Abstract
The identity of the cell adhesive factors in fetal bovine serum, commonly used to supplement growth media, remains a mystery due to the plethora of serum proteins. In the present analyses, we showed that fetuin-A, whose function in cellular attachment in tissue culture has been debated for many years, is indeed a major serum cell attachment factor particularly for tumor cells. We are able to report this because of a new purification strategy that has for the first time given us a homogeneous protein band in colloidal Coomassie-stained gels that retains biological activity. The tumor cells adhered to immobilized fetuin-A and not α2-macroglobulin, its major contaminant. The interaction of cells with fetuin-A was driven mainly by Ca2+ ions, and cells growing in regular medium supplemented with fetal bovine serum were just as sensitive to loss of extracellular Ca2+ ions as cells growing in fetuin-A. Fractionation of human serum revealed that cell attachment was confined to the fractions that had fetuin-A. Interestingly, the tumor cells also took up fetuin-A and secreted it back to the medium using an unknown mechanism that can be observed in live cells. The attachment of tumor cells to fetuin-A was accompanied by phosphatidylinositol 3-kinase/Akt activation that was down-regulated in cells that lack annexin-A6, one of the cell surface receptors for fetuin-A. Taken together, our data show the significance of fetuin-A in tumor cell growth mechanisms in vitro and open new research vistas for this protein.
Keywords: Akt PKB, Annexin, Cell Adhesion, Cell Surface Receptor, MAP Kinases (MAPKs), PI 3-Kinase, Cell Growth, Fetuin-A, Serum Proteins
Introduction
Serum, particularly fetal bovine serum, is widely used as a supplement in culture media that is required for growth of most cells in culture (for a review, see Ref. 1). In the absence of serum, most cells fail to adhere properly, spread, and grow on culture dishes. Serum has a plethora of adhesion (2–4) and growth factors (5). The prevailing assumption over the past two decades has been that integrins are the major cellular receptors for adhesion in cell cultures (6). Cellular adhesion to extracellular adhesion molecules such as vitronectin, fibronectin, and laminin using integrins requires the divalent ions Mg2+/Mn2+ (7). In addition, cell signaling cues for spreading and growth mechanisms in anchorage-dependent cells emanate from the interaction of cells with the adhesion molecules (8). Although it is easy to define such signals in situations where cells are allowed to adhere to known purified extracellular matrix proteins such as fibronectin and collagen (9), adhesion in the presence of fetal bovine serum is complex in that any one of the myriad attachment proteins and or growth factors has the potential to mediate adhesion and growth signals.
Ever since it was first purified and described, fetuin-A/ahsg2 was suspected of being the principal cell adhesion molecule in serum (1, 10). Fetuin-A isolated and purified from serum by the Pedersen method (hereafter referred to as Pedersen fetuin-A) demonstrated cell adhesive properties in the presence of divalent ions (11). The Pedersen fetuin-A, however, is not considered pure because it is contaminated with a number of proteins including α2-macroglobulin (α2M) (12). Fetuin-A purified using the Spiro method on the other hand gave a more pure fraction but lacked attachment properties (1, 13). These discrepancies raised serious doubts as to whether fetuin-A plays any role in cellular adhesion. Previous studies from our laboratory and others demonstrated that the cellular attachment to Pedersen fetuin-A was mediated by annexins, particularly annexin-A2 and -A6 (14, 15). This adhesion is Ca2+ ion-dependent and requires the sialic acid moieties on fetuin-A because asialofetuin-A lacks this property (16).
Fetuin-A is a serum glycoprotein synthesized and secreted by the liver and to a lesser extent the kidneys, placenta, and the tongue (17). It has a high content of sugar moieties including sialic acid residues (1, 18). Its molecular mass ranges from 51 to 67 kDa depending on the carbohydrate content (19). It is a member of the cystatin family of proteins although it lacks cysteine protease-inhibitory capacity (20). A number of studies suggest that fetuin-A is a multifunctional protein (21). A key physiological function attributed to the protein, determined with the aid of fetuin-A knock-out mice, is its ability to inhibit ectopic calcification (22). We demonstrated that fetuin-A is capable of binding to matrix metalloproteinases, particularly MMP-9, and protects this enzyme from autolytic degradation (23). This interaction is most likely mediated by the cystatin domains in fetuin A because other members of the family such as cystatin C also interact with matrix metalloproteinases (23). Interestingly, it has been shown that fetuin-A is also able to stabilize m-calpain, a cytoplasmic cysteine proteinase (24). Other studies in our laboratory demonstrated that the Pedersen fetuin-A mediates the activation of PI 3-kinase/Akt (16). Furthermore, a critical functional role of this protein may depend on its rapid uptake by cells as well as its ability to act as an opsonin in the blood (14, 25).
To revisit the question of whether or not fetuin-A plays any role in cellular adhesion and signaling, we developed a purification protocol in which the less pure Pedersen preparation was further purified by glycerol gradient centrifugation. With this purification strategy, we separated fetuin-A from α2M (its major co-purifying protein) and other contaminating proteins including inter-α (globulin) inhibitor H2 (identified by MALDI-TOF mass spectrometry) starting with the less pure Pedersen preparation. We also resolved human serum on a glycerol gradient and determined that cell attachment and growth are associated with the fractions that contain ahsg/fetuin-A.
EXPERIMENTAL PROCEDURES
Materials
Partially purified fetuin-A (Pedersen fetuin-A) was purchased from either Sigma or Calbiochem. Polyclonal antibodies to α2M were purchased from Sigma. Antibodies to fetuin-A, annexin A2 (AnxA2), annexin A6 (AnxA6), and ERK2 were purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). Antibodies to phospho-ERK1/2 and phospho-Akt (Ser-473) were from Cell Signaling Technology (Danvers, MA). GFP-AnxA2 expression plasmid was kindly donated to us by Dr. Carl E. Creutz (University of Virginia).
Cells
The breast carcinoma cell line (BT-549) was purchased from ATCC (Manassas, VA). A derivative of BT-549 stably overexpressing Galectin-3, herein denoted as BT-Gal3, was kindly donated by Dr. Avraham Raz (Karmanos Cancer Research Institute, Detroit, MI). These cell lines were propagated in Dulbecco's modified Eagle's medium/nutrient F-12 (DMEM/F-12) supplemented with 10% heat-inactivated fetal bovine serum, 2 mmol/liter l-glutamine, 100 units/ml penicillin, and 50 units/ml streptomycin in a 95% air and 5% CO2 incubator at 37 °C. DMEM/F-12 lacked Mg2+ and Ca2+, and serum was the sole source for these ions. Where indicated, serum-free medium consisted of DMEM/F-12 in which fetal bovine serum (FBS) was replaced with 0.1% bovine serum albumin (BSA).
Knockdown of Annexins
For down-regulation of AnxA2 and AnxA6, BT-549 cells were transfected with small hairpin RNAs (shRNAs) in plasmid pSM2c (Open Biosystems, Huntsville, AL) targeting the coding sequences of these proteins. The sequences of the targeted sense region are as follows: for AnxA6, 5′-CACTCGGACCAATGCTGA-3′ and for AnxA2, a mixture of 5′-CCTGCTTTCAACTGAATTGTT-3′, 5′-GCAGGAAATTAACAGAGTCTA-3′, 5′-CTGTACTATTATATCCAGCAA-3′, and 5-CGGGATGCTTTGAACATTGAA-3′. The parental controls were transfected with the empty vector. Transfected cells were selected in complete medium containing 2.5 μg/ml puromycin, cloned, and expanded. Clones in which the down-regulation of these proteins was most efficient as tested by immunoblotting were selected for further studies.
