in fresh medium (1:400 dilution) in the absence (for uninduced conditions) or presence (for induced conditions) of Atc. These cultures were incubated at 37 °C with shaking at 260 rpm for 8 h (h). Growth was monitored hourly by measuring the absorbance at 600 nm (A600) of each sample using a VersaMax microplate reader (Molecular Devices, Sunnyvale, CA). The assays were carried out in triplicate. To compare the toxicity associated with various IbsC mutants, the A600 of each sample measured at t = 8 h was normalized against the A600 of the plasmid control at this time point in order to derive values for relative A600.
The toxicity of these constructs was also confirmed by lethality screens in which we plated cells carrying pNYL-MCS11 with ibsC mutants on LB agar with or without Atc. The effects of the overexpression of these constructs on growth were observed following overnight incubation at 37 °C.
Screening for Toxic IbsC Mutants in Random Sequence Libraries
Upon transforming vectors with each pool of ibsC mutants with randomized codons into DH5αZ1 cells, we selected a collection of colonies to build the various IbsC mutant libraries. We picked around 100, 200, 350, and 450 colonies to establish each single, double, triple, and quadruple substitution library respectively. To screen for toxic IbsC derivatives in each of these libraries, each clone was cultured overnight and diluted 1:400 in LB media supplemented with antibiotics and Atc the following day. These samples were then incubated at 37 °C for 6 h with shaking. The growth assay was carried out in duplicate on two separate 96-well culture plates. A600 measurements were taken at this time point because we consistently observed the greatest difference in growth between the plasmid and the IbsC controls at 6 h post-induction. A600 measurements were normalized against the average A600 corresponding to positive (μp) and negative (μn) controls. These values were denoted “Normalized Growth,” and they were calculated using Equation 1.
We sent all of the constructs from the libraries of mutants with single amino acid substitutions for sequencing (Bio Basic Inc., Markham, ON, Canada). We selected 19 mutants from each library covering all of the possible amino acid substitutions at each position. These mutants were subjected to follow-up growth assays to confirm our observations from the initial screens. The assays were repeated at least three times. We considered mutants with %N.G. <40% to be active, as this value represented 3 standard deviations below the average toxicity of 25 negative control samples (data not shown). From the libraries of mutants with multiple amino acid substitutions, all active mutants were sent for sequencing. We also sequenced 10 inactive mutants from each library to confirm sequence coverage. Growth assays were repeated in triplicate for sequenced mutants.
Dye Uptake Assay
Cells carrying the plasmid control and the plasmid with ibsC or selected ibsC mutants were grown overnight and diluted 1:200 in fresh media. These cultures were grown at 37 °C with shaking until the A600 of the samples were ∼0.3. Cells were subsequently induced with Atc. At 3 h post-induction, cells were harvested and treated with 10 μg ml−1 DiBAC4(3) (Sigma-Aldrich). Cells were incubated with the dye for 20 min before being pelleted and washed with phosphate-buffered saline (PBS). The final pellets were resuspended in different volumes of PBS to achieve uniform cell densities across samples. Fluorescence of each sample was measured using a Tecan Safire microplate reader (excitation at 490 ± 5 nm; emission at 516 ± 5 nm). Relative fluorescence of the mutants was calculated by dividing their fluorescence by the one corresponding to the plasmid control. The assay was repeated a minimum of three times.
Circular Dichroism (CD) Spectroscopy
CD spectra were collected with an AVIV 410 CD instrument (AVIV, Lakewood, NJ). Scans were performed from 260 to 190 nm at a step resolution of 1 nm. A 1-mm pathlength quartz cuvette was used for the measurements. Sample temperature was maintained at 25 °C using a thermostatically controlled cell holder. Peptides were solubilized in 2,2,2-trifluoroethanol (TFE; Sigma-Aldrich) at a concentration of 50 μm. Background signal contributed by the solvent was subtracted for each sample. The CD spectra were converted to mean residual ellipticity. The secondary structure content of each sample was estimated from each spectrum using the CONTIN method available through the CDPro software (23).
RESULTS
Minimization of the IbsC Toxin
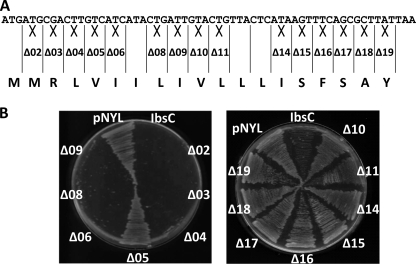
With 19 amino acids, IbsC is one of the smallest bacterial toxins identified to date (1, 19). We were interested in exploring whether this constitutes as the lower size limit of type I toxins or whether shorter peptides can exhibit growth inhibitory potential in E. coli. We generated a series of terminal truncation mutants of IbsC by sequentially removing codons from the 5′- and 3′-ends of its ORF. We eliminated codons 2 through 7 from the 5′ truncation variants (Fig. 1A), while the 3′ truncation variants lacked codons 19 through 13 (Fig. 1B). The start codon was retained in the 5′ truncation mutants to enable translation initiation. Each sequence contained the RBS native to IbsC. The 13 IbsC truncation sequences were introduced into pNYL-MCS11, such that their expression was driven by a tetracycline-inducible promoter (PLtetO-1) (20, 22). E. coli strain DH5αZ1, which constitutively expresses a tetracycline repressor to allow for tight regulation of genes governed by PLtetO-1 (22), were transformed with these constructs. The production of these IbsC derivatives was induced with Atc.
