Abstract
The licensing factor Cdt1 is degraded by CRL4Cdt2 ubiquitin ligase dependent on proliferating cell nuclear antigen (PCNA) during S phase and when DNA damage is induced in G1 phase. Association of both Cdt2 and PCNA with chromatin was observed in S phase and after UV irradiation. Here we used a micropore UV irradiation assay to examine Cdt2 accumulation at cyclobutane pyrimidine dimer-containing DNA-damaged sites in the process of Cdt1 degradation in HeLa cells. Cdt2, present in the nucleus throughout the cell cycle, accumulated rapidly at damaged DNA sites during G1 phase. The recruitment of Cdt2 is dependent on prior PCNA chromatin binding because Cdt2 association was prevented when PCNA was silenced. Cdt1 was also recruited to damaged sites soon after UV irradiation through its PIP-box. As Cdt1 was degraded, the Cdt2 signal at damaged sites was reduced, but PCNA, cyclobutane pyrimidine dimer, and XPA (xeroderma pigmentosum, complementation group A) signals remained at the same levels. These findings suggest that Cdt1 degradation following UV irradiation occurs rapidly at damaged sites due to PCNA chromatin loading and the recruitment of Cdt1 and CRL4Cdt2, before DNA damage repair is completed.
Keywords: Cell Cycle, DNA Damage, DNA Repair, DNA Replication, E3 Ubiquitin Ligase, Protein Degradation, Ubiquitin, Ubiquitylation
Introduction
The integrity of genomic information is critical for proper cell function and cell survival and is maintained by faithful replication during S phase and segregation of duplicated chromosomes during mitosis (1). One of the most important regulatory mechanisms for maintaining genome integrity is the prevention of rereplication of any segment of chromosomal DNA during the cell cycle. Cells are continuously challenged by genotoxic agents and environmental stress and have complex mechanisms to activate DNA damage checkpoints, prevent cell cycle progression, and repair the damaged DNA (2).
An E3 ubiquitin ligase, Cul4-DDB1Cdt2 (also named CRL4 (Cullin ring ligase 4)Cdt2), functions both during the S phase and after DNA damage (3–8). Cullin 4 (Cul4) is a scaffolding component of the complex that interacts with the adaptor protein DDB1, which associates with many substrate-recognizing proteins (9–11). Cdt2, a CRL4 substrate-recognizing protein, targets the replication licensing factor Cdt1 for ubiquitin-mediated proteolysis both in S phase and following DNA damage. Cdt1 and Cdt2 orthologues were originally identified as Cdc10-dependent transcript 1 and 2 in fission yeast (12). Cdt1 has a critical role in establishing licensing for DNA replication in G1 phase (13–16). Cdt1 associates with origin-bound origin recognition complex (ORC)2 and operates together with Cdc6 to load the MCM2-7 complex onto chromatin, thereby licensing DNA for an additional round of DNA replication. DNA replication is initiated upon activation of the S phase cyclin-dependent kinases, and thereafter relicensing of replicated regions is prevented. After initiation of S phase, the function of Cdt1 is strictly prevented by multiple mechanisms, such as proteolysis by two ubiquitin ligases and inhibition by Geminin binding in mammalian cells (13, 14). S phase cyclin-dependent kinase associates with Cdt1 through its Cy motif and phosphorylates Cdt1 to create a phosphodegron recognized by SCFSkp2, also known as CRL1Skp2 (7, 8, 17, 18). In addition, Cdt1 has a proliferating cell nuclear antigen (PCNA)-binding motif (PIP-box) in its N-terminal end. Upon initiation of DNA replication, Cdt1 associates with PCNA and is ubiquitinated by another ubiquitin ligase CRL4Cdt2, creating a feedback control system to block licensing (3–8). On the other hand, when cells are exposed to DNA-damaging agents, a DNA damage response is induced that includes activation of DNA damage checkpoints and repair of damaged DNA. Degradation of Cdt1 is also induced through the same PCNA-dependent CRL4Cdt2 pathway (3–8). Detailed analyses using Xenopus egg extracts demonstrated that upon initiation of replication or incubation with damage-containing DNA, PCNA is loaded on the chromatin, and then Cdt1 associates with PCNA through its PIP-box, and Cdt2 is recruited to the chromatin dependent on PCNA and the PIP-box as well as the specific downstream residue of Cdt1 for ubiquitination (4, 6, 19). Another target of CRL4Cdt2 in mammalian cells is the cyclin-dependent kinase inhibitor p21 (20–22). UV irradiation induces the rapid degradation of p21. Both Cdt1 and p21 possess a PIP-box, which is essential for connecting to the CRL4Cdt2 complex.
UV irradiation induces helix-distorting DNA damage, such as cyclobutane pyrimidine dimers and 6-4 photoproducts. Nucleotide excision repair (NER) is a versatile system for repairing such DNA lesions (23, 24). The repair process requires the coordinated action of multiple proteins, such as those that detect the lesion (UV-DDB and XPC (xeroderma pigmentosum, complementation group C)), unwind the DNA around the lesion (XPB, XPD, and XPA), incise on both sides of the lesion to remove the damaged strand (XPF and XPG), and, finally, those that fill in the gap. PCNA appears to participate in the filling step by interacting with DNA polymerases and the ligase (25). In addition to induction of the NER reaction, phosphorylation of H2AX is induced in UV-irradiated cells, which appears to be mediated by the checkpoint kinases ATM (ataxia telangiectasia-mutated)/ATR (ataxia-telangiectasia and Rad3-related) and is dependent on NER factors, such as XPA and XPC (26).
To evaluate how Cdt2 spatially and temporally responds to the induction of DNA damage for ubiquitination of Cdt1 in mammalian cells, we used a UV-micropore irradiation assay and examined the dynamic response of Cdt2 after UV irradiation. Our findings indicate that both Cdt1 and Cdt2 rapidly accumulate at the sites of DNA damage in G1 phase cells, depending on their interactions with PCNA, and that Cdt1 is degraded quickly before DNA damage repair is completed.
EXPERIMENTAL PROCEDURES
Cell Culture
HeLa cells and 293T cells were cultured in Dulbecco's modified Eagle's medium with 10% fetal bovine serum. For synchronization in early S phase, HeLa cells were blocked using the double thymidine block method. Proteasome inhibitor MG132 was used at 25 μm. UV-C (254 nm) irradiation of whole cells in dishes was performed at 20–50 J/m2 using a UV cross-linker (FS-800, Funakoshi). To analyze the DNA content, flow cytometry was performed as described previously (27). For synchronization in S phase, cells were treated with 2 mm hydroxyurea for 18 h and in M phase with 2 μg/ml nocodazole for 18 h.