Ultrafiltration
Pedersen fetuin-A was dissolved in Hepes-buffered saline (HBS; 11 mm Hepes, 137 mm NaCl, 4, mm KCl, 1 mm glucose) and transferred to 100,000 molecular weight cutoff filter units (Millipore, Billerica, MA) and centrifuged at 10,000 × g until totally filtered. The flow-through was transferred to new tubes; the retentate was reconstituted in an equal volume. The protein concentration in the fractions was determined using the Bradford assay, and equal amounts of protein were analyzed in 4–12% SDS gels and visualized by colloidal Coomassie staining (Sigma).
Affinity Purification of Pedersen Fetuin-A
Wheat germ agglutinin-agarose beads (Sigma) were washed twice with HBS followed by incubation with Pedersen fetuin-A for 4 h at 4 °C. The beads were abundantly washed with the starting buffer and then eluted with 0.1 m N-acetylglucosamine in HBS. Equal volumes of the eluates were resolved by SDS-PAGE as above.
Glycerol Gradient Centrifugation
Glycerol step gradients (10-15-30-45-60%) were made in 13-ml ultracentrifugation tubes (Beckman) from 60% glycerol buffered with 10 mm Tris-HCl, pH 7.4, 150 mm NaCl, and 0.5 mm MgCl2. About 10 mg of Pedersen fetuin-A, fractionated fetal bovine serum as described recently (26), or human serum (Sigma) in a total volume of 1.0 ml were layered on the step gradient. This was then centrifuged at 35,000 rpm (Beckman, SW40Ti rotor) for 18 h at 4 °C. Gradients were fractionated from the top. The fractions were analyzed by SDS-PAGE in 4–12% gels followed by staining with colloidal Coomassie or Western blotting with the indicated antibodies. Before use in other experiments, glycerol was removed by centrifugation through a 3000 molecular weight cutoff ultrafiltration unit, and the retentate was washed with HBS and reconstituted in the initial volume of HBS. Fractions from six identical gradients containing identical protein profiles were pooled and dialyzed against HBS. These were designated from the top of the gradient as fractions S1, S2, and S3 and stored in aliquots at −80 °C until required for use.
Cell Attachment Assays
Wells of 96-well plates were coated in quadruplicates with glycerol gradient fractions (diluted 1:2 in HBS) or the indicated concentrations of the pooled and dialyzed fractions overnight at 4 °C. Prior to use in cell attachment/spreading assays, the coating solutions were discarded, and 1 × 104 cells in serum-free DMEM/F-12 were added to each well and incubated overnight (for 16–24 h) at 37 °C. As controls, cells were seeded in DMEM/F-12 containing Pedersen fetuin-A or serum-free DMEM/F-12. Cells were photographed using a DCM200 digital camera equipped with Scopephoto software, and cell numbers and viability were estimated by Alamar Blue colorimetric assay (absorbance read at 570 nm or fluorescence read at 595 nm after excitation at 530 nm). In other experiments, the cells were fixed in either cold methanol or 2.2% (w/v) paraformaldehyde, stained with crystal violet, and photographed.
Immunoblotting
Cells were grown in 15-cm dishes until 80–90% confluent in complete DMEM/F-12. The cells were washed once in ice-cold PBS and harvested by scraping. Cells were lysed in radioimmune precipitation assay buffer (50 mm Tris-HCl, pH 7.4, 1% Nonidet P-40, 0.1% sodium deoxycholate, 150 mm NaCl, 1 mm EDTA) containing protease inhibitor mixture (Sigma) and phosphatase inhibitors (20 mm sodium fluoride, 50 mm β-glycerophosphate, and 1 mm sodium orthovanadate). Cell lysates or various fractions of glycerol gradients were separated in 4–12% SDS-polyacrylamide gels and transferred to nitrocellulose membranes. The membranes were subsequently probed with the indicated antibodies and revealed by enhanced chemiluminescence as described previously (26).
Uptake of Labeled Purified Fetuin-A by BT-549 Breast Carcinoma Cells
Purified fetuin-A (fraction S1) was labeled with rhodamine isothiocyanate (Sigma) as described previously (26). For real time imaging of rhodamine-labeled purified fetuin, BT-Gal3 cells were transfected with GFP-annexin A2. Following 24 h in culture, the cells were trypsinized and replated on microscope coverslips in 6-well culture plates. Cells were subsequently serum-starved for another 24 h, then washed twice with HBS without Ca2+ and Mg2+, and incubated with 0.8 μm purified fetuin-A (fraction S1) labeled with rhodamine isothiocyanate in HBS containing 2 mm Ca2+. The uptake of rhodamine-labeled fetuin-A (red) by annexin A2-transfected cells (green) was followed in real time for up to 30 min using a Nikon A1R confocal microscope (Nikon, Melville, NY). GFP was excited with a 488 nm laser (emission filter, 505–550 nm band pass), and rhodamine was excited with a 561 nm laser (emission filter, 575–650 nm).
Adherent cells were also grown on microscope coverslips, serum-starved for 48 h in DMEM supplemented with 0.1% BSA, and washed twice with Ca2+- and Mg2+-free HBS. Cells were then incubated for 30 min in HBS containing a 1 mm concentration of divalent ions Ca2+/Mg2+ and 10 μg/ml rhodamine-labeled fetuin-A. The cells were rinsed twice with cold PBS, fixed for 10 min with −20 °C cold methanol, and again rinsed twice with cold PBS. After washing, the coverslips were mounted on slides using Prolong Gold with DAPI (Invitrogen). Uptake was visualized by fluorescence microscopy using a Nikon Eclipse TE-2000-E inverted microscope equipped with NIS-Elements AR software (Nikon).
RESULTS
Ultrafiltration and Wheat Germ Agglutinin Purification of Pedersen Fetuin-A
The rationale behind using ultrafiltration as a strategy to further purify Pedersen fetuin-A was to separate uncomplexed fetuin-A from its high molecular weight aggregates formed with its contaminants. This approach was unable to separate fetuin-A from its higher molecular weight contaminants (data not shown). Similarly, a purification strategy for fetuin-A using wheat germ agglutinin-agarose affinity chromatography also failed to offer an improvement over the Pedersen method. The fractions eluted by N-acetylglucosamine had numerous protein bands by colloidal Coomassie Blue staining, although only one homogeneous band was reported when stained with regular Coomassie (27). More importantly, the dominant α2M contaminant was not separated from fetuin-A (data not shown).
Resolution of Bovine Serum on Glycerol Gradients
In addition to the soluble proteins such as fetuin-A, serum contains nanovesicles known as exosomes that are frequently purified by glycerol or sucrose gradient centrifugation. We recently showed that fetuin-A is equally distributed between the exosome-free and exosome-enriched fractions of bovine serum (26). We therefore sought to examine whether fetuin-A in the fractionated serum could be separated from its contaminants by floating these fractions on glycerol step gradients. To do this, bovine exosome-free serum and exosome-enriched serum were prepared as described recently (26). These were layered on glycerol step gradients, separated by centrifugation, and then fractionated from the top. As shown in Fig. 1A, the fractionation yielded one major protein peak. However, analysis of fetuin-A or α2M by Western blotting in the exosome-free serum (Fig. 1B) surprisingly revealed that fetuin-A was eluted in the less rapidly sedimenting fractions (fractions 3–6), whereas α2M came off in the rapidly sedimenting fractions (fraction 8–11). It is interesting to note that this strategy effectively separated the majority of fetuin-A from its major contaminant α2M and that the separation was more efficient when exosome-free serum was used. This suggests that to purify soluble fetuin-A from serum it is necessary to remove serum exosomes by differential centrifugation and then resolve the exosome-free serum on glycerol gradients. Fetuin-A was associated with serum exosomes that also eluted in the less rapidly sedimenting fractions (fractions 3–7) (Fig. 1C). We previously reported that serum exosomes contain fetuin-A (26).