FIGURE 1.
Toxicity of IbsC 5′ and 3′ truncation mutants. A, amino acid sequence of wild-type IbsC and schematics of the 5′ truncation mutants. Codons 2–7 were sequentially removed from the ORF of ibsC in these constructs. B, schematics of the 3′ truncation mutants in which codons 19 through 13 were removed. C, toxicity of 5′ truncation mutants were assessed by comparing the relative A600 of each mutant to that associated with the positive (IbsC) and negative (pNYL) controls at 8 h post-induction. Mutant IbsC (6–19), which lacked amino acids 2–5, was found to be the minimal toxic derivative of IbsC. D, toxicity of 3′ truncation mutants. Relative A600 of each mutant was compared with that of the controls at 8 h post-induction. Removal of any of the amino acids near the C terminus of IbsC was found to be deleterious to its toxicity. n = 3.
Growth of cells carrying plasmids expressing the IbsC truncation mutants was monitored over 8 h following the induction of each IbsC truncation mutant (supplemental Figs. S1 and S2). Cell density was determined by measuring the A600. To facilitate comparison of the toxicity of each mutant, the A600 of each sample measured at t = 8 h was normalized with the A600 of cells carrying pNYL-MCS11, which served as our negative control. As observed in Fig. 1C, IbsC 5′ truncation mutants lacking amino acids 2–5 remained growth suppressive, indicating that these residues close to the N terminus of the peptide are not essential for toxicity. Removal of amino acids beyond the fifth residue led to inactive constructs, as observed with mutants IbsC (7–19) and IbsC (8–19), which lacked amino acids 2–6 and amino acids 2–7, respectively. On the other hand, elimination of amino acids near the C terminus was not tolerated, and cells expressing the 3′ truncation mutants grew to similar extents as the plasmid control (Fig. 1D). The data presented here suggests that the stretch of hydrophobic residues at the middle of the peptide and the few polar residues near the C terminus may have functional significance for IbsC. We also demonstrated that this toxin may be minimized to 15 amino acids without resulting in significant loss of toxicity.
Contribution of Individual Amino Acids to Toxicity
Data from our sequence truncation studies suggests that amino acids from positions 6–19 of IbsC are required for its toxicity. However, we were uncertain if all of the amino acids in this region are required for toxicity. To probe into amino acids that are potentially important for the function of IbsC, we designed a set of 15 IbsC deletion mutants, each lacking an individual amino acid (Fig. 2A). For amino acids that occur in tandem, such as the two isoleucine residues at positions 6 and 7, only the codon for the first amino acid was removed. Expression of the IbsC deletion mutants was induced in E. coli grown on LB-agar supplemented with Atc (Fig. 2B). Consistent with results from our sequence truncation analysis, IbsC derivatives lacking any of the last 10 amino acids failed to suppress growth following overexpression (mutants Δ10-Δ19), but amino acids Met-2, Arg-3, and Leu-4 can be deleted without interfering with the toxicity of IbsC (mutants Δ2-Δ4). Interestingly, mutants lacking residues Ile-6 (mutant Δ6) remained growth inhibitory when overexpressed although 5′ truncation mutants lacking Ile-6 were non-toxic. While 5′ truncation mutant IbsC (6–19), which lacked amino acids Met-2 to Val-5, was found to be toxic, removal of amino acid Val-5 on its own led to a loss of toxicity (mutant Δ5). This observation was confirmed upon monitoring the effect of overexpressing this deletion mutant in cells grown in liquid medium over 8 h (supplemental Fig. S3). It is possible that the removal of residue Val-5 perturbed the hydrophobicity in its adjacent region or the active structure of the peptide.
FIGURE 2.
Effect of single amino acid deletions on the toxicity of IbsC. A, design of IbsC deletion mutants. Starting from codon 2, codons were individually removed from ibsC. For amino acids that occur in tandem, such as Ile-6 and Ile-7, only the codon of the first amino acid was removed. B, phenotypic changes associated with the overexpression of each deletion mutant in E. coli DH5αZ1. Bacteria carrying pNYL-MCS11 with the deletion mutants were plated on LB-agar with Atc and incubated at 37 °C overnight. With the exception of amino acid Val-5, elimination of any amino acids from positions 2–9 did not seem to affect the toxicity of IbsC (left panel). Removal of any of the last 10 amino acid led to a loss of toxicity (right panel).