Antibodies, Western Blotting, and Immunofluorescence
For Western blotting, whole cell lysates were prepared by lysing cell pellets directly in SDS-PAGE buffer. For immunofluorescence, HeLa cells were fixed in 4% paraformaldehyde (WAKO) for 10 min, permeabilized in 0.25% (v/v) Triton X-100 in phosphate-buffered saline (PBS), and stained with the indicated antibodies as described previously (7). For double staining, Alexa488-conjugated anti-mouse and Alexa592-conjugated anti-rabbit antibodies were used as secondary antibodies with Hoechst 33258 to visualize DNA. The following antibodies were used: Cdt1 (rabbit; described in Ref. 27), Cdt2 (rabbit; described in Ref. 20), cyclin A (mouse, Neomarkers, Ab-6; rabbit, H-432, Santa Cruz Biotechnology, Inc. (Santa Cruz, CA)), γH2AX (mouse, Upstate), Myc (rabbit, Santa Cruz Biotechnology, Inc.), DDB1 (Bethyl), Cul4A (Bethyl), XPA (FL-273, Santa Cruz Biotechnology, Inc.), PCNA (PC10, Santa Cruz Biotechnology, Inc.), and cyclobutane pyrimidine dimer (CPD) (mouse, TDM-2, Cosmo Bio). To separate phosphorylated proteins on SDS-PAGE, Phos-tag (28) was purchased from the NARD Institute (AAL-107) and used according to the manufacturer's instructions.
RNAi Knockdown Experiments
The following double-stranded RNAs were made by Dharmacon and transfected at 100 μm using Oligofectamine (Invitrogen) or HiPerFect (Qiagen), and cells were cultured for 3 days. siRNA for PCNA was CGGUGACACUCAGUAUGUC, siRNA for siLuc, known as GL2 (Dharmacon), was used as a control siRNA.
Micropore UV Irradiation Assay
We used a previously described method to induce DNA photoproducts within localized areas of the cell nucleus (29). Cells were cultured on glass coverslips in a 35-mm dish and incubated for 48 h. To perform micropore UV irradiation, the cells on the coverslip were washed twice with PBS, covered with an isopore polycarbonate membrane filter (Millipore) having a pore size of either 3 or 5 μm in diameter, and irradiated. UV irradiation was achieved using a UV lamp (SUV-16, As One, Japan) with a dose rate of 0.4 J/m2·s, which was monitored with a UV radiometer (UVX radiometer, UVP) at 254 nm. The filter was removed, and cells were then either fixed or cultured for the indicated time before fixation and processed for immunofluorescence.
Chromatin Fractionation Assay
Cell extracts were prepared using 0.1% Triton X-100 mCSK buffer (10 mm Pipes, pH 7.9, 100 mm NaCl, 300 mm sucrose, 0.1% (v/v) Triton X-100, 1 mm phenylmethylsulfonyl fluoride, 10 mm β-glycerophosphate, 1 mm Na3VO4, 10 mm NaF). Approximately 5 × 105 cells were washed with ice-cold PBS and lysed with 0.1 ml of 0.1% Triton X-100 mCSK buffer for 15 min on ice. After centrifugation (15,000 rpm for 15 min at 4 °C), the precipitate was washed once with the same volume of ice-cold 0.1% Triton X-100 mCSK buffer and suspended in SDS sample buffer.
For protein phosphatase treatment, cell extracts were prepared as described above and treated with λ-phosphatase (P9614, Sigma) and incubated at 30 °C for 30 min, and the reaction was subsequently stopped by adding EDTA (final concentration, 1 mm).
RESULTS
Cdt2 Is Present throughout the Cell Cycle and Associates with Chromatin following UV Irradiation
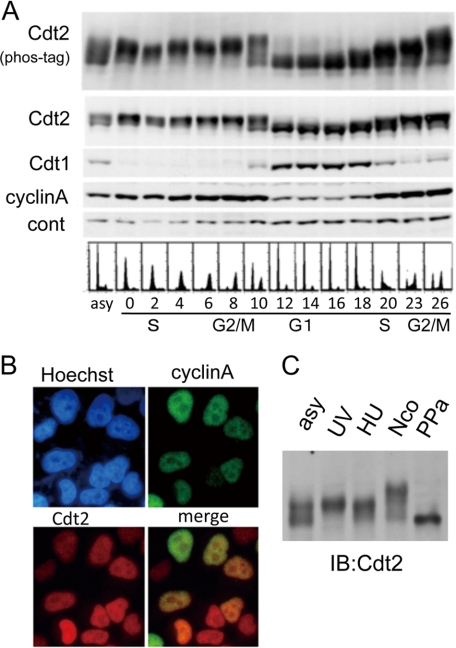
Because CRL4Cdt2 targets Cdt1 for proteolysis both during DNA replication in S phase and after UV irradiation in G1 phase, we first examined Cdt2 protein levels during the cell cycle. HeLa cells blocked in early S phase by a double thymidine block were synchronously released, and cells were collected every 2 h. Consistent with previous reports, Cdt1 was present only in the G1 phase (27). In contrast, Cdt2 protein was present throughout the cell cycle, and its levels remained almost constant (Fig. 1A). Immunostaining showed that the Cdt2 protein is present in the nucleus in all cells, irrespective of the cell cycle stage (Fig. 1B). This is not specific to HeLa cells, which have a defect in both p53 and Rb, because Cdt2 was also detected in all asynchronously growing U2OS and HCT116 cells, both of which are p53-positive and Rb-positive cell lines (supplemental Fig. S1). Cells collected during S phase and until the end of M phase had slower migrating forms of Cdt2 on SDS-PAGE. This slower migration was enhanced when protein samples were run on Phos-tag-containing SDS-PAGE (Fig. 1A, phos-tag), suggesting that it is due to phosphorylation (28). Slower migrating Cdt2 forms were also observed in synchronized 293T cells (Fig. 1C). Following treatment with λ-phosphatase, Cdt2 mobility increased, consistent with reduced mobility due to phosphorylation (Fig. 1C, PPa).