FIGURE 1.
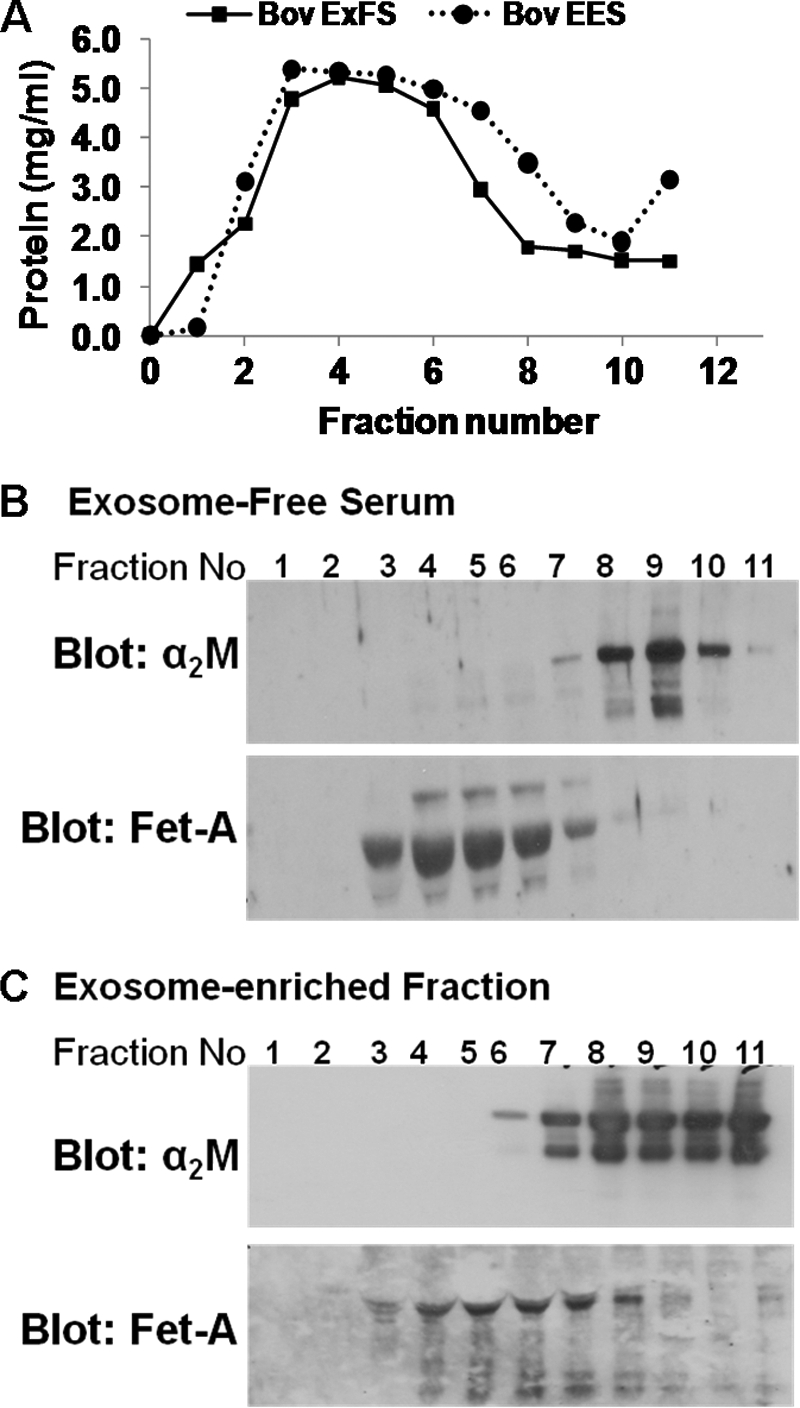
Glycerol gradient separation of fetuin-A from α2M in fetal bovine serum. A, exosome-free (Bov ExFS) and exosome-enriched (Bov EES) fractions of bovine serum were prepared by ultracentrifugation of fetal bovine serum. After reconstitution of the exosome-enriched serum in serum-free DMEM/F-12, 10 mg/ml total protein was layered on a glycerol step gradient, centrifuged, and fractionated from the top (1.0-ml fractions). The protein content in each fraction was measured by the Bradford assay. B and C, equal volumes of each fraction containing separated exosome-free (B) or exosome-enriched (C) proteins were analyzed by SDS-PAGE in 4–12% gradient gels, blotted onto nitrocellulose membranes, and probed with antibodies to fetuin-A (Fet-A) or α2M.
Purification of Pedersen Fetuin-A by Glycerol Gradient Centrifugation
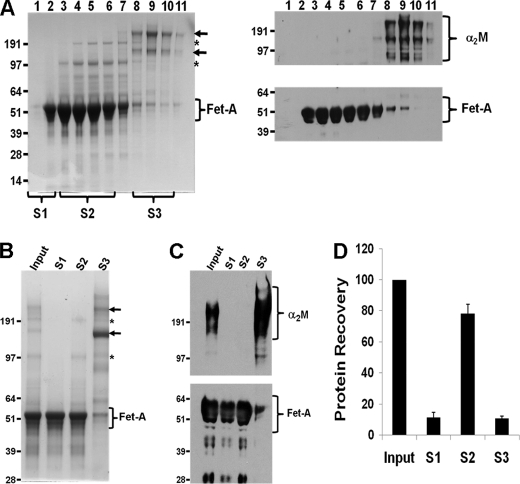
This is the first purification protocol in our hands that has been able to separate fetuin-A in Pedersen fetuin from its major contaminant α2M in solution as depicted by the colloidal Coomassie-stained gel (Fig. 2A). It was critical to resolve each of the fractions in reducing gels followed by colloidal Coomassie staining, the sensitivity of which approaches that of silver staining. Thus, a single homogeneous band in colloidal Coomassie-stained gels is considered pure. Fetuin-A came off in the less rapidly sedimenting fractions (Fig. 2A, lanes 2–6), whereas α2M banded in the rapidly sedimenting fractions (Fig. 2A, lanes 7–11). From the fractionation, we designated as pure fetuin-A (pooled fraction S1) eluted in tubes 1 and 2 (Fig. 2A, lanes 1 and 2), pooled fraction S2 as fetuin-A contaminated with inter-α (globulin) inhibitor H2 (ITIH2) and other minor contaminants eluted in tubes 3–6 (Fig. 2A, lanes 3–6), and lastly pooled fraction S3 as α2M and other contaminants eluted in tubes 8–11 (Fig. 2A, lanes 8–11). The separation of fetuin-A from α2M was confirmed by Western blotting (Fig. 2A, right panel). The tubes containing pure fetuin-A (pooled fraction S1), fetuin-A slightly contaminated with ITIH2 (pooled fraction S2), or mainly α2M (pooled fraction S3) were once more analyzed by Coomassie staining and Western blotting (Fig. 2, B and C, respectively). In terms of recovery, ∼10% of the proteins in Pedersen fetuin were recovered in S1, 80% were recovered in S2, and 10% were recovered in S3 (Fig. 2D). These fractions were then used for adhesion and cell signaling assays.