Variability of Amino Acid Val-5
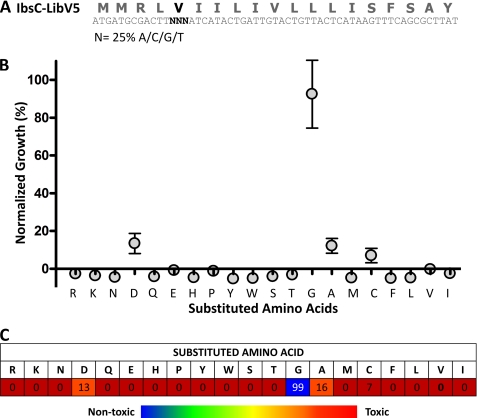
The significance of residue Val-5 in full-length IbsC was examined using a sequence randomization strategy. The sequence encoding the open reading frame of ibsC was designed with mutations at codon 5, such that each nucleotide in the codon was synthesized using a mixture of 25% adenine, cytosine, thymine, and guanine phosphoramidites (Fig. 3A). This pool (or library) of ibsC mutants was subsequently cloned into pNYL-MCS11, downstream of the tetracycline-inducible promoter. E. coli DH5αZ1 cells carrying these recombinant vectors were grown in liquid media supplemented with Atc to screen for growth suppression associated with the overexpression of these IbsC derivatives. Based on our design, we would theoretically need to sample 64 (43) sequences in order to cover all the possible mutations in this codon. If the randomization was unbiased, examining 64 mutants would encompass all 20 amino acids and the three stop codons. To account for unligated vectors and the isolation of identical mutants, we selected ∼100 clones to be screened using a growth assay.
FIGURE 3.
Randomization of the codon encoding amino acid Val-5 in IbsC. A, design of the IbsC-V5 library. Each nucleotide in codon 5 was synthesized with 25% adenine, guanine, cytosine, and thymine phosphoramidites (denoted by N). B, growth assay of IbsC-V5 mutants with the 20 amino acid substitutions. One mutant with each amino acid substitution was selected from the library and was subjected to a follow-up growth assay in which E. coli expressing each mutant was grown for 6 h. A600 of each sample was measured thereafter, and these values were normalized against the growth of bacteria expressing IbsC and the plasmid control (referred to as “normalized growth” or %N.G.). The data points and error bars represent average %N.G. and standard deviation calculated from triplicate. C, average toxicities of IbsC mutants with each of the 20 amino acid substitutions at position Val-5. The amino acids substituted at this position are noted in row 2 of the table. The number in each box in row 3 represents the average %N.G. associated with the overexpression of each mutant calculated from 3 experiments. Amino acid substitutions giving rise to active sequences (%N.G. <10%) are depicted by red boxes, those that led to peptides with intermediate toxicities (%N.G. between 10–20%) are indicated by orange boxes, and those that led to a loss of toxicity (%N.G. >40%) are shown in blue.
In this assay, the A600 of each sample (A600(mutant)) was measured at 6 h post-induction. These values were normalized against the average A600 corresponding to cells carrying pNYL-MCS11 (the negative control, μn) and to cells carrying pNYL-MCS11 with wild-type IbsC (the positive control, μp). The normalized A600 was denoted “Normalized Growth” (%N.G.) and was calculated using Equation 1.
We observed that ∼70% of the sequences that were sampled in this screen appeared active, as their overexpression suppressed growth to levels that were comparable to the overexpression of wild-type IbsC. Following the screen, the constructs were sequenced and amino acid substitutions at position 5 were determined. We were able to isolate IbsC derivatives with substitutions for each of the 20 amino acids at this position. We selected individual mutants for each substitution and subjected them to a follow-up screen to confirm their toxicities. As detailed in Fig. 3, B and C, position 5 of IbsC can withstand substitution by most amino acids regardless of their hydrophobicity, size, and charge. However, glycine substitution was not tolerated at this position and overexpression of the V5G mutant did not suppress growth. The loss of toxicity in this mutant and in the Δ5 deletion mutant suggests that the presence of a functional group in the amino acid at the fifth position may be important to the activity of IbsC.
Variability of C-terminal Amino Acids
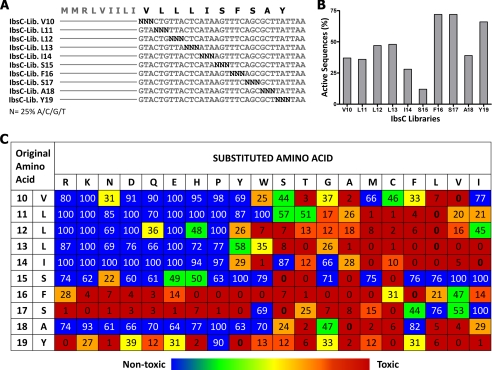
In addition to amino acid Val-5, our single amino acid deletion experiments revealed that the loss of any of the last 10 amino acids in IbsC was deleterious to its toxicity. As such, we carried out a complete substitution analysis of each amino acid in this region using the aforementioned sequence randomization strategy to deduce the amino acid requirements in this region of IbsC (Fig. 4A). Following their synthesis, the 10 pools of mutant ibsC sequences were separately cloned into pNYL-MCS11. Each group of recombinant plasmids was then transformed into DH5αZ1 cells. Approximately 100 constructs were selected from each pool to establish our 10 libraries of ibsC derivatives, and the toxicity of sequences from each library was independently assessed using growth assays. Active sequences were defined as those that can suppress growth by >60% when overexpressed (%N.G. <40%).
FIGURE 4.
