Abstract
The maintenance of eukaryotic telomeres requires telomerase, which is minimally composed of a telomerase reverse transcriptase (TERT) and an associated RNA component. Telomerase activity is tightly regulated by expression of human (h) TERT at both the transcriptional and post-translational levels. The Hsp90 and p23 molecular chaperones have been shown to associate with hTERT for the assembly of active telomerase. Here, we show that CHIP (C terminus of Hsc70-interacting protein) physically associates with hTERT in the cytoplasm and regulates the cellular abundance of hTERT through a ubiquitin-mediated degradation. Overexpression of CHIP prevents nuclear translocation of hTERT and promotes hTERT degradation in the cytoplasm, thereby inhibiting telomerase activity. In contrast, knockdown of endogenous CHIP results in the stabilization of cytoplasmic hTERT. However, it does not affect the level of nuclear hTERT and has no effect on telomerase activity and telomere length. We further show that the binding of CHIP and Hsp70 to hTERT inhibits nuclear translocation of hTERT by dissociating p23. However, Hsp90 binding to hTERT was not affected by CHIP overexpression. These results suggest that CHIP can remodel the hTERT-chaperone complexes. Finally, the amount of hTERT associated with CHIP peaks in G2/M phases but decreases during S phase, suggesting a cell cycle-dependent regulation of hTERT. Our data suggest that CHIP represents a new pathway for modulating telomerase activity in cancer.
Keywords: Aging, Cell Cycle, Chaperone Chaperonin, Nuclear Translocation, Proteasome, Protein Degradation, Telomere, Ubiquitin Ligase
Introduction
Telomeres, the specialized nucleoprotein complexes at the ends of eukaryotic chromosomes, are essential for the maintenance of chromosome integrity (1, 2). In most organisms, telomere DNA consists of long tracts of duplex telomere repeats (TTAGGG in vertebrates) with 3′ single-stranded G overhangs (3) and is tightly associated with the six-protein complex, shelterin, which protects chromosome termini from being recognized as sites of DNA damage (4, 5). Loss of telomere function results in chromosome end fusions, degradation, and other inappropriate reactions, leading to cell senescence, apoptosis, or abnormal cell proliferation (6, 7). In the absence of a telomere maintenance pathway, dividing somatic cells show a progressive loss of telomeric DNA during successive rounds of cell division because of a DNA end replication problem (8, 9). Thus, telomere shortening functions as a control mechanism that regulates the proliferative capacity of cells. Although recombination-mediated telomere elongation has been demonstrated for replenishing telomere DNA (10, 11), the major mechanism to offset telomere erosion is based on telomerase (12, 13). In humans, telomerase is strongly repressed in normal somatic tissues but is expressed in most cancer cells, suggesting that the activation of telomerase is a critical step in human oncogenesis (14, 15).
Although the enzymatic activity of telomerase is regulated by hTERT2 at the transcriptional level (16, 17), several lines of evidence have suggested a post-translational regulation of telomerase activity. A number of telomerase-associated proteins have been identified in vertebrates and appear to regulate telomerase assembly (18), localization (19, 20), and enzymatic function (21, 22). hTERT is positively or negatively regulated through a succession of post-translational modifications, including phosphorylation and ubiquitination. PKC and Akt have been shown to phosphorylate hTERT and function as a positive regulator of telomerase (23, 24). In contrast, telomerase activity is inhibited by c-Abl kinase-mediated phosphorylation of hTERT (25). In the case of ubiquitination, MKRN1 is the first factor identified as an E3 ubiquitin ligase for hTERT in mammalian cells (26). MKRN1 directly binds hTERT both in vitro and in vivo and promotes ubiquitination of hTERT, thereby reducing telomerase activity as well as telomere length. The molecular chaperone Hsp90 and p23 associate with hTERT, and their associations are required for the assembly of active telomerase (18, 27). Hsp90 is a highly conserved and abundant molecular chaperone found in all eukaryotes and is required for proper folding and maturation of its substrate proteins (28). The co-chaperone p23 binds the ATP-bound dimeric form of Hsp90 and stabilizes the Hsp90-substrate complexes (29). Disruption of Hsp90 by geldanamycin inhibits telomerase activity through the ubiquitin-mediated degradation of hTERT (26).
CHIP (C terminus of Hsc70-interacting protein) is a co-chaperone protein identified through its interaction with Hsc/Hsp70 (30, 31). CHIP possesses a tetratricopeptide repeat (TPR) domain responsible for chaperone binding, a charged domain, and a U-box domain essential for E3 ubiquitin ligase activity (32, 33). Through its interaction with molecular chaperones, CHIP has been shown to mediate ubiquitination and degradation of various chaperone-bound proteins. Because hTERT is highly sensitive to Hsp90 disruption (26, 34), it is possible that CHIP may serve as the chaperone-associated ubiquitin ligase capable of targeting hTERT for ubiquitination. It is still not clear, however, whether CHIP plays a physiological role in the regulation of telomerase activity. Here, we present evidence that CHIP interacts with hTERT in the cytoplasm and promotes hTERT ubiquitination. Furthermore, we show that the binding of CHIP and Hsp70 to hTERT inhibits nuclear translocation of hTERT by dissociating p23. The resulting cytoplasmic hTERT is rapidly ubiquitinated and degraded by the proteasome. We also found that the amount of hTERT associated with CHIP peaks in G2/M phases during which telomerase does not act on telomeres but decreases during S phase, suggesting a cell cycle-dependent regulation of hTERT by CHIP. These results suggest that CHIP represents a new pathway for modulating telomerase activity in cancer.
EXPERIMENTAL PROCEDURES
Cell Culture and Plasmids
The human lung carcinoma cell line H1299 was cultured in RPMI 1640 medium, and the human cervical carcinoma cell line HeLa S3 was cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin in 5% CO2 at 37 °C. The expression vector for GST-CHIP was constructed by inserting the whole coding region from pcDNA3-CHIP into pGEX-5X-1, and the GST fusion proteins were purified by glutathione-Sepharose beads according to the manufacturer's instructions (Amersham Biosciences). The CHIP-His expression vectors were kindly provided from Neckers and co-workers (35), and the hTERT-HA expression vector was from Seimiya et al. (20). The FLAG-hTERT, MKRN1-V5, and HA-ubiquitin expression vectors have been described previously (26).
GST Pulldown, Immunoprecipitation, and Immunoblotting
GST pulldown, immunoprecipitation, and immunoblotting were performed as described previously (22). Briefly, the expression vectors were transfected into H1299 cells using Lipofectamine 2000 (Invitrogen) for 24 h followed by lysis. For GST pulldown assay, lysates were precleared with glutathione-Sepharose 4B (Amersham Biosciences) and incubated with glutathione-Sepharose beads containing GST fusion proteins for 2 h at 4 °C. For immunoprecipitation, lysates were preincubated with protein A-Sepharose (Amersham Biosciences) and incubated with primary antibodies precoupled with protein A-Sepharose beads for 2 h at 4 °C. Immunoprecipitation and immunoblotting were performed using anti-FLAG (Sigma), anti-HA (Santa Cruz Biotechnology), anti-hTERT (Rockland), anti-CHIP (Santa Cruz Biotechnology), anti-Hsp90 (Santa Cruz Biotechnology), anti-Hsp70 (Santa Cruz Biotechnology), anti-p23 (Abcam), anti-dyskerin (Santa Cruz Biotechnology), anti-proliferating cell nuclear antigen (Santa Cruz Biotechnology), and anti-actin (Sigma) antibodies as specified. All the immunoblots are representatives of at least three experiments that demonstrated the similar results.
Telomerase Assay
The telomeric repeat amplification protocol (TRAP) was used as described previously (36). Briefly, cell extracts (200 ng of protein) were added to the telomerase extension reactions and incubated for 20 min at 37 °C. PCR was performed using the HTS primer and HACX primer for 30 cycles (denaturation at 94 °C for 30 s, annealing at 62 °C for 30 s, and extension at 72 °C for 30 s). As an internal telomerase assay standard, NT and TSNT primers were added to the PCR mixture as described previously (37). Telomerase products were resolved by electrophoresis on a 12% nondenaturing polyacrylamide gel. Bands were then visualized by staining with SYBR Green (Molecular Probes), and the signal intensity was quantified with a LAS-4000 Plus Image analyzer (Fuji Photo Film).