FIGURE 1.
Cdt2 protein during the cell cycle. A, Cdt2 protein levels during the cell cycle. HeLa cells synchronized at early S phase by a double thymidine block were synchronously released, collected at the indicated times (in h), and analyzed by flow cytometry and Western blotting using antibodies specific for Cdt2, Cdt1, and cyclin A. Nonspecific bands were used as a loading control (cont). Cell extracts run on Phos-tag-containing SDS-PAGE and blotted with Cdt2 are shown in the upper panel (Cdt2, phos-tag). B, Cdt2 is present in the nucleus in all phases of the cell cycle. Asynchronously growing HeLa cells were stained with antibodies for cyclin A and Cdt2. C, phosphorylation states of Cdt2 during the cell cycle. Cell extracts prepared from 293T cells, asynchronously growing (asy), UV-irradiated and collected 1 h later (UV), hydroxyurea-arrested (HU), or nocodazole-arrested (Nco), were run on a Phos-tag-containing SDS-polyacrylamide gel and blotted (IB) with anti-Cdt2 antibodies. Cell extracts prepared from asynchronously growing cells and treated with λ-protein phosphatase prior to electrophoresis were also included (PPa).
The kinetics of the Cdt2 association with chromatin are similar to those of PCNA during replication in Xenopus egg extract (6, 19). In a HeLa cell culture synchronized by a double thymidine block and release, PCNA associated with chromatin during S phase but not during G1 phase (Fig. 2A). Similar to PCNA, Cdt2 was detected in the chromatin-containing fraction during S phase but not during G1 phase.
FIGURE 2.
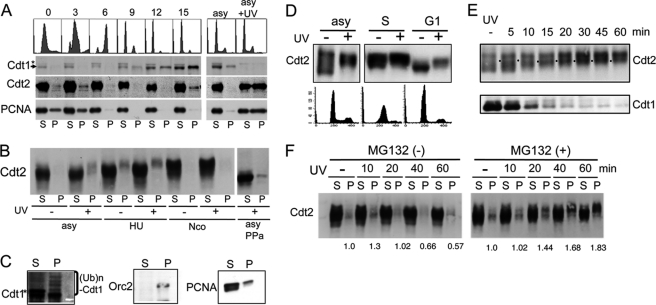
Chromatin association of Cdt2. A, Cdt2 associates with chromatin during S phase and following UV irradiation. Cell extracts were prepared from HeLa cells synchronized with a double thymidine block and release and collected at the indicated time points (in h) following release or asynchronously growing cells irradiated (asy + UV) or not irradiated (asy), fractionated into soluble (S) and chromatin-containing insoluble pellets (P), and blotted with antibodies for Cdt1, Cdt2, and PCNA. Arrow, Cdt1 band; asterisk, cross-reacting band. B, phosphorylated forms of Cdt2 are recovered in chromatin-containing fractions. 293T cells asynchronously growing (asy) or arrested with hydroxyurea (HU) or nocodazole (Nco) were incubated in the presence of MG132 for 1 h and UV-irradiated (+) or not irradiated (−). 1 h after UV irradiation, cell extracts were prepared, separated into soluble (S) and insoluble (P) fractions, and run on a Phos-tag-containing SDS-polyacrylamide gel. Half of the lysate (asy) was treated with protein phosphatase (asy PPa) and separated into soluble and insoluble fractions. C, fractionated cell extracts prepared from asynchronously growing 293T cells treated with MG132 and UV-irradiated as above were blotted with antibodies for Cdt1, Orc2, and PCNA. Asterisk, cross-reacting bands with Cdt1 antibodies. D, Cdt2 in G1 phase cells is phosphorylated following UV irradiation. HeLa cells asynchronously growing (asy), synchronized at early S phase by a double thymidine block, and released for 2 h (S) or 16 h (G1) were UV-irradiated (+) or not (−), and 1 h later, whole cell lysates were prepared and separated on a Phos-tag gel. E, time course analysis of Cdt2 phosphorylation and Cdt1 degradation after UV irradiation. Asynchronous HeLa cells were not irradiated (−) or were irradiated with UV, and cells were collected at the indicated time points. Phosphorylation levels of Cdt2 were examined on a Phos-tag gel. F, chromatin association of Cdt2 after UV irradiation. 293T cells pretreated with MG132 for 1 h (+) or not treated (−) were UV-irradiated, and cells were collected at the indicated time points and separated into soluble (S) and chromatin-containing pellet (P). Relative intensity of Cdt2 in the chromatin fraction was shown.
When asynchronous HeLa cells were irradiated with UV, the fraction of chromatin-bound Cdt2 increased (Fig. 2A, compare asy and asy + UV), similar to PCNA. A similar increase in chromatin-bound Cdt2 was observed in asynchronous 293T cells irradiated with UV (Fig. 2B). Notably, chromatin-bound Cdt2 migrated more slowly on a Phos-tag gel, both following UV treatment and during S phase (Fig. 2B, hydroxyurea (HU)-treated cells), indicative of hyperphosphorylation. In those cultures, cells were pretreated with MG132 to inhibit protein degradation and permit efficient detection of the chromatin association of Cdt2. When lysates prepared from UV-irradiated cells were treated with phosphatase, Cdt2 both in the soluble and pellet fractions was dephosphorylated (Fig. 2B, asy PPa). A multiply ubiquitinated ladder of Cdt1 was recovered in the chromatin fraction of asynchronous UV-irradiated 293T cells (Fig. 2C). This finding is consistent with the fact that Cdt1 ubiquitination by CRL4Cdt2 occurred on the chromatin in Xenopus egg extract, where PCNA and the ubiquitinated form of Cdt1 were recovered on the damage-containing DNA beads (19).
As shown in Fig. 1A, levels of Cdt2 phosphorylation are low during the G1 phase of the cell cycle, when Cdt1 levels are high. We examined if Cdt2 was phosphorylated upon UV irradiation in G1 phase. HeLa cells were synchronized in G1 phase and UV-irradiated. On a Phos-tag gel, Cdt2 migration was retarded in UV-irradiated cells, indicating that Cdt2 was phosphorylated in G1 phase after UV irradiation (Fig. 2D), although its phosphorylation levels were not as high as those in S phase. We carried out a time course analysis of Cdt2 phosphorylation and Cdt1 degradation after UV irradiation using asynchronously growing HeLa cells. A decrease in Cdt1 levels was detected by 5 min postirradiation (Fig. 2E). At this time point, retarded forms of Cdt2 appeared at a position corresponding to the G1-specific phosphorylated form (Fig. 2E, labeled by dots). During the time course, this form of phosphorylated Cdt2 increased as Cdt1 was degraded to a lower level. In 293T cells, Cdt2 phosphorylation and Cdt1 degradation occurred in a similar time course (data not shown).