Complete amino acid substitution of the last 10 amino acids of IbsC. A, randomization of codons encoding amino acids Val-10 to Tyr-19. Each nucleotide in the codon to be randomized (denoted by N) was synthesized with 25% adenine, guanine, cytosine, and thymine phosphoramidites. Using this approach, 10 ibsC citalize random sequence libraries were generated. B, percentage of active sequences observed in each library. Approximately 100 mutants were present in each library. The effect of overexpressing each mutant in the libraries was determined by measuring the A600 of cells 6 h following their induction. Growth of each sample was normalized against the A600 of cells expressing IbsC and the plasmid control. Mutants whose overexpression led to normalized growth (%N.G.) of 40% or less in the two growth assays were considered as active and were tabulated. C, average toxicities of IbsC mutants with each of the 20 amino acid substitutions at positions 10–19. One mutant with each of the 19 amino acid substitutions was selected from each library to assemble a comprehensive set of single amino acid substitution mutants. The effect of overexpressing these mutants on the growth of E. coli was evaluated 6 h post-induction. In the table, the first two columns depict the position and original amino acid at the residue of interest. Row 2 depicts the amino acid substitution at each position. The number in each box represents the average %N.G. calculated from three growth assays. Toxicity of each mutant is color coded: active sequences (%N.G. <10%) are depicted by red boxes, mutants with intermediate activities (%N.G. between 10–20%) are indicated by orange boxes, weakly active mutants (%N.G. between 20–40%) are highlighted in amber/yellow, and inactive mutants (%N.G. >40%) are shown in green/blue.
From our initial screen, we observed that amino acid substitutions were tolerated at most positions in this segment of IbsC. An average of 40% of the sequences across the 10 libraries were found to be active (Fig. 4B). Amino acids near the C terminus of the peptide, namely positions Phe-16, Ser-17, and Tyr-19, were found to be more mutatable than the rest of the sequence. Libraries corresponding to these amino acids contained nearly 70% of active sequences. On the other hand, the library corresponding to position Ser-15 displayed a much lower frequency of active sequences (12%). We sequenced all of the constructs that were examined. Upon analyzing the amino acid substitutions in each library, we noticed that our randomization approach generated near comprehensive sequence coverage. Amino acids that were absent from each library were often coded by non-degenerate codons, such as methionine and tryptophan (data not shown). We rationally designed and synthesized ibsC mutants that were not isolated from the initial screen in order to complete the set of substitutions. To verify the activity of each mutant, we selected 20 mutants from each library, each with a unique amino acid substitution, and repeated growth assays using these constructs (supplemental Fig. S4).
As presented in Fig. 4C, amino acids located near the center of IbsC, principally the ones at positions 10–14, exhibited a strong preference for hydrophobic residues (with Leu, Phe, Met>Cys>Val>Ile). Hydrophilic and charged residues are generally not favored at these positions. Substitutions by charged amino acids near the core of IbsC often led to a complete loss of toxicity. In contrast, positions 16, 17, and 19 demonstrated a propensity to accept hydrophilic and ionic residues. However, strongly hydrophobic residues, such as leucine, valine, and isoleucine, are not favorable substitutions at position 16 and 17. Consistent with the high percentage of active sequences observed in our initial screen, positions 16, 17, and 19 were able to withstand more than 15 of the 19 amino acid substitutions. Position 15, which gave rise to few active sequences in the screen, had only three neutral amino acid substitutions. Like serine, the parent amino acid at this position, the accepted substitutions were small and mostly polar (Thr, Cys, and Ala).
Multiple Amino Acid Substitutions in IbsC
We have established that single amino acid substitutions are generally well-tolerated in IbsC. Here, we seek to determine whether this peptide can accept mutations at multiple locations. We are interested in examining if the identity of an amino acid at one location is dependent on that at another. Because of its hydrophobicity and its propensity to interact with the inner membrane (19), IbsC is hypothesized to adopt an α-helical structure. As such, we have applied the sequence randomization approach to mutate multiple amino acids on the same face of the predicted helix.
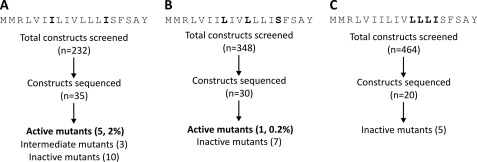
We first mutagenized the isoleucine residues located at positions 7 and 14, which are predicted to reside on one face of the helix (Fig. 5A). We carried out growth assays with 232 constructs that make up the double mutation library. Compared with the screens conducted with the single mutation libraries, we observed remarkably fewer active sequences. We sequenced 35 constructs from this library. This encompasses all of the constructs that exhibited full and partial toxicities in addition to 10 constructs that were found to be non-toxic. We removed sequences with base insertions or deletions, as well as those that appeared to be contaminated with more than one population of mutants. The remaining sequences were subjected to additional growth assays to confirm our observations. From this library, only 5 sequences with toxicities comparable to IbsC were isolated (supplemental Fig. S5A). The %N.G. associated with these mutants was <10%. We also isolated 3 sequences that displayed intermediate activity (with %N.G. between 10 and 40%). From these sequences, we note that hydrophobic residues (Cys, Val, Leu, and Ile) are favored at both position 7 and 14. When the amino acids at these two loci were replaced by more hydrophilic residues, the toxicity of the resulting peptide diminished, although these mutants were still able to suppress growth by 60% or more. We also obtained the sequences of 10 inactive mutants. Most of these constructs were found to contain charged residues (Lys, Arg, Asp, or Glu) at one or both of these positions.