Immunofluorescence Microscopy
Cells were grown on glass coverslips fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 10 min and permeabilized in 0.4% Triton X-100 in PBS for 20 min. Cells were then blocked in 5% bovine serum albumin and incubated with goat anti-hTERT antibody or mouse anti-His antibody overnight at 4 °C. After washing with PBS, cells were incubated with Alexa Fluor 488 rabbit anti-goat immunoglobulin and Alexa Fluor 568 goat anti-mouse immunoglobulin (Molecular Probes). DNA was stained with 4,6-diamino-2-phenylindole (Vectashield, Vector Laboratories) for 1 h at room temperature. Fluorescence images were captured by using an Olympus BX61 fluorescence microscopy.
RNA Interference
The siRNA target sequences specific for CHIP were 5′-CGCUGGUGGCCGUGUAUUA-3′ for siCHIP-1 and 5′-AGCUGGAGAUGGAGAGCUA-3′ for siCHIP-2. The siRNA target sequences specific for Hsp70 were 5′-CUGUGUUUGCAAUGUUGAATT-3′ for siHsp70-1 and 5′-AGAUGAAUUUAUACUGCCATT-3′ for siHsp70-2. The siRNA target sequences specific for p23 were 5′-GCUUAGGGAAAGAGAAUAATT-3′ for sip23-1 and 5′-GAUAUGCUGUAUUUGCCUATT-3′ for sip23-2. The siRNA duplexes were transfected into H1299 cells using RNAiMax transfection reagent (Invitrogen). The scrambled sequence (5′-AATCGCATAGCGTATGCCGTT-3′) was used as a control and did not correspond to any known gene in the data bases. For long term treatment of siRNA, siRNA was transfected to H1299 cells, and transfection was repeated at 3-day intervals.
In Vivo Ubiquitination Assay
H1299 cells were transfected with HA-ubiquitin, FLAG-hTERT, and CHIP-His expression vectors, followed by treatment with 10 μm MG132 to inhibit proteasome function. Lysates were subjected to immunoprecipitation with anti-FLAG antibody, followed by immunoblotting with anti-HA antibody to illuminate ubiquitin-modified hTERT.
Double Thymidine Block of HeLa Cells
HeLa S3 cells were grown in Dulbecco's modified Eagle's medium containing 10% fetal bovine serum. Cells were cultured to logarithmic phase and incubated in medium containing 2 mm thymidine (Sigma). After 16 h, cells were washed twice with PBS and incubated with regular medium for 8 h before a second incubation in 2 mm thymidine for 16 h. Cells were released and collected at 2-h intervals after release from the second thymidine block.
Fluorescence-activated Cell Sorter (FACS) Analysis
Cells were washed with PBS and fixed for 30 min in ice-cold 70% ethanol. The fixed cells were resuspended in PBS containing RNase A (200 μg/ml) and propidium iodide (50 μg/ml) and incubated in the dark for 30 min at room temperature. Cell cycle distribution was examined by flow cytometry using a FACScan flow cytometer (BD Biosciences).
RESULTS
CHIP Interacts with hTERT in the Cytoplasm
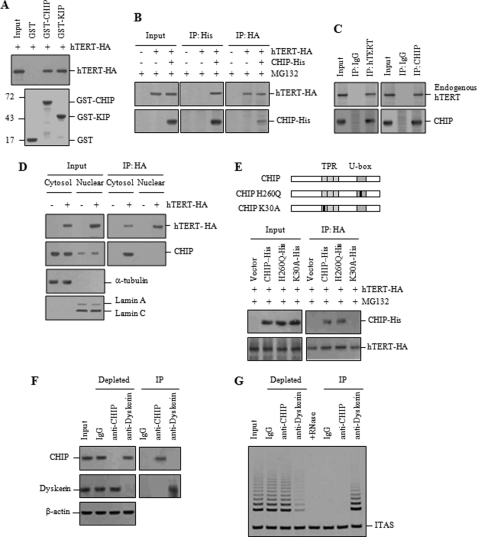
To investigate the role of CHIP in the regulation of telomerase activity, we first examined the direct interaction between hTERT and CHIP by a GST pulldown experiment. GST-CHIP, but not the control GST, precipitated hTERT-HA expressed in H1299 cells (Fig. 1A). KIP, which was known to interact with hTERT, was used as a positive control (22). To determine whether hTERT and CHIP associate in vivo, H1299 cells co-transfected with hTERT-HA and CHIP-His were treated with the proteasome inhibitor MG132 to block degradation of ubiquitinated hTERT and subjected to immunoprecipitation. hTERT-HA was detected in anti-His immunoprecipitates when CHIP-His was expressed (Fig. 1B). Likewise, CHIP-His was recovered in anti-HA immunoprecipitates. Endogenous hTERT and CHIP were immunoprecipitated by endogenous CHIP and hTERT in H1299 cells, respectively (Fig. 1C), indicating that hTERT interacts with CHIP in mammalian cells. To determine whether these two proteins have any opportunity to interact in intact cells, cytoplasmic and nuclear extracts were separately collected from H1299 cells expressing hTERT-HA. Whereas hTERT-HA was predominantly localized to the nucleus, the majority of endogenous CHIP signal was detected in the cytoplasm (Fig. 1D). Co-immunoprecipitation experiments showed that CHIP was immunoprecipitated by hTERT-HA in the cytoplasmic fraction, but not in the nuclear fraction, suggesting that CHIP is capable of interacting with hTERT in the cytoplasm.
FIGURE 1.
CHIP interacts with hTERT in the cytoplasm. A, GST, GST-CHIP, and GST-KIP were immobilized on glutathione-Sepharose and incubated with exogenously expressed hTERT-HA, followed by immunoblotting with anti-HA antibody. GST fusions were visualized by Coomassie Blue staining. Molecular mass markers are shown in kilodaltons. B, H1299 cells co-transfected with hTERT-HA and CHIP-His were treated with 10 μm MG132 for 2 h and subjected to immunoprecipitation (IP) with either anti-His or anti-HA antibodies, followed by immunoblotting as indicated. C, H1299 cells were subjected to immunoprecipitation with either anti-hTERT or anti-CHIP antibodies, followed by immunoblotting as indicated. IgG antibody was used as a negative control. D, cytoplasmic and nuclear extracts were separately collected from H1299 cells transfected with hTERT-HA and subjected to immunoprecipitation with anti-HA antibody. Duplicate blots were immunolabeled with anti-tubulin (for cytoplasmic fraction) and anti-lamin (for nuclear fraction) antibodies to confirm the absence of the cross-contamination in each fraction. E, H1299 cells were co-transfected with hTERT-HA, along with His-tagged wild-type or mutant CHIP as indicated and treated with 10 μm MG132 for 2 h. Immunoprecipitation was carried out with anti-HA antibody to pull down CHIP-His. F, H1299 cell lysates were incubated with anti-CHIP, anti-dyskerin, or mouse IgG antibodies and incubated with protein G-Sepharose. The supernatants (depleted) and immunoprecipitates (IP) were analyzed by immunoblotting with antibodies against CHIP and dyskerin. G, supernatants and immunoprecipitates were analyzed for telomerase activity by the TRAP assay. To test RNA-dependent extension, RNase A (0.25 mg/ml) was added to the extracts before the primer extension reaction. ITAS represents the internal telomerase assay standard.
CHIP contains a three TPR repeat domain at the N terminus, required for its interaction with chaperones, and a U-box domain at the C terminus, required for ubiquitin ligase activity (32, 33). The K30A mutation in the TPR domain disrupts interaction with Hsp90 or Hsp/Hsc70 chaperones, and the H260Q mutation in the U-box domain abolishes the ubiquitin ligase activity (35). To examine the effect of the CHIP mutants on their interaction with hTERT, H1299 cells were co-transfected with hTERT-HA and either wild-type or mutant CHIP-His and treated with MG132. Co-immunoprecipitation experiments revealed that the K30A mutation severely impaired the interaction between hTERT and CHIP, whereas the H260Q mutation demonstrated a strong interaction with hTERT (Fig. 1E). These results indicate that the chaperone binding activity of CHIP is required for the interaction with hTERT.