Next, we performed a cell fractionation assay to examine the chromatin association of Cdt2 during this time course. It appeared that the amount of Cdt2 associated with chromatin increased 10 min after UV irradiation and then decreased (Fig. 2F, left). In the presence of MG132, however, both the total amount of phosphorylated Cdt2 and the levels of phosphorylated Cdt2 in the chromatin fraction increased during the time course (Fig. 2F, right).
Accumulation of Cdt1 and Cdt2 at Sites of DNA Damage after UV Irradiation in G1 Cells
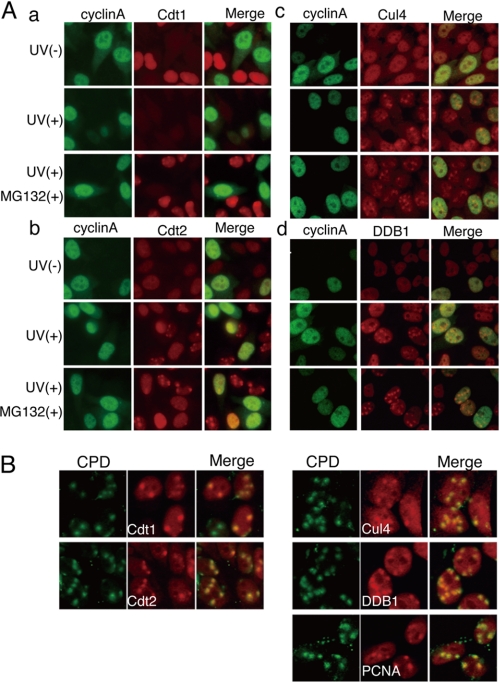
Degradation of Cdt1 started soon after UV irradiation on Western blotting (Fig. 2E). As shown above, Cdt2 is present during G1 and associates with chromatin following UV irradiation, suggesting that a complex for Cdt1 ubiquitination assembles at the sites of DNA damage. To directly analyze the spatial regulation of Cdt2 following DNA damage, we used a micropore UV irradiation assay to generate damage at specific sites within the nucleus (29), followed by Cdt2 protein immunofluorescence. HeLa cells were covered with a polycarbonate isopore membrane filter having a 3-μm pore and irradiated with UV at 50 J/m2. As reported, CPD formation and accumulation of γH2AX were detected at locally UV-irradiated sites (supplemental Fig. S2) (26, 29, 30). In these experimental conditions, we examined Cdt1 and Cdt2 protein accumulation at damaged sites 30 min following irradiation. Cdt1 protein, present in non-irradiated cyclin A-negative G1 cells, was degraded 30 min after UV irradiation, although the cells were covered with a micropore filter and only locally irradiated (Fig. 3A, a). In contrast, Cdt2 staining was detected as spots in the nucleus (Fig. 3A, b), suggesting that the Cdt2 protein accumulated at damaged sites. To examine Cdt1 localization in the absence of degradation, cells were first treated with the proteasome inhibitor MG132 and irradiated. In the presence of MG132, Cdt1-positive spots were clearly detected. Additionally, Cdt2 signal at the damaged sites became clearer when MG132 was added. Co-staining with cyclin A indicated that both Cdt1 and Cdt2 were detected in spots in cyclin A-negative cells (Fig. 3A, a and b), whereas CPD signals were detected irrespective of the presence of cyclin A (supplemental Fig. S2). This finding is consistent with Cdt1 being present only during G1 phase and indicates that Cdt2 only accumulates at damaged sites during G1. In contrast, Cul4 and DDB1 spots were detected irrespective of the presence of cyclin A (Fig. 3A, c and d).
FIGURE 3.
Accumulation of Cdt1, Cdt2, and PCNA at sites of local DNA damage. A, Cdt1-positive or Cdt2-positive spots in the nucleus were formed in G1 phase cells in response to local DNA damage. Cells pretreated with the proteasome inhibitor MG132 for 1 h (MG132(+)) or not treated were locally UV-irradiated and 30 min later were co-stained with antibodies for cyclin A and Cdt1 (a), Cdt2 (b), Cul4A (c), or DDB1 (d). Cul4A and DDB1 spots were detected in both G1 phase and S-G2 phase cells. B, locally irradiated cells treated as above were co-stained with antibodies for CPD and Cdt1, Cdt2, PCNA, Cul4A, or DDB1. To detect Cdt1, cells were pretreated with MG132 for 1 h.
The above findings suggested that both Cdt1 and Cdt2 were recruited to the damaged sites, where Cdt1 was then ubiquitinated and degraded. To confirm this, HeLa cells were treated as above and stained with antibodies against Cdt1, Cdt2, PCNA, and the CRL4Cdt2 components Cul4A and DDB1, together with anti-CPD antibodies. Co-staining showed that both Cdt1 and Cdt2 colocalized at CPD-stained spots (Fig. 3B). PCNA, which is involved in DNA repair and acts as a co-factor for CRL4Cdt2-mediated ubiquitination of Cdt1, was also detected at CPD-stained spots. Cul4A and DDB1 were also detected at CPD-stained spots (Fig. 3B). These findings suggested that Cdt1 is a mobile protein in the nucleus and is recruited to damage sites where PCNA and CRL4Cdt2 also accumulate to ubiquitinate Cdt1.