FIGURE 2.
Separation of fetuin-A in Pedersen fetuin from its major contaminants by glycerol gradient centrifugation. A, Pedersen fetuin-A was layered on a glycerol step gradient and centrifuged for 18 h at 100,000 × g, and the gradients were fractionated from the top. The distribution of fetuin-A and its contaminants was analyzed by SDS-PAGE in 4–12% gradient gels and either stained with colloidal Coomassie (left panel) or blotted onto nitrocellulose membranes and probed with antibodies to fetuin-A (Fet-A) or α2M (right panels). Notice the α2M peak in fraction 9 and the fetuin-A peak in fraction 4. The α2M bands are indicated by arrows, and ITIH2 is indicated by asterisks. B, fractionated fetuin-A fractions were pooled as indicated in A above and exhaustively dialyzed against HBS at 4 °C. Equal amounts of the pooled fractions were analyzed by SDS-PAGE in 4–12% gradient gels and either stained with colloidal Coomassie (B) or blotted onto nitrocellulose membranes and probed with antibodies to fetuin-A or α2M (C). D, densitometric analysis of protein recovery after glycerol gradient centrifugation. Bars represent recovered protein relative to the input from at least three determinations (mean ± S.E.).
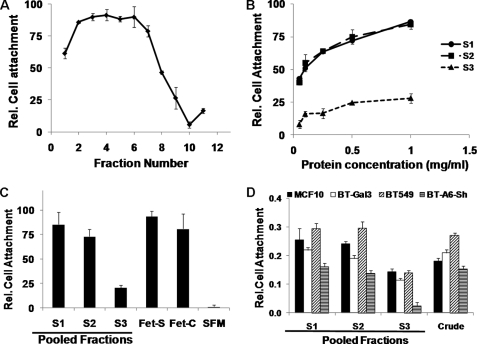
We tested the ability of proteins in each fraction of the glycerol gradient in the attachment of breast cancer cells. Although the protein concentration of fraction 1 was quite low compared with the other fractions (Fig. 2A), it effectively supported adhesion of BT-549 cells (Fig. 3A). However, it contained the purest fetuin-A because a longer exposure of the Western blot revealed the fetuin-A band in this fraction. More importantly, the pooled fraction S1 had the highest relative cell attachment potential (Fig. 3C). Despite having some impurities, S2 supported cell adhesion more or less to the same extent as S1 (Fig. 3, B and C). Because S3 (enriched in α2M) had the lowest cell attachment potential, it can be concluded that this serum protein is not involved in the adhesion and spreading of breast tumor cells. Similarly, the inability of fractions containing ITIH2 and other minor contaminants (S2) to affect adhesion relative to S1 shows that cellular adhesion and spreading are vested in fetuin-A. Interestingly, tumor cells that lacked annexin A6 (BT-A6-sh), one of the cell surface receptors for fetuin-A (15), had the poorest adhesion to the three pooled fractions and to Pedersen fetuin-A from two independent vendors (Sigma and Calbiochem) (Fig. 3D). Slight adhesion of cells to S3 is likely due to the small amount of contaminating fetuin-A. The optimum adhesion of cells to immobilized fetuin-A was realized in the presence of 1 mm Ca2+ ions.
FIGURE 3.
Cell attachment assays on glycerol gradient-purified fetuin-A. Partially purified fetuin-A from bovine serum (Pedersen fetuin-A) at 10 mg/ml in TBS was layered on a 10–60% glycerol step gradient and centrifuged, and equal fractions were collected from the top. Triplicate wells of a microtiter plate were coated overnight at 4 °C with 100 μl of a 1:2 dilution of each fraction (A); the indicated concentration of the pooled and dialyzed fractions S1, S2, and S3 (B); and a 0.5 mg/ml concentration of each of the pooled and dialyzed fractions S1, S2, and S3 as well as the crude preparation of commercially available fetuin-A (C and D). BT-549 cells stably expressing Galectin-3 (BT-Gal3) (A–D) or the indicated breast cancer cell lines (D) were harvested, washed, and resuspended in serum-free medium. The cells (1 × 104 cells/well) were then plated on the precoated wells, and the cells were allowed to attach overnight at 37 °C. Unattached cells were removed along with the medium by aspiration, and the cell counts and viability were estimated using the Alamar Blue assay. Bars represent attachment/viability of cells relative (Rel.) to attachment/viability in serum-free medium (mean ± S.E.). Fet-S, fetuin-A from Sigma; Fet-C, fetuin-A from Calbiochem.
Attachment of Breast Tumor Cells to Wells Coated with Human Serum Fetuin-A
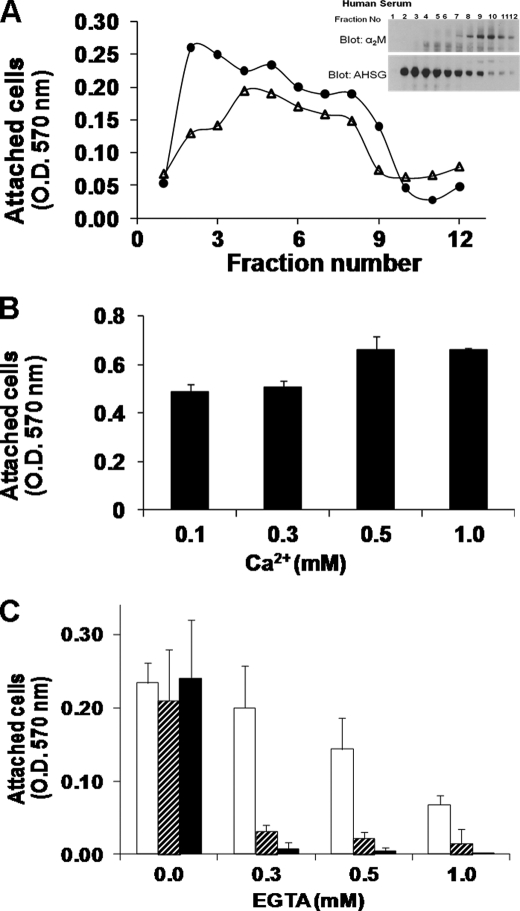
The goal of this experiment was to fractionate human serum into fractions rich in human fetuin-A (ahsg) and fractions devoid of the protein and to determine which serum fractions have cell attachment properties. Serum exosomes and exosome-free serum were resolved on glycerol gradients, and each of the fractions was tested for its ability to support cell attachment in the presence of Ca2+ ions. As expected, the serum fractions that were positive for fetuin-A (Fig. 4, solid circles and inset), supported the most attachment (fractions 1–5). Serum exosomes eluting in the less rapidly sedimenting fractions 2–7 (Fig. 4A, triangles) similarly supported cellular attachment as reported previously (26). The serum fractions that contained α2M (Fig. 4A, inset) on the other hand did not support cellular adhesion. Cell attachment in the presence of fetuin-A was supported by a wide range of Ca2+ ion concentration (0.1–1 mm) (Fig. 4B).
FIGURE 4.