To ensure accurate cellular telomerase activity, hTERT should be properly assembled and translocated into the nucleus. Our observation that the hTERT-CHIP interaction occurred in the cytoplasm prompted us to hypothesize that CHIP may associate with an intermediate or immature hTERT, which is biologically nonfunctional for telomerase activity. To test this hypothesis, we performed immunoprecipitation using antibodies against CHIP and dyskerin, each of which efficiently depleted its cognate protein from H1299 cell lysates (Fig. 1F). Lysates depleted of either CHIP or dyskerin were analyzed for telomerase activity by the TRAP assay. Whereas dyskerin depletion resulted in removal of most of telomerase activity, CHIP depletion did not reduce the overall level of telomerase activity (Fig. 1G). Conversely, telomerase activity was associated with dyskerin immunoprecipitates, but not with CHIP immunoprecipitates. These results suggest that, although dyskerin resides in the active telomerase complex, CHIP associates with the catalytically inactive telomerase complex in the cytoplasm.
CHIP Inhibits the Nuclear Localization of hTERT and Promotes the Proteasomal Degradation of hTERT
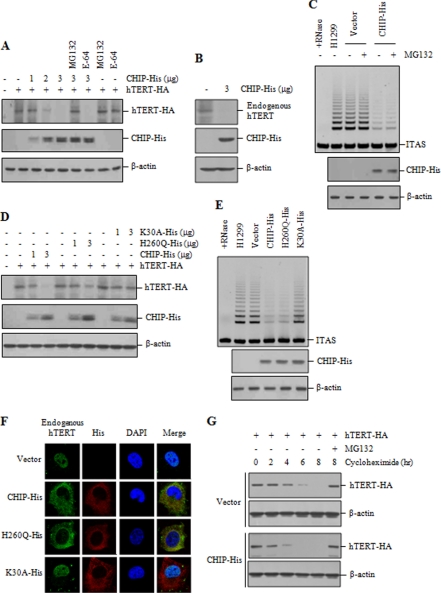
Because CHIP has been shown to possess E3 ubiquitin ligase activity (33, 35), we investigated the involvement of CHIP in hTERT degradation. Overexpression of CHIP led to a clear reduction in the levels of hTERT-HA in a dose-dependent manner (Fig. 2A). Incubation of cells with MG132 rescued the CHIP-induced reduction of hTERT, whereas the treatment with the lysosomal proteolysis inhibitor E-64 had no effect on hTERT level. These results indicate that hTERT degradation proceeds via the proteasome. We also show that overexpression of CHIP significantly decreased endogenous hTERT protein level (Fig. 2B). However, overexpression of CHIP had no effect on hTERT mRNA level (supplemental Fig. 1A). We next examined whether CHIP influences telomerase activity. Telomerase activity was significantly reduced in cells expressing CHIP compared with the control cells (Fig. 2C). However, reduction in telomerase activity was not rescued by MG132 treatment, further suggesting that CHIP-bound hTERT is nonfunctional for telomerase activity in vivo.
FIGURE 2.
CHIP inhibits nuclear localization of hTERT and enhances hTERT degradation. A, H1299 cells were co-transfected with hTERT-HA and increasing amounts of CHIP-His and treated with or without 10 μm MG132 or E-64 for 2 h as indicated. The hTERT levels were measured by immunoblotting with anti-HA antibody. B, H1299 cells were transfected with or without CHIP-His, and lysates were analyzed by immunoblotting for the expression of endogenous hTERT and CHIP-His. C, H1299 cells transfected with CHIP-His or the empty vector were treated with or without 10 μm MG132 for 2 h, and lysates were analyzed for telomerase activity by the TRAP assay. D, H1299 cells were co-transfected with hTERT-HA and increasing amounts of CHIP-His, H260Q-His, or K30A-His. The hTERT levels were measured by immunoblotting with anti-HA antibody. E, H1299 cells transfected with CHIP-His, H260Q-His, or K30A-His were analyzed for telomerase activity by the TRAP assay. F, H1299 cells transfected with CHIP-His, H260Q-His, or K30A-His were treated with 10 μm MG132 for 2 h and subjected to indirect immunofluorescence with anti-hTERT (green) or anti-His (red) antibodies, followed by fluorescent microscopic observation. The nuclei were stained with 4,6-diamino-2-phenylindole (DAPI, blue). G, H1299 cells were co-transfected with hTERT-HA, along with CHIP-His or the empty vector, and treated with 100 μg/ml cycloheximide for the indicated times. Lysates were analyzed by immunoblotting with anti-HA or anti-actin antibodies. Cells were pretreated with 10 μm MG132 as indicated. ITAS represents the internal telomerase assay standard.
We next examined the effect of the CHIP mutants on hTERT degradation. Although overexpression of wild-type CHIP significantly reduced the hTERT level, the H260Q mutant is partially defective in degrading hTERT-HA (Fig. 2D). This could be due to lack of ubiquitin ligase activity in the H260Q mutant. In contrast, the K30A mutant did not reduce the hTERT level, presumably because it failed to interact with hTERT. In the TRAP assay with the CHIP mutants, we observed that overexpression of H260Q resulted in a significant reduction in telomerase activity compared with the vector control (Fig. 2E). However, the K30A mutant did not reduce the overall level of telomerase activity. These results suggest that the inhibitory effect of CHIP on telomerase activity is mediated through the chaperone binding activity of CHIP but not through its ubiquitination activity.
Because the nuclear localization of hTERT is required for telomerase activity to elongate telomeric DNA in vivo, we examined whether CHIP affects the nuclear localization of hTERT. H1299 cells transfected with wild-type or mutant CHIP were treated with MG132 and subjected to indirect immunofluorescence staining. Whereas endogenous hTERT was predominantly localized to the nucleus in the control cells, transfection of wild-type CHIP or H260Q resulted in a cytoplasmic accumulation of hTERT (Fig. 2F). However, the K30A mutant did not affect the nuclear localization of hTERT. Identical results were observed in HeLa S3 cells (supplemental Fig. 2). Because telomerase activity was not detected in the cytoplasmic fraction (supplemental Fig. 3), these results support the idea that CHIP-bound hTERT fails to translocate into the nucleus and that the resulting cytoplasmic hTERT is not assembled into active telomerase and could be subsequently degraded by the proteasome.
We further examined that the amount of CHIP is involved in regulating the protein stability of hTERT. The protein stability was monitored in cells expressing CHIP-His or the empty vector after cycloheximide treatment to inhibit new protein synthesis. Overexpression of CHIP-His significantly reduced the half-life of hTERT compared with the control cells (Fig. 2G). In both cases, hTERT was stabilized upon treatment with MG132, indicating that CHIP regulates the half-life of hTERT through the proteasome-dependent degradation.