Time Course Analysis of Cdt1 and Cdt2 Signal at DNA-damaged Sites
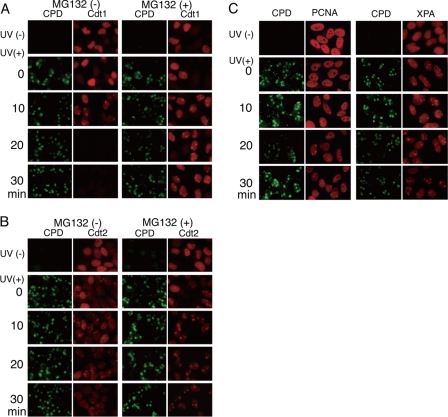
Because the Cdt1 signal was lost 30 min after UV irradiation through micropores, we examined Cdt1 signals at the sites of DNA damage at earlier time points at 10-min intervals. Although no dotlike signal of Cdt1 was detected just after UV irradiation (Fig. 4A, 0 min), clear signals of Cdt1 colocalizing with CPD were detected at 10 min after irradiation, indicating that Cdt1 was accumulating at damaged sites (Fig. 4A). At later time points (20 and 30 min), the Cdt1 signal disappeared from the whole nucleus, indicating that Cdt1 was degraded. In contrast, the intensity of the CPD signal remained at the same level for 30 min after UV irradiation. When MG132 was included, a clear spot signal of Cdt1 was also detected at 10 min, and its intensity remained at the same level at 30 min. These results indicate that Cdt1 accumulates at damaged sites prior to its degradation and suggest that Cdt1 accumulation was saturated at 10 min after UV irradiation.
FIGURE 4.
Time course analysis of Cdt1, Cdt2, PCNA, and XPA accumulation at sites of DNA damage. A and B, cells growing asynchronously (UV(−)) or cells UV-irradiated locally (UV(+)) were fixed at the indicated time points for immunostaining with antibodies for CPD and Cdt1 (A) or Cdt2 (B). Half of the cultures were incubated with MG132 for 1 h, irradiated with UV, and treated as described above (MG132(+)). C, time course analysis of PCNA and XPA accumulation. Cells growing asynchronously (UV(−)) or cells UV-irradiated locally (UV(+)) were fixed at the indicated time points for immunostaining with antibodies for CPD and PCNA or XPA.
We then examined Cdt2 accumulation at the damaged sites. Cdt2 spots were detected soon after UV irradiation (Fig. 4B, time 0). The Cdt2 spot signals at the sites of DNA damage became stronger at 10 min postirradiation and then appeared to decrease (Fig. 4B, 30 min), in parallel with Cdt1 degradation. When cells were treated with MG132, the Cdt2 signal remained at the same level throughout the time course.
Because PCNA is required for Cdt1 degradation and DNA damage repair, we followed the accumulation of PCNA at damage sites. PCNA spot signals were evident 10 min after UV irradiation, at around the time when both Cdt1 and Cdt2 had accumulated (Fig. 4C). At a later time (30 min), although the Cdt1 and Cdt2 signals disappeared or were reduced to lower levels, the PCNA and the CPD signals remained at a similar level at 10 min. Similar results were obtained for NER factor; XPA localized at the damaged sites, as reported previously (31). These observations suggested that Cdt1 degradation is an early response to UV irradiation compared with the repair process. Taken together, our data indicate that Cdt1 accumulates at damaged sites early during the DNA damage response and is modified on damaged chromatin for subsequent degradation.
Cdt1 Accumulation at Damage Sites Depends on its PIP-box
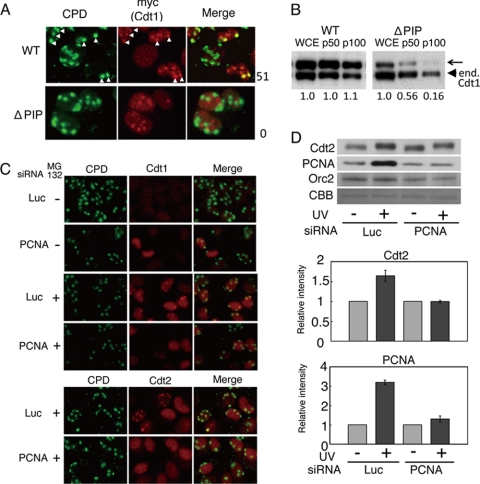
Cdt1 degradation by CRL4Cdt2 requires the association of Cdt1 with PCNA through its PIP-box located at its N-terminal end (6–8). On the other hand, Cdt1 possesses intrinsic DNA binding activity and binds the ORC (32, 33). To confirm that Cdt1 recruitment at damaged sites depends on the PIP-box, but not on its increased affinity to damaged DNA, the accumulation of a PIP-box mutated form of Cdt1 was examined. For this experiment, we used a previously described HeLa cell line stably expressing PIP-box-mutated Cdt1 (ΔPIP) (A6-Cdt1-NLS-Myc) (7), and as controls, we used cell lines expressing wild-type Cdt1. The WT-Cdt1 accumulated at CPD sites (Fig. 5A). In contrast, the PIP-box-mutated Cdt1 (ΔPIP) failed to accumulate at CPD-containing damaged sites, indicating that Cdt1 accumulated at damage sites through interaction with PCNA. Note that exogenously expressed Myc-tagged Cdt1, both wild-type and ΔPIP forms, showed prominent spot signals in the nucleus that probably correspond to nucleoli, where endogenous Cdt1 also accumulates (27). To confirm that the PIP-box of Cdt1 was required for chromatin association, cells were UV-irradiated, and the affinity of WT and ΔPIP Cdt1-NLS-Myc to chromatin was examined after extracting the lysate with sequentially increasing the concentration of salts in the extraction buffer. The exogenously expressed Cdt1 (WT)-NLS-Myc showed the same affinity to chromatin as endogenous Cdt1 as salt concentration was increased. In contrast, Cdt1 (ΔPIP) in the chromatin-containing fractions was reduced to a lower level, indicating that Cdt1 (ΔPIP) had lost chromatin association (Fig. 5B).
FIGURE 5.