FIGURE 5.
Toxicities of IbsC mutants with multiple amino acid substitutions. A, library of IbsC double mutants in which codons encoding amino acids Ile-7 and Ile-14 were randomized using the sequence randomization approach. B, library of IbsC triple mutants with randomized codons Leu-8, Leu-11, and Ser-15. C, library of IbsC quadruple mutants with randomized codons Leu-11, Leu-12, Leu-13, and Ile-14. Amino acids encoded by the randomized codons in each library are indicated in black. The number of constructs that were screened and sequenced is presented in the flowcharts. The number of sequenced mutants displaying high (%N.G. <10%), intermediate (%N.G. between 10–40%), and low (%N.G. >40%) toxicities in each library are indicated.
In addition to generating a library of double mutants, we screened triple mutants by randomizing the codons encoding amino acids Leu-8, Leu-11, and Ser-15 (Fig. 5B). These amino acids are hypothesized to be on the side of the putative α-helix opposite of Ile-7 and Ile-14. We expected to detect very few toxic triple mutants from our screen. Thus, we subjected nearly 350 constructs to growth assays. From this screen, we identified one active sequence with the expected length (supplemental Fig. S5B). However, further sequence analysis indicated that this mutant only contained two amino acid substitutions (L8G and S15A). The remaining mutants that we sequenced were inactive. Many of these mutants were found to contain proline at positions 8 and 15. Consistent with the inactive mutants we examined in the double mutant library, we observed that positively and negatively charged residues were generally disfavored in the triple mutants.
From our single amino acid substitution study, we observed that positions 11–14 of IbsC demonstrated a strong preference for hydrophobic residues (see Fig. 4C). We speculated that the presence of these consecutive hydrophobic amino acids is required for the toxicity of IbsC. They may be involved in mediating the interaction between IbsC and the inner membrane. Thus, we randomized the 12 nucleotides corresponding to codons 11–14 to examine the amino acid requirements in this region (Fig. 5C). Because of the scarcity of active sequences isolated from our double and triple mutant libraries, we examined the effect of the overexpression of around 450 sequences on the growth of E. coli. We did not isolate any active clones through this screen. Twenty constructs from this library were selected and sequenced. We eliminated sequences that appeared to have base insertions, deletions, or contaminations, and the remaining sequences were subjected to additional growth assays. We noticed that hydrophilic and charged residues were prevalent in these inactive mutants (supplemental Fig. S5C). One mutant, IbsC (L11V, L12L, L13I, I14V), was found to be inactive even though the amino acids at positions 11–14 of this mutant remained highly hydrophobic, akin to the residues in the original sequence. This suggests that drastic changes to the sequence of IbsC are deleterious to its toxicity.
Structural Analysis of IbsC and Selected Derivatives
To further examine whether the activity of IbsC mutants is dependent on their structures, CD spectroscopy was carried out. Wild-type peptide and mutants IbsC (6–19) and IbsC (L8G, L11L, S15A) were chemically synthesized and subjected to CD analysis. The non-toxic mutant, IbsC (L11R), was also analyzed for comparison. With hydrophobicities of >75%, the peptides were insoluble in aqueous solvents. As such, 2,2,2-trifluoroethanol (TFE) was used as the solvent. The CD spectra collected for each peptide were analyzed using a secondary structure prediction program. As hypothesized, IbsC predominantly existed as an α-helix (supplemental Fig. S6, A–D). Interestingly, truncation mutant IbsC (6–19) and triple mutant IbsC (L8G, L11L, S15A), which displayed comparable toxicities as IbsC, were found to be comprised of β-aggregates along with α-helices. On the other hand, IbsC (L11R), the inactive point mutant, also seemed to favor the α-helical conformation under these conditions. Our structural analysis suggests that IbsC does preferentially adopt an α-helical conformation. However, the toxicities associated with these peptides may not be solely dependent on their secondary structures.
Overexpression of IbsC and Its Toxic Derivatives Causes Membrane Depolarization
IbsC has been proposed to interact with the inner membrane of E. coli. Its overexpression has been proposed to induce pore formation and elicit membrane depolarization (19). It is uncertain whether toxic IbsC derivatives disrupt the integrity of the inner membrane like their wild-type counterpart and if non-toxic variants have lost their ability to cause membrane defects. To examine the mechanism of toxicity of IbsC mutants, we subjected E. coli expressing a subset of these mutants to a dye uptake assay. We monitored the ability of cells to take up a fluorescent dye, DiBAC4(3) [Bis-(1,3-dibarbituric acid)-trimethine oxonal], following the overexpression of 14 active and inactive IbsC variants. Following membrane depolarization, DiBAC4(3) is able to enter cells, localize to highly hydrophobic environments, and interact with cytoplasmic proteins (24). This subsequently results in an increase in fluorescence, which is expected to be proportional to the change in membrane potential (24). In this assay, we induced the expression of IbsC and its derivatives in DH5αZ1 cells for 3 h before they were incubated with DiBAC4(3). We chose to assess dye uptake at 3 h post-induction, because maximal increase in fluorescence was detected at this time point following the induction of wild-type IbsC (data not shown).