CHIP Knockdown Increases the Level of Cytoplasmic hTERT but Does Not Affect Telomerase Activity
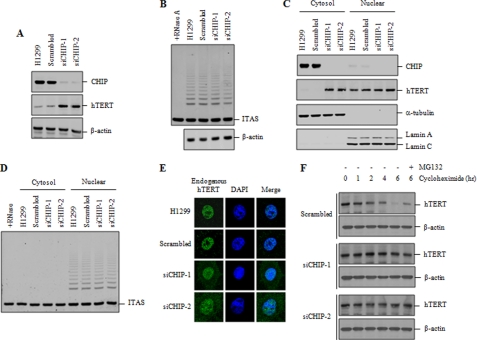
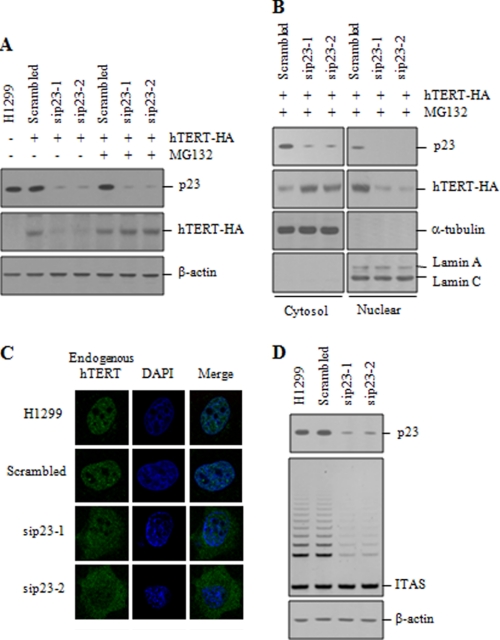
To examine the role of CHIP in a more physiological setting, the expression of endogenous CHIP was depleted using two different small interfering RNA (siRNA) duplexes. CHIP knockdown resulted in a clear increase in the level of endogenous hTERT (Fig. 3A), further confirming that CHIP negatively regulates the stability of hTERT protein in mammalian cells. As measured by RT-PCR analysis, an increase in the level of hTERT protein was not due to a CHIP-related increase in the transcription of the hTERT gene (supplemental Fig. 1B). We next examined whether CHIP knockdown affects telomerase activity. Intriguingly, no significant difference was observed in telomerase activity between CHIP knockdown cells and the control cells (Fig. 3B).
FIGURE 3.
CHIP knockdown increases the level of cytoplasmic hTERT but does not affect telomerase activity. A, H1299 cells were transfected with scrambled or CHIP siRNA (siCHIP-1 or siCHIP-2). The protein levels of endogenous CHIP and hTERT were measured by immunoblotting with anti-CHIP and anti-hTERT antibodies. B, H1299 cells were transfected with scrambled or CHIP siRNA, and lysates were analyzed for telomerase activity by the TRAP assay. C, cytoplasmic and nuclear extracts were separately collected from H1299 cells transfected with scrambled or CHIP siRNA, and lysates were analyzed by immunoblotting for the expression of endogenous CHIP and hTERT. D, cytoplasmic and nuclear extracts were separately collected from H1299 cells transfected with scrambled or CHIP siRNA, and lysates were analyzed for telomerase activity by the TRAP assay. E, H1299 cells transfected with scrambled or CHIP siRNA were subjected to indirect immunofluorescence with anti-hTERT (green) antibody, followed by fluorescent microscopic observation. The nuclei were stained with 4,6-diamino-2-phenylindole (DAPI, blue). F, H1299 cells transfected with scrambled or CHIP siRNA were treated with 100 μg/ml cycloheximide for the indicated times, and lysates were analyzed by immunoblotting with anti-hTERT or anti-actin antibodies. Cells were pretreated with 10 μm MG132 as indicated. ITAS represents the internal telomerase assay standard.
Because CHIP interacts only with cytoplasmic hTERT, we determined whether CHIP knockdown affects the subcellular localization of hTERT. Cytoplasmic and nuclear extracts were separately collected from H1299 cells and subjected to immunoblot analysis to assess endogenous hTERT level. Whereas CHIP knockdown did not affect the level of nuclear hTERT, it caused a significant increase in the level of cytoplasmic hTERT (Fig. 3C). Telomerase activity was exclusively detected in the nuclear extracts but not in the cytoplasmic extracts (Fig. 3D). Consistently, telomerase activity in the nuclear extracts was not affected by CHIP knockdown. These results indicate that an increase in cytoplasmic hTERT by CHIP knockdown did not affect the overall level of telomerase activity.
Because telomerase elongates telomeres, the best readout for a change in telomerase activity is telomere length. Therefore, we compared telomere length in control versus CHIP siRNA-treated cells over time. We repeatedly treated H1299 cells with CHIP siRNA at 3-day intervals for 2 weeks. The same method has been used to study the role of the DNA replication factors in telomere maintenance (38, 39). Whereas about 80–90% of endogenous CHIP was depleted during the course of repetitive siRNA transfection (supplemental Fig. 4A), telomerase activity was not affected by CHIP knockdown (supplemental Fig. 4B). In addition, telomere length was not significantly altered in CHIP siRNA-treated cells compared with the control cells over this long term treatment (supplemental Fig. 4C). These results suggest that telomerase activity and telomere length were not significantly affected by CHIP knockdown.
To further confirm the effect of CHIP knockdown on the subcellular localization of hTERT, CHIP knockdown cells were subjected to indirect immunofluorescence staining to monitor the localization of endogenous hTERT. Consistent with the immunoblot results in Fig. 3C, a clear accumulation of hTERT in the cytoplasm was observed in CHIP knockdown cells compared with the control cells (Fig. 3E). However, CHIP knockdown did not significantly change the nuclear localization of hTERT.
To examine the effect of CHIP knockdown on the turnover of hTERT protein, H1299 cells were transfected with scrambled or CHIP siRNA, incubated with cycloheximide to block new protein synthesis, and then analyzed by immunoblotting with hTERT antibody. As shown in Fig. 3F, endogenous hTERT was turned over with a half-life of ∼4 h in the control cells. CHIP knockdown significantly extended the half-life of hTERT. Turnover of hTERT was blocked by the MG132 treatment, indicating that hTERT degradation is mediated by the proteasome.
CHIP Enhances hTERT Ubiquitination
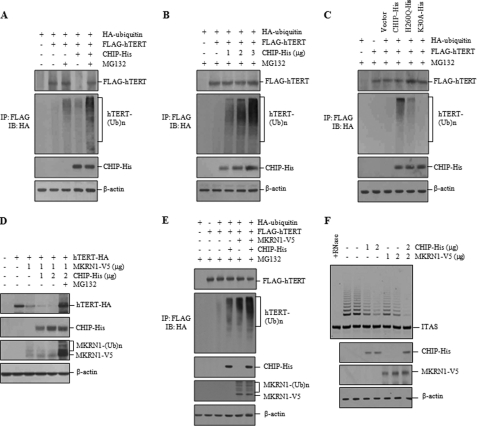
To investigate whether hTERT is ubiquitinated prior to its proteasome-dependent degradation, H1299 cells were co-transfected with FLAG-hTERT, HA-ubiquitin, and CHIP-His. CHIP overexpression decreased the levels of FLAG-hTERT, but MG132 reversed this trend (Fig. 4A). To illuminate ubiquitin-modified hTERT, anti-FLAG immunoprecipitates were evaluated by immunoblotting with anti-HA antibody. Ubiquitinated hTERT was elevated by CHIP expression even in the absence of MG132. This modification was further enhanced in cells treated with MG132 (Fig. 4A). In addition, hTERT displayed enhanced CHIP-induced ubiquitination in a dose-dependent manner in the presence of MG132 (Fig. 4B). In an experiment with the CHIP mutants, the H260Q mutant caused a marked increase in hTERT ubiquitination compared with the vector control but a slight decrease compared with wild-type CHIP (Fig. 4C). In contrast, the K30A mutant did not stimulate hTERT ubiquitination, suggesting that chaperone binding activity of CHIP is necessary for hTERT ubiquitination. Because the H260Q mutant is unable to catalyze protein ubiquitin conjugation, its ability to promote hTERT ubiquitination suggests that CHIP-bound hTERT could be ubiquitinated by another E3 ligase that targets hTERT.
FIGURE 4.