PCNA-dependent accumulation of Cdt1 and Cdt2 at damaged sites. A, accumulation of Cdt1 requires its PCNA-interacting motif (PIP-box). HeLa cells stably expressing Myc-tagged wild-type Cdt1-NLS-Myc (WT) or Cdt1 mutated at its PIP-box-NLS-Myc (ΔPIP) were treated with MG132 for 1 h, locally irradiated, and 1 h later fixed and stained with CPD and Myc antibodies. White arrowheads indicate spots co-stained with both Myc (Cdt1) and CPD antibodies. The frequency of such double positive spots is shown on the right (as a percentage). B, chromatin association requires the PIP-box. HeLa cells stably expressing WT Cdt1-NLS-Myc or Cdt1 (ΔPIP)-NLS-Myc were pretreated with MG132 for 1 h and UV-irradiated. 1 h later, whole cell lysate (WCE) was prepared, extracted sequentially in the presence of 50 and 100 mm NaCl, and pellet fractions were obtained (p50 and p100, respectively). Pellet fractions were examined for the levels of Cdt1 (WT) and Cdt1 (ΔPIP). An arrowhead shows the endogenous Cdt1. The arrow shows the ectopically expressed Cdt1 (WT)- or Cdt1 (ΔPIP)-NLS-Myc. Relative intensity of Cdt1 (WT)- or Cdt1 (ΔPIP)-NLS-Myc to the endogenous Cdt1 for each lane is shown at the bottom. C, PCNA was required for accumulation of Cdt1 and Cdt2 at the sites of DNA damage. HeLa cells were transfected with siRNAs for luciferase (Luc) or PCNA and cultured for 3 days. Cells cultured in the presence (+) or absence (−) of MG132 for 1 h were processed for the micropore assay using antibodies for CPD and Cdt1 or Cdt2 1 h post UV-irradiation. D, Cdt2 chromatin association is dependent on PCNA. 293T cells were transfected with siRNAs for luciferase or PCNA and 3 days later were treated with MG132 for 1 h and UV-irradiated (+) or not (−). 1 h later, cell extracts were prepared, and chromatin-containing fractions were processed for immunoblotting with the indicated antibodies. The relative intensity of bands is normalized by the Orc2 signal.
Cdt1 and Cdt2 Accumulation at Damaged Sites Is Dependent on PCNA
We then investigated the requirement of PCNA for Cdt1 and Cdt2 accumulation at damaged sites by silencing PCNA expression. In control cells transfected with siRNA for luciferase, Cdt1 was degraded 30 min after micropore UV irradiation. In PCNA-silenced cells, although Cdt1 was not degraded, no accumulation of Cdt1 was detected at the sites of DNA damage (Fig. 5C, top, second row). To confirm that Cdt1 cannot accumulate at the sites of DNA damage in the absence of PCNA, cells were pretreated with MG132 and then irradiated. In contrast to cells transfected with siRNA for luciferase, which showed an accumulation of Cdt1 at the sites of DNA damage, Cdt1 accumulation was not detected in PCNA-silenced cells (Fig. 5C, top, third and fourth rows). Similarly, Cdt2 was accumulated at damage-containing sites in control cells, but the Cdt2 accumulation was completely blocked in PCNA-silenced cells (Fig. 5C, bottom).
Finally, we examined the effect of PCNA silencing on the chromatin association of Cdt2 after UV irradiation by fractionating chromatin. Cell extracts prepared from siLuc- or siPCNA-transfected cells were separated into soluble and insoluble fractions, and chromatin-containing insoluble fractions were examined (Fig. 5D). In control cells, the accumulation of PCNA and Cdt2 on chromatin was increased after UV irradiation. In contrast, when PCNA was silenced, this increase in PCNA and Cdt2 on chromatin was not observed. The levels of Orc2 protein on chromatin did not change after UV irradiation, irrespective of the silencing of PCNA.
DISCUSSION
The licensing factor Cdt1 is degraded through CRL4Cdt2, not only following DNA replication initiation but also after inducing DNA damage. Previous findings demonstrated that Cdt1 was degraded when cells were exposed to γ-irradiation or UV irradiation or when Xenopus egg extract was incubated with damage-containing DNA, but how Cdt1 and Cdt2 respond temporally and spatially following induction of DNA damage has been unclear. Here, we showed that both Cdt1 and Cdt2 accumulate at sites of DNA damage soon after UV irradiation in mammalian cells. The accumulation was dependent on the presence of PCNA and a PIP-box on Cdt1. As Cdt1 levels decreased at later time points following DNA damage, Cdt2 signals at the damaged sites decreased in parallel, suggesting that Cdt2 accumulation at damaged sites depends on its substrates. This is consistent with our observation that Cdt2 spots in the nucleus of locally irradiated cells were detected in G1 phase cells but not S phase cells (Fig. 3) because Cdt1 is present only in G1 phase, whereas Cdt2 is present throughout the cell cycle. PCNA and XPA were also recruited to damaged sites with similar kinetics to Cdt1 and Cdt2 but remained at the sites of DNA damage after Cdt1 degradation. PCNA recruitment is a prerequisite for Cdt1 and Cdt2 association with damaged sites and the subsequent degradation of Cdt1. Consistently, ubiquitinated forms of Cdt1 were recovered in the chromatin-containing fraction when proteasomal degradation was inhibited.
Our data suggest that Cdt1 is continuously recruited to sites of DNA damage, modified, and degraded, until all of the Cdt1 protein disappears (Fig. 4). This indicates that Cdt1 is not stably associated with chromatin at fixed sites during G1 phase. This finding is consistent with previous studies showing that Cdt1 interacts with chromatin, but its interaction is transient and dynamically changes during G1 phase (34, 35). When cells are exposed to UV irradiation and DNA lesions are formed, the NER pathway is initiated to remove the DNA lesion-containing segment and fill in the gap. Current NER models indicate that PCNA is loaded at repair sites for DNA repair synthesis and the final ligation process (25). Cdt1 dynamically associating with chromatin will interact with chromatin-loaded PCNA and will be docked. The order of the Cdt1 and CRL4Cdt2 interaction with PCNA is not well known. Cdt1 protein bound to PCNA might be recognized by CRL4Cdt2, or Cdt1 weakly interacting with Cdt2 might be fixed upon PCNA interaction. Experiments with Xenopus egg extracts argue for the former possibility, because Cdt1 associates with chromatin in the absence of Cdt2 (19). We observed that Cdt1 signals at the sites of DNA damage were reduced when Cdt2 expression was silenced (data not shown). This finding suggests that Cdt1 weakly bound to PCNA would be fixed by subsequent binding of Cdt2. The data in Fig. 4 show that, as compared with the spot signals for Cdt1 and PCNA, Cdt2 signals were detected just after irradiation (Fig. 4, 0 min). We do not think that Cdt2 was recruited to the sites of DNA damage before PCNA and Cdt1 were recruited. Rather, Cdt1 and PCNA signals might be obscured due to the surrounding nuclear staining for each protein. Actually, Cdt2 accumulation at damaged sites depends on the presence of PCNA (Fig. 5C) and its substrates, at least Cdt1; first, as Cdt1 was degraded at locally damaged sites, Cdt2 signals at the sites were reduced, and second, in S phase when Cdt1 and p21 are degraded, no accumulation of Cdt2 was detected at locally damaged sites.