We subjected 8 IbsC point mutants to the dye uptake assay. This set of mutants contained constructs that exhibited full activity [IbsC (V5R, V10T, S15C, and S17E)], intermediate activity [IbsC (V5D and V10N)], and no activity [IbsC (S15V and S17I)] in our growth assays. The overexpression of IbsC led to a 4-fold increase in fluorescence relative to the plasmid control (pNYL). Comparable to the wild-type toxin, active mutants led to a 3–4-fold fluorescence increase (Fig. 6A). The two inactive mutants demonstrated a slight increase in fluorescence (∼1.4-fold relative to the negative control). It is possible that the accumulation of these small peptides resulted in stress on the inner membrane, yet the defect may not be severe enough to cause growth suppression. Overexpression of the IbsC (V10N) mutant did not result in apparent damage of the inner membrane. In previous growth assays, we saw that there is often variability in the toxicity of intermediately active constructs (supplemental Fig. S3). Here, our results suggest that this variability in toxicity may be attributed to impaired membrane interaction or membrane penetration by these mutants.
FIGURE 6.
Overexpression of toxic IbsC derivatives is disruptive to the integrity of the inner membrane. A, effect of IbsC point mutants on membrane potential. Eight IbsC point mutants with different levels of observed toxicity were overexpressed in E. coli DH5αZ1. At 3 h after inducing the expression of each mutant, E. coli was incubated with DiBAC4(3), a potential-sensitive fluorescent dye. DiBAC4(3) penetrates cells with compromised inner membranes, resulting in an increase in green fluorescence (λem = 516 nm). Similar to the overexpression of IbsC, cells expressing toxic mutants with V5D, V5R, V10T, S15C, and S17E substitutions were ∼3–4-fold more fluorescent relative to the plasmid control. B, change in membrane polarization following the overexpression of IbsC combination mutants. We selected 3 IbsC double mutants and 3 IbsC triple mutants with varying toxicities and tested their effect on the inner membrane using the DiBAC4(3) uptake assay. Inducing the expression of active mutants IbsC (I7C, I14V) and IbsC (L8G, L11L, and S15A) dissipated the proton motive force, resulting in dye uptake and fluorescence enhancements of ∼2.5–4-fold. The tables below each graph compare the toxicities (denoted by %N.G.) with the relative fluorescence (R.F.) observed following the overexpression of each construct. n = 3.
We further selected 6 double and triple mutants with varying toxicities and examined their effect on the integrity of the inner membrane (Fig. 6B). Change in fluorescence observed following the induction of the three inactive mutants approximated the change associated with the plasmid control. The expression of mutant IbsC (I7P, I14M), which displayed moderate toxicity, led to a 2-fold increase in fluorescence. Overexpression of mutant IbsC (I7C, I14V) resulted in fluorescence increase of ∼2.5-fold. Consistent with this observation, this mutant was 10% less toxic than IbsC. We also examined one mutant, IbsC (L8G, L11L, S15A), that appeared as toxic as IbsC in our initial screen. Similar to IbsC, this construct elicited a 4-fold increase in fluorescence after 3 h of Atc induction. Overall, the extent of membrane depolarization associated with the overexpression of these mutants coincided with their toxicities.
DISCUSSION
The Ibs family of toxins is well-conserved in E. coli and other families of Gram-negative bacteria. Nevertheless, our study revealed that IbsC can withstand extensive mutations. Despite having only 19 amino acids, we found, through sequence truncation experiments, that the length of this peptide can be further minimized to 15 residues. With the exception of amino acid Val-5 and those at the last 10 positions of the peptide, amino acids can be individually removed without compromising the toxicity of IbsC. We systematically introduced mutations in the codons encoding these 11 amino acids using a sequence randomization strategy, and generated over 200 IbsC point mutants. Through growth and dye uptake assays, we found that a number of IbsC mutants exhibited growth inhibition and membrane disruption activities that are comparable to that of their wild-type counterpart when they are overexpressed. We are curious as to why amino acid substitutions are not more frequently observed in the native Ibs homologs. The sequences of the five Ibs homologs in E. coli K-12 (19) and those derived from Salmonella typhimurium LT2 (25) are very well conserved. Amino acid substitutions that are found at the more variable positions often share similar properties (e.g. Arg being substituted by Lys; Leu being substituted by Val or Ile).
Considering that the Sib antitoxins are encoded on the antisense strand of Ibs, the nucleotide sequences of both genes may have coevolved in a manner that can favor the structure of the sRNA antitoxins and prevent non-cognate sRNA-mRNA interactions. In a recent study, it was reported that the codon encoding amino acid Ser-17 of IbsC, which is not conserved among the five Ibs homologs in E. coli K-12, serves as a part of a recognition site between cognate sib-ibs pairs (26). In our present study, where the SibC antitoxin is not overexpressed, we also observed a high frequency of amino acid substitutions at this position in our toxic mutants.
The sequence conservation may also imply a possible sequence-function relationship in the peptide. The toxic phenotype we observed may be a consequence of IbsC overexpression. Under physiological conditions, the expression of the Ibs peptides may rarely surpass the threshold at which it starts to become deleterious to the cell. As such, the native Ibs sequences may be favored because they are better suited for their potential biological functions and for protein-protein or protein-membrane interactions.