Attachment of tumor cells to human serum proteins and fetuin-A. A, human serum exosomes and exosome-free serum were fractionated on glycerol gradients as described under “Experimental Procedures.” The wells of a 96-well microtiter plate were coated with the aliquots of the gradient fractions (100 μl/well) overnight at 4 °C. The wells were washed once with SFM, and BT-549 cells were added to the wells (2 × 104 cells/well) in SFM containing 1 mm Ca2+. The cells were allowed to attach for 3 h at 37 °C, the non-adherent cells were washed off with SFM, and the cells were once more incubated in SFM containing Alamar Blue (1:10). After 6–8 h of incubation, the plates were read at 570 nm to determine the number of attached and viable cells. Wells were coated with exosomal fractions (triangles) and with exosome-free serum (circles). Inset, the Western blots of the glycerol gradient fraction of exosome-free serum probed with anti-ahsg and anti-α2M. B, BT-549 cells were seeded in the wells of a 96-well microtiter plate (2 × 104 cells/well) in fetuin-A dissolved in SFM (0.25%, w/v) and containing different concentrations of Ca2+ (0.1–1 mm). C, BT-549 cells were allowed to attach to the wells of a microtiter plate (2 × 104 cells/well) in either complete medium (hatched bars) or 0.25% (w/v) fetuin-A in SFM (solid bars) for 6 h. Some of the wells of a microtiter plate were precoated with fibronectin (2 μg/well), and then BT-549 cells were added to them (2 × 104 cells/well) in complete medium (open bars) and incubated for 6 h. At the end of the incubation, the wells were washed once with HBS containing 1 mm Ca2+ and then incubated with HBS containing graded doses of EGTA (0–1 mm) for 30 min. The detached cells were washed twice with HBS containing 1 mm Ca2+, and finally cells were incubated in the respective incubation medium containing Alamar Blue (1:10) for 4–6 h or until color change to monitor the attached cells. Bars represent mean ± S.E.
Contribution of Serum Proteins to in Vitro Cell Attachment
Fetuin-A at 0.25% (w/v) supported the attachment of tumor cells to plastic wells to the same extent as the serum proteins at various concentrations of Ca2+ ions (Fig. 4C). The chelation of calcium ions in the medium drastically reduced the attachment of cells in the presence of either complete medium or 0.25% (w/v) fetuin-A at all concentrations of EGTA (Fig. 4C). Cells in the wells that were precoated with fibronectin on the other hand were less sensitive to the removal of Ca2+ by EGTA (Fig. 4C, open bars). The data suggest that in the presence of complete medium integrins are not the major adhesion receptors; otherwise, the cells would be less sensitive to EGTA chelation. More importantly, the data underscore the significance of Ca2+ in the adhesion of cells to plastic in the presence of medium supplemented with fetal bovine serum. Under these conditions, fetuin-A appears to be a major player in the adhesive process. However, this does not rule out the contribution of serum factors such as some glycans that can also mediate adhesion in the presence of Ca2+ ions (28).
Uptake of Labeled Purified Fetuin-A by BT-549 Breast Carcinoma Cells
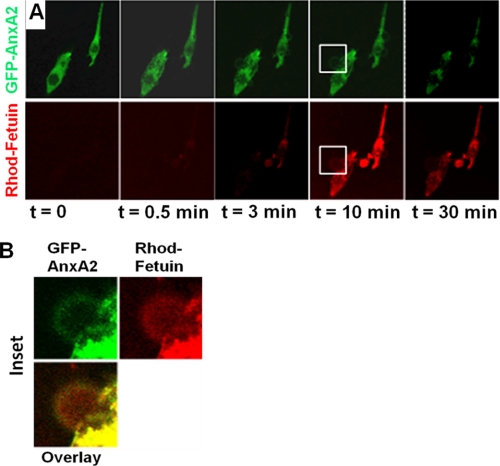
We next questioned whether fetuin-A can be taken up by the tumor cells and whether once inside fetuin-A can interact with intracellular AnxA2 or AnxA6. Breast carcinoma cells were transiently transfected with GFP-AnxA2 (green) and then incubated with rhodamine-labeled fetuin-A (red). The uptake of labeled fetuin-A was then followed in live cells. The data show that fetuin-A was taken up within seconds (Fig. 5A). By 10 min, the uptake was almost complete, and the cells began to pump out excess fetuin-A using an unknown mechanism that involved formation of membrane blebs (Fig. 5B). Interestingly, the fetuin-A in the blebs did not appear to co-localize with GFP-AnxA2. The data also show that internalized fetuin-A co-localized with GFP-AnxA2 inside the cells (Fig. 5B).
FIGURE 5.
Uptake of purified fetuin-A by BT-549 breast cancer cells. A and B, real time imaging of fetuin-A uptake. BT-549 cells overexpressing Galectin-3 (clone 11-9-1-4) were transfected with GFP-AnxA2. At 24 h post-transfection, the cells were trypsinized and replated on microscope coverslips in 6-well culture plates. Cells were subsequently serum-starved for 24 h, then washed twice with HBS without Ca2+ and Mg2+, and incubated with 0.8 μm rhodamine-labeled (Rhod) fetuin-A (fraction S1) in HBS containing 2 mm Ca2+. The uptake of rhodamine fetuin-A (red) by GFP-AnxA2-expressing cells (green) was followed in real time for up to 30 min using a Nikon A1R confocal microscope (A). The inset boxes in A are expanded in B, which shows the co-localization of rhodamine-labeled fetuin-A and GFP-AnxA2.
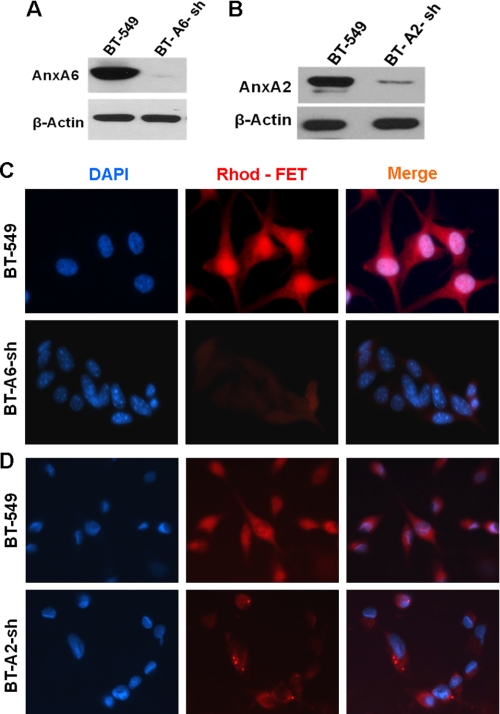
To determine the mechanisms that mediate the uptake of fetuin-A, we questioned whether annexins play a role in this process. The annexins on the cell surface may interact with fetuin-A in solution to mediate its uptake as shown for other cell types (14). To test this, we incubated rhodamine-labeled fetuin-A (fraction S1) with BT-549 breast carcinoma cells in which AnxA2 and AnxA6 had been knocked down and renamed BT-A2-sh and BT-A6-sh, respectively (Fig. 6, A and B). As shown in Fig. 6, C and D, depletion of AnxA6 and AnxA2, respectively, in BT-549 cells inhibited the uptake of fetuin-A compared with the parental cells.
FIGURE 6.
Influence of annexin-A2 and annexin-A6 on uptake of fetuin-A by tumor cells. BT-549, AnxA6-depleted BT-549 (BT-A6-sh; A), and AnxA2-depleted BT-549 (BT-A2-sh; B) cells were grown on glass coverslips in 6-well culture plates for 24 h. Cells were subsequently serum-starved for 48 h, washed twice with HBS, and treated with 10 μg/ml rhodamine labeled fetuin-A (Rhod-FET) (fraction S1) in HBS for 30 min at 37 °C. The cells were once again washed in ice-cold HBS and then fixed with paraformaldehyde. The coverslips were mounted in Prolong Gold with DAPI and visualized by fluorescence microscopy as described under “Experimental Procedures” (C and D).