Cdt1, recruited to sites of DNA damage, is degraded soon after UV irradiation. As degradation proceeds, Cdt2 accumulation at damaged sites is reduced. PCNA and XPA remain at the site of DNA damage, however, after Cdt1 is degraded (Fig. 4). It was reported that replication protein A, for example, which binds to single-stranded DNA and is an intermediate formed during NER, was detected at DNA-damaged sites at 8 h, and a significant fraction of DNA damage remained unrepaired even at 24 h post-UV irradiation (31, 36, 37). Thus, degradation of Cdt1 is rapidly completed as compared with the repair of damage. PCNA might interact with many proteins involved not only in repair but also in chromatin reassembly. Among them, Cdt1 may have a higher affinity to PCNA than other proteins, which could be assisted by Cdt2 binding. Recently, the monoubiquitination of PCNA by CRL4Cdt2 was reported (37). Monoubiquitinated PCNA has a role in helping in translesion DNA synthesis (37). It is also possible that monoubiquitination of PCNA will modify the affinity of PCNA to Cdt1 and other PCNA-interacting proteins so that a specific partner of PCNA is selected at an appropriate step in early or late stages of DNA repair.
There is a good correlation between phosphorylation of Cdt2 and degradation of Cdt1. Cdt2 is phosphorylated in the S phase during normal cell cycle progression, when Cdt1 is degraded through CRL4Cdt2 (Fig. 1A). In addition, phosphorylation of Cdt2 was induced after UV irradiation. In G1 phase, when Cdt1 is present, the levels of Cdt2 phosphorylation were low. Upon UV irradiation, as Cdt1 was degraded, higher phosphorylated forms of Cdt2 were detected (Fig. 2, D and E). Hyperphosphorylated forms of Cdt2 were detected in chromatin-containing fractions, where ubiquitinated Cdt1 was also recovered (Fig. 2, B and C). These results suggest that when Cdt2 is phosphorylated, Cdt1 is efficiently ubiquitinated. The ubiquitination activity of CRL4Cdt2 might be up-regulated, or its interactions with substrates might be increased by Cdt2 phosphorylation. It is not known whether phosphorylation of Cdt2 is required for chromatin association nor whether Cdt2 is phosphorylated following its chromatin association at the sites of DNA damage. In the time course experiments following UV irradiation, the amount of phosphorylated Cdt2 continued to increase as Cdt1 was degraded to lower levels. During the same time course, it appeared that the levels of chromatin-bound Cdt2 increased at 10 min but decreased at later time points (Fig. 2, E and F). This is consistent with the observation that Cdt2 localization at sites of DNA damage following localized UV irradiation became stronger at 10 min postirradiation and then appeared to decrease (Fig. 4B). Taken together, these data suggest that phosphorylation of Cdt2 may take place off chromatin. On the other hand, in cells treated with MG132, the levels of phosphorylated Cdt2 in the chromatin fraction increased during a time course analysis (Fig. 2F). When extracts prepared from cells having hyperphosphorylated Cdt2 were treated with protein phosphatase, recovery of Cdt2 in the chromatin fraction was decreased (Fig. 2B), suggesting that hyperphosphorylated forms of Cdt2 associate with chromatin with a higher affinity. We speculate that initial phosphorylation of Cdt2 occurs prior to its recruitment to the sites of DNA damage and that Cdt2 is additionally phosphorylated following its recruitment. This is enhanced when Cdt1 proteolysis is blocked.
Previous reports show that UV irradiation induces the activation of kinases and that H2AX phosphorylation is induced during G1 phase, dependent on NER (26, 30). Single-stranded DNA repair intermediates formed at NER sites are recognized by replication protein A, resulting in the activation of ATR (38, 39). Although degradation of Cdt1 occurs in the absence of ATR and ATM (40), the possibility cannot be excluded that ATR activated after UV irradiation phosphorylates CRL4Cdt2 and thereby modulates CRL4Cdt2 activity.
Although our data indicate that the degradation of Cdt1 is an early event carried out during DNA damage repair, the biological role of Cdt1 degradation remains unclear. When cells were treated with MG132, Cdt1 was stabilized. In those cells, we observed that XPA spot signals at the sites of DNA damage were reduced as compared with those in control cells (supplemental Fig. S3). This suggests that Cdt1 degradation might be required so that NER could take place efficiently at DNA-damaged sites, although it is possible that ubiquitylation and/or degradation of other proteins involved in repair was inhibited in MG132-treated cells, resulting in reduced accumulation of XPA. Cdt1 degradation might block illegitimate initiation of DNA replication when cells in the G1 phase, especially those cells initiating entry into the cell cycle from the G0 state, had DNA lesions. Alternatively, the presence of Cdt1 might obstruct repair and chromatin reassembly. DNA damage induces histone modifications and the recruitment of chromatin remodeling factor to loosen the chromatin structure so that repair enzymes can easily access the damaged sites (24). Repair of lesions close to origins might be hindered by the presence of Cdt1, which would recruit more MCM2–7 complex. Further work is required to address the biological meaning of Cdt1 degradation after DNA damage.
Supplementary Material
Acknowledgments
We are grateful to Dr. Z. Lygerou for corrections and critical reading of the manuscript, Dr. M. Wakasugi for advice on the micropore assay, and Drs. K. Sugasawa and R. Nishi for discussions.
This work was supported by Grants-in-aid for Scientific Research (B), Scientific Research on Priority Areas, and the Global Center of Excellence Program from the Ministry of Education, Culture, Sport, Science, and Technology of Japan and the Naito Foundation (to H. N.); and Grants-in-aid for Scientific Research and the Uehara Memorial Foundation (to Y. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S3.
- ORC
- origin recognition complex
- NER
- nucleotide excision repair
- CPD
- cyclobutane pyrimidine dimmer
- PCNA
- proliferating cell nuclear antigen.