CHIP promotes hTERT ubiquitination. A, H1299 cells were co-transfected with HA-ubiquitin, FLAG-hTERT, and CHIP-His and treated with 10 μm MG132 for 2 h as specified. FLAG-hTERT was visualized with anti-FLAG antibody, and immunoprecipitation (IP) was carried out with anti-FLAG antibody before probing with anti-HA antibody. Ub, ubiquitin. B, H1299 cells were co-transfected with HA-ubiquitin, FLAG-hTERT, and increasing amounts of CHIP-His and treated with 10 μm MG132 for 2 h. Immunoprecipitation was carried out with anti-FLAG antibody before probing with anti-HA antibody. C, H1299 cells were co-transfected with HA-ubiquitin and FLAG-hTERT, along with CHIP-His or H260Q-His or K30A-His, and treated with 10 μm MG132 for 2 h. Immunoprecipitation was carried out with anti-FLAG antibody, followed by immunoblotting (IB) with anti-HA antibody. D, H1299 cells were co-transfected with hTERT-HA, MKRN1-V5, and CHIP-His and treated with or without 10 μm MG132 as specified. The protein levels of hTERT-HA were measured by immunoblotting with anti-HA antibody. E, H1299 cells were co-transfected with HA-ubiquitin, FLAG-hTERT, and CHIP-His or MKRN1-V5 and treated with 10 μm MG132 for 2 h as specified. FLAG-hTERT was visualized with anti-FLAG antibody, and immunoprecipitation was carried out with anti-FLAG antibody before probing with anti-HA antibody. F, H1299 cells transfected with CHIP-His and MKRN1-V5 were analyzed for telomerase activity by the TRAP assay as indicated. ITAS represents the internal telomerase assay standard.
Because MKRN1 was shown to be an E3 ligase for hTERT (26), we next investigated whether both CHIP and MKRN1 promote hTERT ubiquitination. Overexpression of MKRN1 caused a clear reduction in the hTERT level, which was further decreased by CHIP overexpression (Fig. 4D). However, hTERT degradation was blocked by MG132 treatment. In the presence of MG132, there was a significant increase in both unmodified and ubiquitinated MKRN1 proteins, suggesting that MKRN1 is rapidly degraded through auto-ubiquitination (Fig. 4D). However, the level of CHIP expression was not altered by MG132 treatment. We also found that the amount of ubiquitinated hTERT was significantly increased in cells co-transfected with both CHIP and MKRN1 compared with cells transfected with either CHIP or MKRN1 alone (Fig. 4E). We next compared the effects of CHIP and MKRN1 on telomerase activity. Overexpression of CHIP or MKRN1 led to a marked decrease in telomerase activity compared with the vector control (Fig. 4F). When both proteins were co-expressed, we observed an additive effect on telomerase activity.
CHIP Remodels the hTERT-Chaperone Complex to Inactive Form
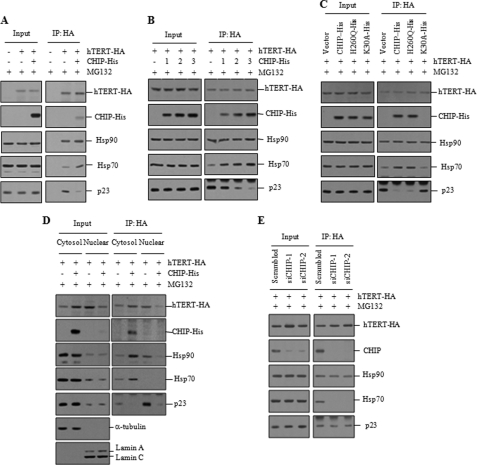
The Hsp90 and p23 molecular chaperones have been shown to associate with hTERT for the assembly of active telomerase (18, 27). Because CHIP-bound hTERT is localized to the cytoplasm and appears to be biologically nonfunctional for telomerase activity, it is conceivable that CHIP may remodel the hTERT-chaperone complex, resulting in a failure of hTERT to mature. To test this possibility, H1299 cells were co-transfected with hTERT-HA and CHIP-His prior to MG132 treatment and subjected to immunoprecipitation. Expression levels of endogenous Hsp90, Hsp70, and p23 proteins were not affected by CHIP overexpression (Fig. 5A). In the absence of CHIP overexpression, all three chaperones interacted with hTERT. However, the hTERT-Hsp70 interaction was significantly increased by CHIP overexpression, and p23 was dissociated from hTERT. Hsp90 binding to hTERT was not affected by CHIP overexpression (Fig. 5A). To examine these findings in closer detail, H1299 cells were co-transfected with hTERT-HA and increasing amounts of CHIP-His prior to MG132 treatment. CHIP overexpression resulted in a substantial increase in association of Hsp70 with hTERT but promoted dissociation of p23 from hTERT in a dose-dependent manner (Fig. 5B). We next investigated the effect of the CHIP mutants on the hTERT-chaperone complexes. The H260Q mutant led to an increase in association of Hsp70 with hTERT but a dissociation of p23 from hTERT to a similar extent with wild-type CHIP (Fig. 5C). In contrast, the K30A mutant was unable to interact with hTERT and did not affect the association of Hsp70 and p23 with hTERT.
FIGURE 5.
CHIP remodels the hTERT-chaperone complexes. A, H1299 cells were co-transfected with hTERT-HA and CHIP-His and treated with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates (IP) were analyzed by immunoblotting with anti-HA, anti-His, anti-Hsp90, anti-Hsp70, and anti-p23 antibodies. B, H1299 cells were co-transfected with hTERT-HA and increasing amounts of CHIP-His and treated with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates were analyzed by immunoblotting. C, H1299 cells were co-transfected with hTERT-HA and CHIP-His, H260Q-His, or K30A-His and treated with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates were analyzed by immunoblotting. D, cytoplasmic and nuclear extracts were separately collected from H1299 cells co-transfected with hTERT-HA and CHIP-His prior to treatment with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates were analyzed by immunoblotting. E, H1299 cells transfected with hTERT-HA and scrambled or CHIP siRNA were treated with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates were analyzed by immunoblotting.
To further study the effect of CHIP overexpression on the subcellular localization of the hTERT-chaperone complexes, we examined the levels of chaperones bound to hTERT in cytoplasmic and nuclear fractions (Fig. 5D). In the absence of CHIP overexpression, the majority of Hsp90 and p23 proteins associated with hTERT were localized to the nucleus. In contrast, Hsp70 bound to hTERT was only detected in the cytoplasm. When CHIP was overexpressed, Hsp90 bound to hTERT was accumulated in the cytoplasm, but p23 was dissociated from hTERT. The amount of Hsp70 bound to hTERT was remarkably increased in the cytoplasm, suggesting that Hsp70 is actively recruited to hTERT by CHIP overexpression. These results support the notion that hTERT interacts with CHIP and Hsp70 in the cytoplasm as it progresses toward the degradative pathway. We next examined the effect of CHIP knockdown on the interaction of hTERT with chaperone proteins. Consistent with data presented above, Hsp90, Hsp70, and p23 proteins interacted with hTERT in H1299 cells transfected with the scrambled siRNA (Fig. 5E). However, Hsp70 interaction with hTERT was significantly inhibited by CHIP knockdown, although binding of Hsp90 and p23 to hTERT was not affected. These results indicate that CHIP is required for Hsp70 recruitment to hTERT.
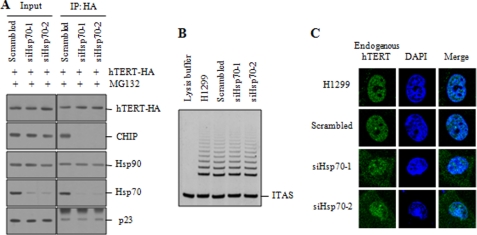
Because CHIP associates with Hsp70 and functions as a chaperone-dependent ubiquitin ligase (40, 41), we tested whether CHIP is sufficient to interact with hTERT in the absence of Hsp70. The expression of endogenous Hsp70 was depleted using siRNAs (siHsp70-1 and siHsp70-2). The interaction between hTERT and CHIP was dramatically reduced by Hsp70 knockdown (Fig. 6A), suggesting that hTERT-CHIP interaction depends on Hsp70. When telomerase activity was compared in control versus Hsp70 siRNA-treated cells, no significant difference was observed in telomerase activity (Fig. 6B), consistent with the results shown in CHIP knockdown cells (Fig. 3B). To determine the effect of Hsp70 knockdown on the subcellular localization of hTERT, Hsp70 knockdown cells were treated with MG132 and subjected to indirect immunofluorescence staining. The nuclear localization of hTERT was not significantly affected by Hsp70 knockdown (Fig. 6C). We note that Hsp70 knockdown led to a clear accumulation of hTERT in the cytoplasm. These findings are consistent with the results shown in CHIP siRNA-treated cells (Fig. 3E).