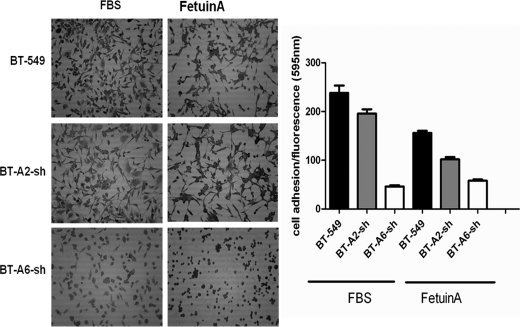
To further investigate the adhesive interactions between carcinoma cells and fetuin-A, we analyzed the attachment of parental BT-549 transfected with empty vector and BT-549 cells in which AnxA2 (BT-A2-sh) and AnxA6 (BT-A6-sh) had been knocked down to microtiter wells coated with either fetal bovine serum or fetuin-A in SFM. We determined in three separate experiments that whereas parental BT-549 adhered strongly to FBS- and fetuin-A-coated wells after 8 h of incubation both BT-A2-sh and BT-A6-sh had reduced adhesion to these substrata (Fig. 7). The reduced adhesion of cells to both FBS and fetuin-A was more drastic in AnxA6- compared with AnxA2-depleted cells. More importantly, lack of AnxA6 prevented cell spreading on either FBS or fetuin-A. Loss of AnxA2 on the other hand did not affect cell spreading (Fig. 7).
FIGURE 7.
Roles of AnxA2 and AnxA6 in adhesion and cell spreading on wells coated with either FBS or fetuin-A. The wells of a 96-well culture plate were coated overnight with either 10% FBS or 100 μg/ml purified fetuin-A in SFM. The wells were then washed once with HBS, and then cells (BT-549, BT-A2-sh, and BT-A6-sh) were added (2 × 104 cells/well) in SFM containing 1 mm Ca2+ and Mg2+ and incubated overnight at 37 °C. The detached cells were washed once with SFM, then fresh SFM including Alamar Blue (1:10) was added, and cells were incubated for another 4 h. The plate was read at 595 nm in a fluorescence plate reader after excitation at 530 nm. Each bar represents the average reading of six wells (mean ± S.E.). The SFM was subsequently removed, and cells were fixed in cold methanol and stained with crystal violet. Excess dye was washed off with water, the plate was dried, and cells were photographed.
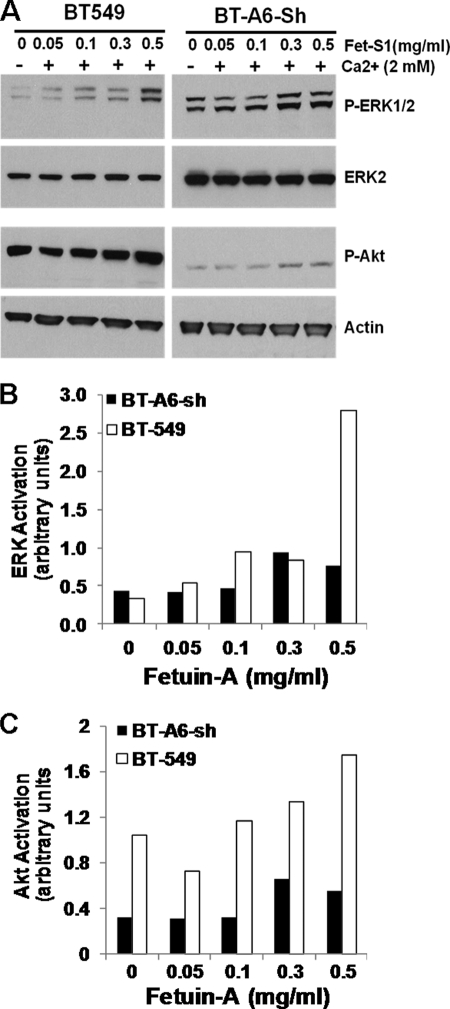
Lastly, we investigated the signaling mechanisms that are turned on upon interaction of breast carcinoma cells with purified fetuin-A. Purified fetuin-A fraction (S1) was incubated with BT-549 cells for 10 min in the absence or presence of graded doses of the protein, and then activation of the mitogen-activated protein kinase and PI 3-kinase/Akt pathways was monitored by measuring the levels of phospho-ERK1/2 and phospho-Akt (Ser-473) as readouts. A higher concentration of fetuin-A (fraction S1) was able to activate ERK to varying extents in the presence of Ca2+ (Fig. 8A). Interestingly, BT-549 cells that lacked AnxA6 showed almost negligible activity of the PI 3-kinase/Akt pathway compared with the parental cells (Fig. 8A). However, the mitogen-activated protein kinase pathway was highly active in this cell line even in the absence of Ca2+ (Fig. 8A).
FIGURE 8.
Fetuin-A-dependent activation of MAPK in breast carcinoma cells requires annexins and extracellular Ca2+. A, parental BT-549 or AnxA6-depleted BT-549 (BT-A6-sh) cells were serum-starved for 24 h, then washed two times with HBS, and incubated in HBS with or without 2 mm Ca2+ and the indicated concentrations of purified fetuin-A (fraction S1) (Fet-S1). Cells were subsequently harvested using a cell scraper, washed in ice-cold HBS, and lysed in ice-cold radioimmune precipitation assay buffer containing protease and phosphatase inhibitors as described under “Experimental Procedures.” Equal amounts of proteins were analyzed by Western blotting using antibodies to phospho-ERK1/2 (P-ERK1/2), phospho-Akt (Ser-473) (P-Akt), and total ERK2 or β-actin as the loading controls. B and C, densitometric analysis of ERK and Akt phosphorylation in the total cell lysates. Bars represent ERK1/2 phosphorylation normalized to total ERK2 (B) or Akt phosphorylation normalized to β-actin (C) from a representative.
DISCUSSION
Serum is known to have a number of adhesion platforms, which include the classical extracellular matrix proteins such as laminins, collagens, fibronectin, and vitronectin. More recent studies have revealed that chondroitin sulfate chains can also mediate cellular adhesion (28). We recently demonstrated that nanovesicles, known as exosomes, also form an important part of the cellular adhesion repertoire in serum and are more relevant in the anchorage-independent growth of tumor cells (26).
Fetuin-A is a notoriously sticky molecule that co-purifies with a number of unrelated proteins and other factors (1, 18). Separating these contaminating proteins from fetuin-A has been a technical challenge ever since this bovine protein and its human homologue (ahsg) were first described (1). Our goal in this study was to revisit an old question and ask whether or not fetuin-A plays a role in cellular adhesion in culture. From the moment it was first described until the report by Spiro (29), it was generally assumed that fetuin-A plays a role in the in vitro attachment and growth of cells. Using the standard techniques of the day, Spiro (29) demonstrated that highly purified fetuin-A lacked the ability to support cellular attachment in vitro. The results suggested that cellular attachment and growth were mediated by serum proteins that associate with fetuin-A (29). To further support this notion, Nie et al. (30) isolated a factor in fetal bovine serum that could replace Pedersen fetuin-A as a growth factor for human skeletal muscle satellite cells. Subsequent studies suggested that the reagents used in the Spiro method, namely Zn2+ ions, altered the structure of fetuin-A and consequently its ability to support cellular attachment and growth (1). An interesting report by Yu and Tsai (31) demonstrated that when Pedersen fetuin-A was incubated with Zn2+ followed by dialysis to remove the salts and then injected with breast tumor cells into nude mice fetuin-A induced apoptosis in the cells, which then failed to form tumors compared with untreated controls (without Zn2+). Their data underscored the ability of Zn2+ ions to alter the structure and consequently the biological properties of fetuin-A.