At present, the exact cellular target(s) of IbsC and its mechanism of action are unclear. Our mutagenesis data provided some insight into the residues that may be important for the function of this peptide. IbsC was proposed to adopt an α-helical conformation and insert into membranes (19). Structural data presented in our present study support that hypothesis. Growth analyses conducted with the IbsC point mutants suggest that the hydrophobic residues spanning the middle of the peptide may be required for the proposed transmembrane localization of IbsC. From the combination mutants, we found that valine, leucine, and isoleucine are highly favored at multiple positions. From our mutagenesis studies, we noticed that polar and ionizable amino acids are well-tolerated at positions flanking the hydrophobic residue-rich region in the middle of the peptide. If IbsC is indeed a transmembrane peptide, it is possible that these hydrophilic and charged residues can facilitate initial contact between the peptide and polar head groups of the bilayer. These residues may further play a role in anchoring the peptide and stabilizing its interaction with the inner membrane. While we observed that an inactive point mutant with an arginine substitution at the core of the peptide was capable of adopting an α-helical structure in solvent, the presence of this charged residue may impede its interaction with the lipid bilayer. In the same experiments, we noticed that mutants IbsC (6–19) and IbsC (L8G, L11L, S15A) were not as helical as the wild-type toxin or as the IbsC (L11R) mutant in TFE. However, they retain their hydrophobic cores and may stably insert into the phospholipid bilayer when placed in the context of the membrane. In addition to causing structural changes and disrupting membrane insertion, mutations giving rise to non-toxic mutants may also perturb the peptides' ability to self-aggregate or interact with other cellular targets.
Type I toxins have been implicated in regulatory processes that are important for the individual and communal survival and adaptability of bacteria. These elements are suggested to be abundant in microbial genomes. Currently, a number of biochemical approaches and computer algorithms are implemented in searches for peptides and sRNAs that comprise putative type I TA pairs (18, 27). As more TA pairs are identified and characterized, parameters guiding their searches are refined. Using IbsC as a model, we developed a better understanding of the sequence requirements for small peptide toxins found in prokaryotes. From our sequence truncation analysis, we learned that IbsC can be minimized to 15 amino acids and remain active, suggesting that the lower size limit of toxins can be set at 15 residues. Based on the minimal active truncation mutant, IbsC (6–19), we postulate that this toxin requires a minimum of 10 amino acids with highly hydrophobic side chains (e.g. Met, Cys, Phe, Leu, Val, or Ile) to retain its toxicity. Growth assays carried out with our IbsC point mutants indicated that only substitutions by other hydrophobic residues are tolerated at these positions near the core of the peptide. This is consistent with previous studies on transmembrane proteins, which suggest that 9 to 11 residues are sufficient to promote helix translocation across the membrane (28–30). At positions flanking the hydrophobic core, amino acids with different hydrophilic side chains were found to be able to substitute for native hydrophilic residues. This again demonstrates that many of IbsC residues can be substituted with similar functional groups. This knowledge of the general architecture of IbsC may aid in the design and search for novel toxin/antitoxin systems.
Searches for novel TA systems are further fueled by the therapeutic potential of these elements. For example, expression of the HokC toxin from E. coli was induced in melanoma (31), breast (32), and lung (33) cancer cell lines. This toxin showed promise in hindering the growth and proliferation of these malignant cells. Type I toxins may also be considered as possible antimicrobial targets. Recent studies have shown that treating E. coli with certain antibiotics, such as ciprofloxacin, can induce the expression of toxins that have been linked to the SOS response, thereby promoting the formation of persister cells and enhancing tolerance to these drugs (34). In another study, it was found that the overexpression of membrane-targeting peptides, including IbsC, can increase the sensitivity of bacteria toward tobramycin and other aminoglycosides (35). Given the possible applications of toxin-antitoxin systems, it would be of interest to further probe into the regulation and function of the Ibs family of toxins in the cell and to examine whether IbsC and the derivatives we generated through these studies would be suited for therapeutic purposes.
Supplementary Material
Acknowledgments
We thank Li Laboratory members for helpful discussions with regard to the project design and the manuscript. We are also tremendously grateful to Drs. Richard and Raquel Epand, Dr. Giuseppi Melacini, and Julijana Milojevic for their time, use of equipment, and advice on structural studies. We thank Professor Herman Bujard for the gift of plasmids and the E. coli DH5αZ1 strain.
This work was supported by the Natural Sciences and Engineering Research Council of Canada (NSERC).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Tables S1–S3 and Figs. S1–S6.
- TA
- toxin-antitoxin
- %N.G.
- normalized growth (%)
- Atc
- anhydrotetracycline
- IGR
- intergenic region
- PLtetO-1
- tetracycline-inducible promoter
- RBS
- ribosome binding site
- sRNA
- small RNA
- TFE
- 2,2,2-trifluoroethanol.