FIGURE 6.
Hsp70 is required for the interaction between hTERT and CHIP. A, H1299 cells transfected with hTERT-HA and scrambled or Hsp70 siRNA (siHsp70-1 or siHsp70-2) were treated with 10 μm MG132 for 2 h. Lysates (input) and anti-HA immunoprecipitates (IP) were analyzed by immunoblotting as indicated. B, H1299 cells transfected with scrambled or Hsp70 siRNA were analyzed for telomerase activity by the TRAP assay. C, H1299 cells transfected with scrambled or Hsp70 siRNA were treated with 10 μm MG132 for 2 h and subjected to indirect immunofluorescence with anti-hTERT (green) antibody, followed by fluorescent microscopic observation. The nuclei were stained with 4,6-diamino-2-phenylindole (DAPI, blue). ITAS represents the internal telomerase assay standard.
Because Hsp90 and p23 remain associated with active telomerase in the nucleus (27, 42), we wanted to verify the critical role of p23 in the regulation of telomerase activity. To determine the effect of p23 knockdown on hTERT degradation, H1299 cells were transfected with hTERT-HA and siRNA duplexes targeting p23 (sip23-1 and sip23-2), and we assessed the protein levels. Knockdown of endogenous p23 markedly reduced the hTERT levels (Fig. 7A). However, this hTERT degradation was inhibited by MG132 treatment. We also found that p23 knockdown induced a cytoplasmic accumulation of hTERT-HA in the presence of MG132, whereas hTERT-HA was predominantly localized to the nucleus in cells transfected with the scrambled siRNA (Fig. 7B). Inhibition of nuclear localization of hTERT by p23 knockdown was further confirmed by immunofluorescence staining. Endogenous hTERT was significantly accumulated in the cytoplasm by p23 knockdown (Fig. 7C), indicating that p23 is required for nuclear translocation of hTERT. We next determined the effect of p23 on telomerase activity. p23 knockdown resulted in a significant reduction in telomerase activity compared with the scrambled siRNA control (Fig. 7D).
FIGURE 7.
p23 plays an essential role in the nuclear localization of hTERT. A, H1299 cells transfected with hTERT-HA and scrambled or p23 siRNA (sip23-1 or sip23-2) were treated with or without 10 μm MG132 for 2 h as specified. The protein levels of endogenous p23 and hTERT-HA were measured by immunoblotting as indicated. B, cytoplasmic and nuclear extracts were separately collected from H1299 cells transfected with hTERT-HA and scrambled or p23 siRNA and treated with 10 μm MG132 for 2 h. The protein levels of endogenous p23 and hTERT-HA were measured by immunoblotting. C, H1299 cells transfected with scrambled or p23 siRNA were treated with 10 μm MG132 for 2 h and subjected to indirect immunofluorescence with anti-hTERT (green) antibody, followed by fluorescent microscopic observation. The nuclei were stained with 4,6-diamino-2-phenylindole (DAPI, blue). D, H1299 cells transfected with scrambled or p23 siRNA were analyzed for telomerase activity by the TRAP assay. ITAS represents the internal telomerase assay standard.
CHIP Specifically Interacts with hTERT in G2/M Phases
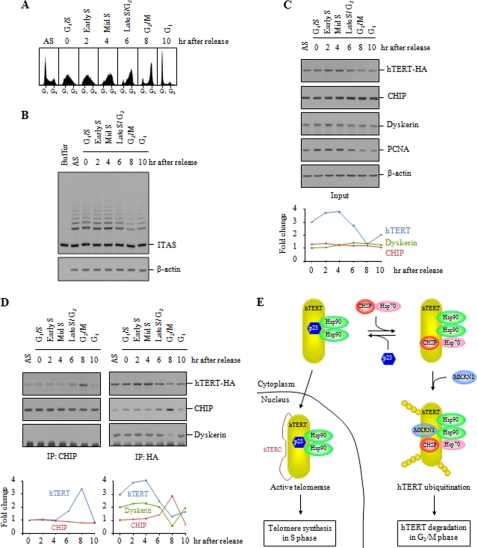
Telomerase elongates telomeric DNA preferentially during S phase of the cell cycle (43). Although several lines of evidence have suggested the cell cycle-dependent regulation of telomerase (44–46), it is unclear how telomerase is restricted to act specifically on telomeres during S phase. Because CHIP associates with catalytically inactive hTERT in the cytoplasm, we examined whether CHIP influences telomerase activity during the cell cycle. The double thymidine block method was used to obtain HeLa S3 cells synchronized at the G1/S transition, and cell cycle progression was monitored upon removal of thymidine by FACS analysis (Fig. 8A). When we performed the TRAP assay with the synchronized cells, telomerase activity peaked in S phase but decreased during G2/M phase (Fig. 8B).
FIGURE 8.
CHIP specifically interacts with hTERT in G2/M phases. A, HeLa S3 cells were synchronized by double thymidine block and harvested at 2-h intervals over a 10-h time course after release. Cell cycle progression was monitored by propidium iodide staining and FACS analysis. The 0-h time point corresponds to blocked cells before release. AS, asynchronous cells. B, telomerase activity peaks in S phase. The synchronized cells were analyzed for telomerase activity by the TRAP assay. C, HeLa S3 cells transfected with hTERT-HA were synchronized by double thymidine block. The expression levels of hTERT-HA, CHIP, and dyskerin were measured by immunoblotting with anti-HA, anti-CHIP, and anti-dyskerin antibodies. Immunoblot for proliferating cell nuclear antigen was used for an independent maker of S phase. Band intensities were quantified and displayed as fold change in the accompanying graph. PCNA, proliferating cell nuclear antigen. D, interaction of hTERT and CHIP during cell cycle progression. The synchronized HeLa S3 cells were subjected to immunoprecipitation (IP) with anti-CHIP or anti-HA antibodies, followed by immunoblot analysis as indicated. Band intensities were quantified and displayed as fold change in the accompanying graph. E, model of chaperone-mediated nuclear import and ubiquitin-mediated degradation of hTERT. p23 recruitment to the hTERT-Hsp90 complexes maintains hTERT in a conformation enabling the nuclear translocation. The binding of CHIP and Hsp70 to hTERT causes p23 to dissociate, leading to cytoplasmic retention of hTERT. Cytoplasmic hTERT is rapidly ubiquitinated by CHIP and/or MKRN1 and subsequently degraded by the proteasome in G2/M phases during which telomerase does not act on telomeres. ITAS represents the internal telomerase assay standard.
We next investigated the interaction of hTERT and CHIP over the course of the cell cycle. HeLa S3 cells transfected with hTERT-HA were synchronized and subjected to immunoblot analysis. Expression of hTERT-HA peaked in S phase whereas expression of endogenous CHIP and dyskerin did not vary through the majority of the cell cycle (Fig. 8C). Immunoblot for proliferating cell nuclear antigen was used for an independent marker of S phase. The levels of hTERT mRNA and human telomerase RNA component transcripts were not changed during cell cycle progression (supplemental Fig. 5). Immunoprecipitation of CHIP revealed that the amount of hTERT-HA associated with CHIP peaked in G2/M phases but decreased during G1 and S phases (Fig. 8D). Likewise, CHIP was specifically immunoprecipitated by hTERT-HA during G2/M phases. Interestingly, dyskerin was specifically dissociated from hTERT in G2/M phases. These results are consistent with the idea that CHIP modulates telomerase activity through dynamic control of the hTERT protein stability in a cell cycle-dependent manner.
DISCUSSION
Telomerase activity is tightly regulated at multiple levels, including hTERT gene expression, post-translational protein-protein interactions, and covalent modifications of hTERT, including phosphorylation and ubiquitination (12, 23). Here, we report the identification of CHIP as a hTERT-interacting protein and provide evidence that CHIP modulates the degradation pathway of hTERT. We show that CHIP promotes hTERT ubiquitination and degradation by the proteasome without affecting the cellular level of hTERT mRNA.