We hereby demonstrate that cellular attachment to Pedersen fetuin-A is mediated by the protein per se and not its contaminating factors as suggested by earlier reports that overwhelmingly supported the “contaminant theory” (1). Clearly, it is important to define the cell adhesion mechanisms that are in play in fetal bovine serum-based tissue culture because it is still the dominant model system that biologists use to study cell growth mechanisms. Our data also support earlier studies that intimated the importance of Ca2+ for tumor cell growth (32). The interaction of cells with serum-derived fetuin-A is mediated by cell surface-expressed annexins (15). This is based on the observation that knockdown of annexins, particularly AnxA6, on cells significantly reduced the adhesion of these cells to fetuin-A-coated wells (Fig. 3) (15). Others have also shown that fetuin-A interacts with AnxA2 and AnxA6 (14, 33). Kojima et al. (34) demonstrated that AnxA4 binds strongly to fetuin-A coupled to Sepharose columns. Our attempts to co-immunoprecipitate fetuin-A with AnxA2 or AnxA6 in cell lysates with antibodies to fetuin-A and vice versa, however, have not given us consistently reliable data. Nevertheless, the adhesive interaction of carcinoma cells with immobilized fetuin-A that depends on the levels of annexins expressed on the cell surface suggests that these proteins could interact more when associated with membranes or immobilized but only interact weakly in solution. However, the cell signals mediated by fetuin-A in solution such as PI 3-kinase/Akt can also be transmitted by immobilized fetuin-A (16).
Herein, we have demonstrated the significance of annexin/fetuin-A interaction in cellular adhesion and growth of cells in media supplemented with fetal bovine serum. The data suggest that whereas annexin family members AnxA2 and AnxA6 play significant roles in the attachment of breast carcinoma cells to fetuin-A it is AnxA6 that mediates cell spreading. Interestingly, AnxA2 has been shown to promote cell spreading in intestinal epithelial cells (35), suggesting that the abilities of AnxA2 and AnxA6 to mediate cell spreading is cell type-dependent. It has been reported that down-regulation of AnxA2 expression in human cells markedly reduced their proliferation (36). The mechanism driving this AnxA2-based cell proliferation was not defined by this report.
The data reported herein intimate that lack of AnxA2 or AnxA6 leads to a reduced interaction of the cells with fetuin-A and consequently altered growth potential in culture. Interestingly, cells that lack AnxA2 and AnxA6 such as LNCaP prostate cancer cells grow poorly in culture with a doubling time of ∼31 h compared with another prostate cancer line (PC3) that expresses AnxA2 and has a doubling time of ∼24 h (37). Whether cell/fetuin-A interaction is also as important for in vivo cell attachment and growth as it is for in vitro adhesion is yet to be defined. We previously determined that Lewis lung carcinoma cells proliferate much more vigorously after transplantation into fetuin-A wild-type syngeneic C57/BL-6 mice relative to the fetuin-A-null mice. The cells also attached and proliferated well in vitro in the presence of fetuin-A (16). The in vivo extracellular matrices are populated with ligands for integrins such as fibronectin, laminin, and collagen, and therefore, one would expect that integrin-mediated adhesion is more relevant in this microenvironment (8). However, there are other extracellular matrices such as liver extracellular matrix that have a higher than normal concentration of fetuin-A. We have also demonstrated that the lung extracellular matrix has a high concentration of fetuin-A.3 Fetuin-A binds to elastin fibers with micromolar affinity, and tumor cells have a tendency to attach to the fibers in the presence of culture medium supplemented with fetal bovine serum (38). Taken together, the elevated levels of fetuin-A in the lung and liver extracellular matrix could be one of the reasons why these organs are considered to be “good soil” for tumor growth (39). The metastatic tumor cells may have a preference for fetuin-A as an adhesion and growth platform. Bone is another microenvironment to which a number of metastatic tumor cells attach and grow (40). Interestingly, fetuin-A has been shown to be concentrated in the bone extracellular matrix (41).
Our data showing the efficient uptake of labeled fetuin-A by tumor cells were interesting. Although we cannot rule out the possibility that some of the fetuin-A taken up is degraded by the tumor cells, the data suggest a sophisticated mechanism of trafficking fetuin-A by tumor cells and possibly other cell types (14). The uptake could be part of deadhesion that is necessary in cellular processes such as motility and cell division (42). Another possibility is that the fetuin-A taken up stabilizes and protects intracellular caspases and calpains from autolytic degradation (21, 24). Lastly, fetuin-A could be taken in, undergo some form of covalent modification, and then be secreted back to the medium as suggested by the data. We are currently doing experiments to address these possibilities. More importantly, our data suggest that both AnxA2 and AnxA6 are involved in fetuin-A uptake mechanisms as shown in other cell types (14).
We previously showed that the interaction of cells with Pedersen fetuin-A leads to the activation of PI 3-kinase/Akt (16). One could argue that because Pedersen fetuin-A is not pure the PI 3-kinase/Akt activation is mediated by α2M, a major contaminant of fetuin-A. This line of argument has merit because purified α2M has been shown to mediate PI 3-kinase/Akt activation (43, 44). The present data, based on purified fetuin-A, now confirm our earlier studies that indeed the fetuin-A mediates the activation of PI 3-kinase/Akt using phospho-Akt (Ser-473) as a readout. More interestingly, the data confirm that PI 3-kinase/Akt is activated upon the interaction of fetuin-A with members of the annexin family of membrane receptors, particularly AnxA6. PI 3-kinase/Akt is one of the principal growth signaling pathways utilized by tumor cells (45). Together, our data demonstrate that the interaction of tumor cells with fetuin-A should be given a serious consideration because this protein is not just a mere bystander in the realm of cell growth mechanisms.
In summary, we herein show that breast carcinoma cells in culture adhere in the presence of fetuin-A via annexins and that the signaling that emanates from this interaction is relevant for cell growth. More importantly, the data suggest that this interaction (annexin/fetuin-A) is just as relevant (if not more) as the interaction between cell surface integrins and the extracellular adhesion proteins such as fibronectin in tissue culture. We also show that tumor cells efficiently take up fetuin-A (via annexins) and that this could be part of an important hitherto unknown growth-related mechanism. Lastly, we show that Pedersen fetuin-A can be purified to homogeneity without sacrificing its cell attachment properties.
This work was supported, in whole or in part, by National Institutes of Health Grants 1SC1CA134018-01 (to J. O.), G12RR03032-19 (to J. O.), and 5U54NS041071 (to S. J. G.). This work was also supported by Department of Defense Grant W81XWH-07-1-0254 (to J. O.).
J. Ochieng, unpublished observations.
- ahsg
- α2HS-glycoprotein
- α2M
- α2-macroglobulin
- SFM
- serum-free medium
- HBS
- Hepes-buffered saline
- AnxA2
- annexin A2
- AnxA6
- annexin A6
- ITIH2
- inter-α (globulin) inhibitor H2
- PI
- phosphatidylinositol.