REFERENCES
- 1.Nurse P. (1994) Cell 79, 547–550 [DOI] [PubMed] [Google Scholar]
- 2.Hoeijmakers J. H. (2001) Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 3.Higa L. A., Banks D., Wu M., Kobayashi R., Sun H., Zhang H. (2006) Cell Cycle 5, 1675–1680 [DOI] [PubMed] [Google Scholar]
- 4.Jin J., Arias E. E., Chen J., Harper J. W., Walter J. C. (2006) Mol. Cell 23, 709–721 [DOI] [PubMed] [Google Scholar]
- 5.He Y. J., McCall C. M., Hu J., Zeng Y., Xiong Y. (2006) Genes Dev. 20, 2949–2954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arias E. E., Walter J. C. (2006) Nat. Cell Biol. 8, 84–90 [DOI] [PubMed] [Google Scholar]
- 7.Nishitani H., Sugimoto N., Roukos V., Nakanishi Y., Saijo M., Obuse C., Tsurimoto T., Nakayama K. I., Nakayama K., Fujita M., Lygerou Z., Nishimoto T. (2006) EMBO J. 25, 1126–1136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senga T., Sivaprasad U., Zhu W., Park J. H., Arias E. E., Walter J. C., Dutta A. (2006) J. Biol. Chem. 281, 6246–6252 [DOI] [PubMed] [Google Scholar]
- 9.O'Connell B. C., Harper J. W. (2007) Curr. Opin. Cell Biol. 19, 206–214 [DOI] [PubMed] [Google Scholar]
- 10.Jackson S., Xiong Y. (2009) Trends Biochem. Sci. 34, 562–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee J., Zhou P. (2007) Mol. Cell 26, 775–780 [DOI] [PubMed] [Google Scholar]
- 12.Hofmann J. F., Beach D. (1994) EMBO J. 13, 425–434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arias E. E., Walter J. C. (2007) Genes Dev. 21, 497–518 [DOI] [PubMed] [Google Scholar]
- 14.Blow J. J., Dutta A. (2005) Nat. Rev. Mol. Cell. Biol. 6, 476–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bell S. P., Dutta A. (2002) Annu. Rev. Biochem. 71, 333–374 [DOI] [PubMed] [Google Scholar]
- 16.Nishitani H., Lygerou Z. (2004) Front. Biosci. 9, 2115–2132 [DOI] [PubMed] [Google Scholar]
- 17.Sugimoto N., Tatsumi Y., Tsurumi T., Matsukage A., Kiyono T., Nishitani H., Fujita M. (2004) J. Biol. Chem. 279, 19691–19697 [DOI] [PubMed] [Google Scholar]
- 18.Liu E., Li X., Yan F., Zhao Q., Wu X. (2004) J. Biol. Chem. 279, 17283–17288 [DOI] [PubMed] [Google Scholar]
- 19.Havens C. G., Walter J. C. (2009) Mol. Cell 35, 93–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishitani H., Shiomi Y., Iida H., Michishita M., Takami T., Tsurimoto T. (2008) J. Biol. Chem. 283, 29045–29052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim Y., Starostina N. G., Kipreos E. T. (2008) Genes Dev. 22, 2507–2519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abbas T., Sivaprasad U., Terai K., Amador V., Pagano M., Dutta A. (2008) Genes Dev. 22, 2496–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gillet L. C., Schärer O. D. (2006) Chem. Rev. 106, 253–276 [DOI] [PubMed] [Google Scholar]
- 24.Dinant C., Houtsmuller A. B., Vermeulen W. (2008) Epigenetics Chromatin 1, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moser J., Kool H., Giakzidis I., Caldecott K., Mullenders L. H., Fousteri M. I. (2007) Mol. Cell 27, 311–323 [DOI] [PubMed] [Google Scholar]
- 26.Marti T. M., Hefner E., Feeney L., Natale V., Cleaver J. E. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 9891–9896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishitani H., Taraviras S., Lygerou Z., Nishimoto T. (2001) J. Biol. Chem. 276, 44905–44911 [DOI] [PubMed] [Google Scholar]
- 28.Kinoshita E., Kinoshita-Kikuta E., Takiyama K., Koike T. (2006) Mol. Cell Proteomics 5, 749–757 [DOI] [PubMed] [Google Scholar]
- 29.Katsumi S., Kobayashi N., Imoto K., Nakagawa A., Yamashina Y., Muramatsu T., Shirai T., Miyagawa S., Sugiura S., Hanaoka F., Matsunaga T., Nikaido O., Mori T. (2001) J. Investig. Dermatol. 117, 1156–1161 [DOI] [PubMed] [Google Scholar]
- 30.Matsumoto M., Yaginuma K., Igarashi A., Imura M., Hasegawa M., Iwabuchi K., Date T., Mori T., Ishizaki K., Yamashita K., Inobe M., Matsunaga T. (2007) J. Cell Sci. 120, 1104–1112 [DOI] [PubMed] [Google Scholar]
- 31.Marteijn J. A., Bekker-Jensen S., Mailand N., Lans H., Schwertman P., Gourdin A. M., Dantuma N. P., Lukas J., Vermeulen W. (2009) J. Cell Biol. 186, 835–847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yanagi K., Mizuno T., You Z., Hanaoka F. (2002) J. Biol. Chem. 277, 40871–40880 [DOI] [PubMed] [Google Scholar]
- 33.Chen S., de Vries M. A., Bell S. P. (2007) Genes Dev. 21, 2897–2907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xouri G., Dimaki M., Bastiaens P. I., Lygerou Z. (2007) Cell Cycle 6, 1549–1552 [DOI] [PubMed] [Google Scholar]
- 35.Xouri G., Squire A., Dimaki M., Geverts B., Verveer P. J., Taraviras S., Nishitani H., Houtsmuller A. B., Bastiaens P. I., Lygerou Z. (2007) EMBO J. 26, 1303–1314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell D. L., Haipek C. A., Clarkson J. M. (1985) Mutat. Res. 143, 109–112 [DOI] [PubMed] [Google Scholar]
- 37.Terai K., Abbas T., Jazaeri A. A., Dutta A. (2010) Mol. Cell 37, 143–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hanasoge S., Ljungman M. (2007) Carcinogenesis 28, 2298–2304 [DOI] [PubMed] [Google Scholar]
- 39.Stokes M. P., Rush J., Macneill J., Ren J. M., Sprott K., Nardone J., Yang V., Beausoleil S. A., Gygi S. P., Livingstone M., Zhang H., Polakiewicz R. D., Comb M. J. (2007) Proc. Natl. Acad. Sci. U.S.A. 104, 19855–19860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Higa L. A., Mihaylov I. S., Banks D. P., Zheng J., Zhang H. (2003) Nat. Cell Biol. 5, 1008–1015 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.