Overexpression of CHIP inhibits nuclear translocation of hTERT and decreases the protein stability of hTERT, thereby inhibiting telomerase activity. In contrast, knockdown of CHIP increases the level of cytoplasmic hTERT. However, it does not affect the level of nuclear hTERT and has no effect on telomerase activity and telomere length. Although cytoplasmic hTERT was accumulated by CHIP knockdown, telomerase activity was detected exclusively in the nuclear extracts but not in the cytoplasmic extracts as measured by the TRAP assay. These findings suggest that cytoplasmic hTERT may not be properly assembled into the active telomerase holoenzyme. Nevertheless, we cannot exclude the possibility that the absence of a change in telomerase activity may be due to the assay used in this experiment, instead of testing endogenous telomerase activity by the conventional method. In addition, we observed no significant change in telomere length by CHIP knockdown. The absence of a change in telomere length may reflect either that 2 weeks of knocking CHIP down is not enough to observe such change or that telomeres in CHIP knockdown cells do not alter as much as observed in the terminal restriction fragment length analysis. However, given that cytoplasmic hTERT is nonfunctional for telomerase activity, it is more likely that this mode of regulating hTERT levels may have no impact on telomere length.
Our data indicate that CHIP physically interacts with hTERT in the cytoplasm in vitro and in vivo. This interaction requires the chaperone-binding TPR domain but is independent of the U-box domain with E3 ubiquitin ligase activity. We have found that CHIP overexpression led to a cytoplasmic retention of hTERT, thereby inhibiting telomerase activity. However, the cytoplasmic retention of hTERT was not induced by a TPR domain point mutant of CHIP (K30A), which is unable to interact with Hsp/Hsc70 or Hsp90. These results suggest that inhibition of telomerase activity by CHIP can be achieved by blocking nuclear import of hTERT. The resulting cytoplasmic hTERT is rapidly degraded by the proteasome. Thus, the ability of CHIP to induce hTERT ubiquitination and degradation correlates with its ability to bind hTERT. Although the U-box domain of CHIP is not required for hTERT binding nor to inhibit nuclear import of hTERT, it is necessary for ubiquitination and degradation of hTERT. Intriguingly, we observed that the H260Q mutant still retains the ability to ubiquitinate hTERT and causes a partial decrease in hTERT level. These results suggest that the U-box domain of CHIP might be dispensable for its function in stimulating hTERT ubiquitination and degradation. Similar results were observed on CHIP-mediated ubiquitination of E47 (47) and Runx2 degradation (48). In the case of E47, CHIP does not act as an E3 ligase for E47 but appears to act more as an adaptor to facilitate the interaction between E47 and Skp2. This complex can join the SCF E3 ligase complex to initiate ubiquitination of E47 (47). Similarly, other factors might be involved in the hTERT ubiquitination process mediated by CHIP.
Previously, MKRN1 has been reported to function as an E3 ubiquitin ligase for hTERT (26). In this work, we provide evidence that CHIP, like MKRN1, can promote hTERT ubiquitination. Overexpression of either MKRN1 or CHIP independently reduced the cellular level of hTERT as well as telomerase activity, which is further decreased in cells co-transfected with both genes. Because MKRN1 and CHIP are each capable of promoting ubiquitination of hTERT, CHIP may target hTERT for ubiquitin-mediated degradation through an MKRN1-independent mechanism. We cannot, however, exclude the possibility that the two proteins may function cooperatively under physiological conditions. When hTERT fails to be translocated into the nucleus by its interaction with CHIP, the cytoplasmic hTERT might be ubiquitinated by MKRN1, together with CHIP. Indeed, we observed that the H260Q mutant causes a partial increase in hTERT ubiquitination. However, whether or not both MKRN1 and CHIP can bind hTERT simultaneously in the cytoplasm is not known. In this study, we also show that the CHIP-hTERT interaction specifically occurs in G2/M phases, suggesting that CHIP negatively regulates the hTERT level in a cell cycle-dependent manner. Therefore, it will be of interest to determine whether the association of hTERT and MKRN1 is cell cycle-dependent. The functional similarity between CHIP and MKRN1 raises many important questions about the physiological significance of the multiple pathways that exert negative control over hTERT. It is yet uncertain exactly how these pathways are specifically regulated and under what situations they are differently activated.
The molecular chaperone network plays a key role in maintaining a balance between folding and degradation of its various substrates (49, 50). Multichaperone complexes containing Hsp90, Hsp70, and other co-chaperones have been shown to assemble on substrate polypeptides through several intermediate assembly steps (51). Prior studies indicated that Hsp90 and p23 interact with hTERT and are required for the assembly of active telomerase (18, 27) and the nuclear localization of hTERT (42). Hsp70 also associates with hTERT but readily dissociates when telomerase is folded into its active form. In this study, we found that CHIP overexpression resulted in a substantial reduction in association of p23 and hTERT, but it does not affect the Hsp90 binding to hTERT. On the other hand, Hsp70 binding to hTERT occurred only in the cytoplasm and was significantly increased by CHIP overexpression. However, the K30A mutant, which cannot interact with Hsp70, failed to bind hTERT and promote hTERT degradation. Furthermore, we found that the interaction between hTERT and CHIP was dramatically reduced by Hsp70 knockdown. These results suggest that Hsp70 binding to CHIP is required for hTERT degradation and that the interaction between CHIP and hTERT is stable only in the presence of CHIP-Hsp70 association. Similar results were recently reported on CHIP-mediated HIF-1α degradation (52). In this case, Hsp70 directly interacts with HIF-1α and mediates HIF-1α degradation through recruitment of CHIP.
Based on previous reports and our data, we propose a model for chaperone-mediated hTERT folding and degradation (Fig. 8E). Dynamic control of the hTERT protein stability involves several chaperone-mediated assembly steps. In this model, Hsp90 appears to associate constitutively with nascent hTERT in the cytoplasm. p23 recruitment to the hTERT-Hsp90 complexes maintains hTERT in a conformation enabling the nuclear translocation. The reduced maturation of hTERT upon p23 knockdown suggests that the integrity of the hTERT-Hsp90-p23 complex is critical for the nuclear localization of active telomerase. The binding of CHIP and Hsp70 to hTERT causes dissociation of p23 from hTERT and induces a failure of hTERT to mature, resulting in cytoplasmic accumulation of an immature form that is incompetent in nuclear translocation. The cytoplasmic hTERT complex is rapidly ubiquitinated by CHIP and/or MKRN1 and subsequently degraded by the proteasome. Thus, both p23, which favors nuclear translocation, and the CHIP-Hsp70 complex, which favors degradation, compete for binding to hTERT. The outcome of this competition likely dictates which of the two fates of hTERT, nuclear translocation or degradation, is favored. Importantly, we found that the association of hTERT with CHIP is cell cycle-dependent. Because the nuclear localization of hTERT preferentially occurs during S phase, the CHIP-mediated hTERT degradation pathway may be specifically activated in G2 and M phases during which telomerase does not act on telomeres (Fig. 8E).
In conclusion, our data provide evidence for an important role of CHIP in regulating telomerase activity by modulating the cellular abundance of hTERT in addition to the known negative regulator MKRN1. This study enriches our knowledge of the dynamic regulation of hTERT protein stability during the cell cycle and suggests alternative approaches for inhibiting telomerase activity in cancer.
Supplementary Material
This work was supported in part by World Class University Fund R31-2009-000-10086-0 from the Korean Ministry of Education, Science, and Technology and by Korea Research Foundation Grants KRF-M1075604000107N560400110 and KRF-20090084897.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. 1–5.
- hTERT
- human telomerase reverse transcriptase
- E3
- ubiquitin-protein isopeptide ligase
- Hsp90
- heat shock protein 90
- Hsp70
- heat shock protein 70
- TPR
- tetratricopeptide repeat
- TRAP
- telomeric repeat amplification protocol.