REFERENCES
- 1.Nie Z. (1992) Am. J. Physiol. Cell Physiol. 263, C551–C562 [DOI] [PubMed] [Google Scholar]
- 2.Hayman E. G., Pierschbacher M. D., Ruoslahti E. (1985) J. Cell Biol. 100, 1948–1954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McInnes C., Knox P., Winterbourne D. J. (1987) J. Cell Sci. 88, 623–629 [DOI] [PubMed] [Google Scholar]
- 4.Burrill P. H., Bernardini I., Kleinman H. K., Kretchmer N. (1981) J. Supramol. Struct. Cell. Biochem. 16, 385–392 [DOI] [PubMed] [Google Scholar]
- 5.Hetzel M., Bachem M., Anders D., Trischler G., Faehling M. (2005) Lung 183, 225–237 [DOI] [PubMed] [Google Scholar]
- 6.Barczyk M., Carracedo S., Gullberg D. (2010) Cell Tissue Res. 339, 269–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mould A. P. (1996) J. Cell Sci. 109, 2613–2618 [DOI] [PubMed] [Google Scholar]
- 8.Ivaska J., Heino J.Cell Tissue Res. 339, 111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rodan S. B., Rodan G. A. (1997) J. Endocrinol. 154, (suppl.) S47–S56 [PubMed] [Google Scholar]
- 10.Fisher H. W., Puck T. T., Sato G. (1958) Proc. Natl. Acad. Sci. U.S.A. 44, 4–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rizzino A., Sato G. (1978) Proc. Natl. Acad. Sci. U.S.A. 75, 1844–1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salomon D. S., Bano M., Smith K. B., Kidwell W. R. (1982) J. Biol. Chem. 257, 14093–14101 [PubMed] [Google Scholar]
- 13.Ham R. G., St Clair J. A., Webster C., Blau H. M. (1988) In Vitro Cell Dev. Biol. 24, 833–844 [DOI] [PubMed] [Google Scholar]
- 14.Chen N. X., O'Neill K. D., Chen X., Duan D., Wang E., Sturek M. S., Edwards J. M., Moe S. M. (2007) Am. J. Physiol. Renal Physiol. 292, F599–F606 [DOI] [PubMed] [Google Scholar]
- 15.Kundranda M. N., Ray S., Saria M., Friedman D., Matrisian L. M., Lukyanov P., Ochieng J. (2004) Biochim. Biophys. Acta 1693, 111–123 [DOI] [PubMed] [Google Scholar]
- 16.Kundranda M. N., Henderson M., Carter K. J., Gorden L., Binhazim A., Ray S., Baptiste T., Shokrani M., Leite-Browning M. L., Jahnen-Dechent W., Matrisian L. M., Ochieng J. (2005) Cancer Res. 65, 499–506 [PubMed] [Google Scholar]
- 17.Denecke B., Gräber S., Schäfer C., Heiss A., Wöltje M., Jahnen-Dechent W. (2003) Biochem. J. 376, 135–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brown W. M., Saunders N. R., Møllgård K., Dziegielewska K. M. (1992) BioEssays 14, 749–755 [DOI] [PubMed] [Google Scholar]
- 19.Brown W. M., Dziegielewska K. M., Saunders N. R., Christie D. L., Nawratil P., Müller-Esterl W. (1992) Eur. J. Biochem. 205, 321–331 [DOI] [PubMed] [Google Scholar]
- 20.Ochieng J., Chaudhuri G. (2010) J. Health Care Poor Underserved 21, 51–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reynolds J. L., Skepper J. N., McNair R., Kasama T., Gupta K., Weissberg P. L., Jahnen-Dechent W., Shanahan C. M. (2005) J. Am. Soc. Nephrol. 16, 2920–2930 [DOI] [PubMed] [Google Scholar]
- 22.Schafer C., Heiss A., Schwarz A., Westenfeld R., Ketteler M., Floege J., Muller-Esterl W., Schinke T., Jahnen-Dechent W. (2003) J. Clin. Investig. 112, 357–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ray S., Lukyanov P., Ochieng J. (2003) Biochim. Biophys. Acta 1652, 91–102 [DOI] [PubMed] [Google Scholar]
- 24.Mellgren R. L., Huang X. (2007) J. Biol. Chem. 282, 35868–35877 [DOI] [PubMed] [Google Scholar]
- 25.Wang H., Zhang M., Bianchi M., Sherry B., Sama A., Tracey K. J. (1998) Proc. Natl. Acad. Sci. U.S.A. 95, 14429–14434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ochieng J., Pratap S., Khatua A. K., Sakwe A. M. (2009) Exp. Cell Res. 315, 1875–1888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cartellieri S., Hamer O., Helmholz H., Niemeyer B. (2002) Biotechnol. Appl. Biochem. 35, 83–89 [DOI] [PubMed] [Google Scholar]
- 28.Takagi H., Asano Y., Yamakawa N., Matsumoto I., Kimata K. (2002) J. Cell Sci. 115, 3309–3318 [DOI] [PubMed] [Google Scholar]
- 29.Spiro R. G. (1960) J. Biol. Chem. 235, 2860–2869 [PubMed] [Google Scholar]
- 30.Nie Z., Jellinek D., Ham R. G. (1991) Biochem. Biophys. Res. Commun. 178, 959–966 [DOI] [PubMed] [Google Scholar]
- 31.Yu C. L., Tsai M. H. (2001) Cancer Lett. 166, 173–184 [DOI] [PubMed] [Google Scholar]
- 32.Kohn E. C., Liotta L. A. (1995) Cancer Res. 55, 1856–1862 [PubMed] [Google Scholar]
- 33.Shroff R. C., McNair R., Figg N., Skepper J. N., Schurgers L., Gupta A., Hiorns M., Donald A. E., Deanfield J., Rees L., Shanahan C. M. (2008) Circulation 118, 1748–1757 [DOI] [PubMed] [Google Scholar]
- 34.Kojima K., Yamamoto K., Irimura T., Osawa T., Ogawa H., Matsumoto I. (1996) J. Biol. Chem. 271, 7679–7685 [DOI] [PubMed] [Google Scholar]
- 35.Babbin B. A., Parkos C. A., Mandell K. J., Winfree L. M., Laur O., Ivanov A. I., Nusrat A. (2007) Am. J. Pathol. 170, 951–966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chiang Y., Rizzino A., Sibenaller Z. A., Wold M. S., Vishwanatha J. K. (1999) Mol. Cell. Biochem. 199, 139–147 [DOI] [PubMed] [Google Scholar]
- 37.Yu C. H., Kan S. F., Pu H. F., Jea Chien E., Wang P. S. (2008) Cancer Sci. 99, 2467–2476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ochieng J., Warfield P., Green-Jarvis B., Fentie I. (1999) J. Cell. Biochem. 75, 505–514 [DOI] [PubMed] [Google Scholar]
- 39.Fidler I. J. (1995) J. Natl. Cancer Inst. 87, 1588–1592 [DOI] [PubMed] [Google Scholar]
- 40.Suva L. J., Griffin R. J., Makhoul I. (2009) Endocr. Relat. Cancer 16, 703–713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dziegielewska K. M., Horny H. P., Valent P., Habgood M. D., Schumacher U. (2001) Histochem. J. 33, 443–451 [DOI] [PubMed] [Google Scholar]
- 42.Evanko S. P., Tammi M. I., Tammi R. H., Wight T. N. (2007) Adv. Drug Deliv. Rev. 59, 1351–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Misra U. K., Deedwania R., Pizzo S. V. (2006) J. Biol. Chem. 281, 13694–13707 [DOI] [PubMed] [Google Scholar]
- 44.Padmasekar M., Nandigama R., Wartenberg M., Schlüter K. D., Sauer H. (2007) Cardiovasc. Res. 75, 118–128 [DOI] [PubMed] [Google Scholar]
- 45.Dy G. K., Adjei A. A. (2009) Proc. Am. Thorac. Soc. 6, 218–223 [DOI] [PubMed] [Google Scholar]