REFERENCES
- 1.Hemm M. R., Paul B. J., Schneider T. D., Storz G., Rudd K. E. (2008) Mol. Microbiol. 70, 1487–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alix E., Blanc-Potard A. B. (2009) Mol. Microbiol. 72, 5–11 [DOI] [PubMed] [Google Scholar]
- 3.Fozo E. M., Hemm M. R., Storz G. (2008) Microbiol. Mol. Biol. Rev. 72, 579–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerdes K., Rasmussen P. B., Molin S. (1986) Proc. Natl. Acad. Sci. U.S.A. 83, 3116–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hayes F. (2003) Science 301, 1496–1499 [DOI] [PubMed] [Google Scholar]
- 6.Magnuson R. D. (2007) J. Bacteriol. 189, 6089–6092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Melderen L., Saavedra De Bast M. (2009) PLoS Genet. 5, e1000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wozniak R. A., Waldor M. K. (2009) PLoS Genet. 5, e1000439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pedersen K., Christensen S. K., Gerdes K. (2002) Mol. Microbiol. 45, 501–510 [DOI] [PubMed] [Google Scholar]
- 10.Ramage H. R., Connolly L. E., Cox J. S. (2009) PLoS Genet. 5, e1000767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Darfeuille F., Unoson C., Vogel J., Wagner E. G. (2007) Mol. Cell. 26, 381–392 [DOI] [PubMed] [Google Scholar]
- 12.Unoson C., Wagner E. G. (2008) Mol. Microbiol. 70, 258–270 [DOI] [PubMed] [Google Scholar]
- 13.Kawano M., Aravind L., Storz G. (2007) Mol. Microbiol. 64, 738–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buts L., Lah J., Dao-Thi M. H., Wyns L., Loris R. (2005) Trends Biochem. Sci. 30, 672–679 [DOI] [PubMed] [Google Scholar]
- 15.Rotem E., Loinger A., Ronin I., Levin-Reisman I., Gabay C., Shoresh N., Biham O., Balaban N. Q. (2010) Proc. Natl. Acad. Sci. U.S.A. 107, 12541–12546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amitai S., Yassin Y., Engelberg-Kulka H. (2004) J. Bacteriol. 186, 8295–8300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Amitai S., Kolodkin-Gal I., Hananya-Meltabashi M., Sacher A., Engelberg-Kulka H. (2009) PLoS Genet. 5, e1000390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fozo E. M., Makarova K. S., Shabalina S. A., Yutin N., Koonin E. V., Storz G. (2010) Nucleic Acids Res. 38, 3743–3759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fozo E. M., Kawano M., Fontaine F., Kaya Y., Mendieta K. S., Jones K. L., Ocampo A., Rudd K. E., Storz G. (2008) Mol. Microbiol. 70, 1076–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mok W. W., Navani N. K., Barker C., Sawchyn B. L., Gu J., Pathania R., Zhu R. D., Brown E. D., Li Y. (2009) Chembiochem 10, 238–241 [DOI] [PubMed] [Google Scholar]
- 21.Hershberg R., Altuvia S., Margalit H. (2003) Nucleic Acids Res. 31, 1813–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lutz R., Bujard H. (1997) Nucleic Acids Res. 25, 1203–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sreerama N., Woody R. W. (2000) Anal. Biochem. 287, 252–260 [DOI] [PubMed] [Google Scholar]
- 24.Epps D. E., Wolfe M. L., Groppi V. (1994) Chem. Phys. Lipids. 69, 137–150 [DOI] [PubMed] [Google Scholar]
- 25.Papenfort K., Pfeiffer V., Lucchini S., Sonawane A., Hinton J. C., Vogel J. (2008) Mol. Microbiol. 68, 890–906 [DOI] [PubMed] [Google Scholar]
- 26.Han K., Kim K. S., Bak G., Park H., Lee Y. (2010) Nucleic Acids Res. 38, 5851–5866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wassarman K. M., Repoila F., Rosenow C., Storz G., Gottesman S. (2001) Genes Dev. 15, 1637–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Calamia J., Manoil C. (1990) Proc. Natl. Acad. Sci. U.S.A. 87, 4937–4941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gurezka R., Langosch D. (2001) J. Biol. Chem. 276, 45580–45587 [DOI] [PubMed] [Google Scholar]
- 30.Krishnakumar S. S., London E. (2007) J. Mol. Biol. 374, 671–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Boulaiz H., Prados J., Melguizo C., Marchal J. A., Carrillo E., Peran M., Rodríguez-Serrano F., Martinez-Amat A., Caba O., Hita F., Concha A., Aránega A. (2008) Br. J. Dermatol. 159, 370–378 [DOI] [PubMed] [Google Scholar]
- 32.Boulaiz H., Prados J., Melguizo C., García A. M., Marchal J. A., Ramos J. L., Carrillo E., Vélez C., Aranega A. (2003) Br. J. Cancer. 89, 192–198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Prados J., Melguizo C., Rama A., Ortiz R., Boulaiz H., Rodriguez-Serrano F., Caba O., Rodriguez-Herva J. J., Ramos J. L., Aranega A. (2008) Int. J. Oncol. 33, 121–127 [PubMed] [Google Scholar]
- 34.Dörr T., Vulić M., Lewis K. (2010) PLoS Biol. 8, e1000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee S., Hinz A., Bauerle E., Angermeyer A., Juhaszova K., Kaneko Y., Singh P. K., Manoil C. (2009) Proc. Natl. Acad. Sci. U.S.A. 106, 14570–14575 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.