REFERENCES
- 1.Smogorzewska A., de Lange T. (2004) Annu. Rev. Biochem. 73, 177–208 [DOI] [PubMed] [Google Scholar]
- 2.Palm W., de Lange T. (2008) Annu. Rev. Genet. 42, 301–334 [DOI] [PubMed] [Google Scholar]
- 3.Griffith J. D., Comeau L., Rosenfield S., Stansel R. M., Bianchi A., Moss H., de Lange T. (1999) Cell 97, 503–514 [DOI] [PubMed] [Google Scholar]
- 4.de Lange T. (2005) Genes Dev. 19, 2100–2110 [DOI] [PubMed] [Google Scholar]
- 5.Liu D., O'Connor M. S., Qin J., Songyang Z. (2004) J. Biol. Chem. 279, 51338–51342 [DOI] [PubMed] [Google Scholar]
- 6.Blasco M. A., Lee H. W., Hande M. P., Samper E., Lansdorp P. M., DePinho R. A., Greider C. W. (1997) Cell 91, 25–34 [DOI] [PubMed] [Google Scholar]
- 7.Lee H. W., Blasco M. A., Gottlieb G. J., Horner J. W., 2nd., Greider C. W., DePinho R. A. (1998) Nature 392, 569–574 [DOI] [PubMed] [Google Scholar]
- 8.Lingner J., Cooper J. P., Cech T. R. (1995) Science 269, 1533–1534 [DOI] [PubMed] [Google Scholar]
- 9.Cerone M. A., Autexier C., Londoño-Vallejo J. A., Bacchetti S. (2005) Oncogene 24, 7893–7901 [DOI] [PubMed] [Google Scholar]
- 10.Bryan T. M., Englezou A., Dalla-Pozza L., Dunham M. A., Reddel R. R. (1997) Nat. Med. 3, 1271–1274 [DOI] [PubMed] [Google Scholar]
- 11.Dunham M. A., Neumann A. A., Fasching C. L., Reddel R. R. (2000) Nat. Genet. 26, 447–450 [DOI] [PubMed] [Google Scholar]
- 12.Autexier C., Lue N. F. (2006) Annu. Rev. Biochem. 75, 493–517 [DOI] [PubMed] [Google Scholar]
- 13.Bianchi A., Shore D. (2008) Mol. Cell 31, 153–165 [DOI] [PubMed] [Google Scholar]
- 14.Hahn W. C., Counter C. M., Lundberg A. S., Beijersbergen R. L., Brooks M. W., Weinberg R. A. (1999) Nature 400, 464–468 [DOI] [PubMed] [Google Scholar]
- 15.Kim N. W., Piatyszek M. A., Prowse K. R., Harley C. B., West M. D., Ho P. L., Coviello G. M., Wright W. E., Weinrich S. L., Shay J. W. (1994) Science 266, 2011–2015 [DOI] [PubMed] [Google Scholar]
- 16.Wang J., Xie L. Y., Allan S., Beach D., Hannon G. J. (1998) Genes Dev. 12, 1769–1774 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Greenberg R. A., O'Hagan R. C., Deng H., Xiao Q., Hann S. R., Adams R. R., Lichtsteiner S., Chin L., Morin G. B., DePinho R. A. (1999) Oncogene 18, 1219–1226 [DOI] [PubMed] [Google Scholar]
- 18.Holt S. E., Aisner D. L., Baur J., Tesmer V. M., Dy M., Ouellette M., Trager J. B., Morin G. B., Toft D. O., Shay J. W., Wright W. E., White M. A. (1999) Genes Dev. 13, 817–826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akiyama M., Hideshima T., Hayashi T., Tai Y. T., Mitsiades C. S., Mitsiades N., Chauhan D., Richardson P., Munshi N. C., Anderson K. C. (2003) Cancer Res. 63, 18–21 [PubMed] [Google Scholar]
- 20.Seimiya H., Sawada H., Muramatsu Y., Shimizu M., Ohko K., Yamane K., Tsuruo T. (2000) EMBO J. 19, 2652–2661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou X. Z., Lu K. P. (2001) Cell 107, 347–359 [DOI] [PubMed] [Google Scholar]
- 22.Lee G. E., Yu E. Y., Cho C. H., Lee J., Muller M. T., Chung I. K. (2004) J. Biol. Chem. 279, 34750–34755 [DOI] [PubMed] [Google Scholar]
- 23.Cong Y. S., Wright W. E., Shay J. W. (2002) Microbiol. Mol. Biol. Rev. 66, 407–425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kimura A., Ohmichi M., Kawagoe J., Kyo S., Mabuchi S., Takahashi T., Ohshima C., Arimoto-Ishida E., Nishio Y., Inoue M., Kurachi H., Tasaka K., Murata Y. (2004) Oncogene 23, 4505–4515 [DOI] [PubMed] [Google Scholar]
- 25.Kharbanda S., Kumar V., Dhar S., Pandey P., Chen C., Majumder P., Yuan Z. M., Whang Y., Strauss W., Pandita T. K., Weaver D., Kufe D. (2000) Curr. Biol. 10, 568–575 [DOI] [PubMed] [Google Scholar]
- 26.Kim J. H., Park S. M., Kang M. R., Oh S. Y., Lee T. H., Muller M. T., Chung I. K. (2005) Genes Dev. 19, 776–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Forsythe H. L., Jarvis J. L., Turner J. W., Elmore L. W., Holt S. E. (2001) J. Biol. Chem. 276, 15571–15574 [DOI] [PubMed] [Google Scholar]
- 28.Whitesell L., Lindquist S. L. (2005) Nat. Rev. Cancer 5, 761–772 [DOI] [PubMed] [Google Scholar]
- 29.Dittmar K. D., Demady D. R., Stancato L. F., Krishna P., Pratt W. B. (1997) J. Biol. Chem. 272, 21213–21220 [DOI] [PubMed] [Google Scholar]
- 30.Murata S., Minami Y., Minami M., Chiba T., Tanaka K. (2001) EMBO Rep. 2, 1133–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Connell P., Ballinger C. A., Jiang J., Wu Y., Thompson L. J., Höhfeld J., Patterson C. (2001) Nat. Cell Biol. 3, 93–96 [DOI] [PubMed] [Google Scholar]
- 32.Ballinger C. A., Connell P., Wu Y., Hu Z., Thompson L. J., Yin L. Y., Patterson C. (1999) Mol. Cell. Biol. 19, 4535–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meacham G. C., Patterson C., Zhang W., Younger J. M., Cyr D. M. (2001) Nat. Cell Biol. 3, 100–105 [DOI] [PubMed] [Google Scholar]
- 34.Keppler B. R., Grady A. T., Jarstfer M. B. (2006) J. Biol. Chem. 281, 19840–19848 [DOI] [PubMed] [Google Scholar]
- 35.Xu W., Marcu M., Yuan X., Mimnaugh E., Patterson C., Neckers L. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 12847–12852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kim J. H., Kim J. H., Lee G. E., Lee J. E., Chung I. K. (2003) Mol. Pharmacol. 63, 1117–11124 [DOI] [PubMed] [Google Scholar]
- 37.Kim N. W., Wu F. (1997) Nucleic Acids Res. 25, 2595–2597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deng Z., Dheekollu J., Broccoli D., Dutta A., Lieberman P. M. (2007) Curr. Biol. 17, 1989–1995 [DOI] [PubMed] [Google Scholar]
- 39.Sampathi S., Bhusari A., Shen B., Chai W. (2009) J. Biol. Chem. 284, 3682–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayer M. P., Bukau B. (2005) Cell. Mol. Life Sci. 62, 670–684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jiang J., Ballinger C. A., Wu Y., Dai Q., Cyr D. M., Höhfeld J., Patterson C. (2001) J. Biol. Chem. 276, 42938–42944 [DOI] [PubMed] [Google Scholar]
- 42.Lee J. H., Chung I. K. (2010) Cancer Lett. 290, 76–86 [DOI] [PubMed] [Google Scholar]
- 43.Marcand S., Brevet V., Mann C., Gilson E. (2000) Curr. Biol. 10, 487–490 [DOI] [PubMed] [Google Scholar]
- 44.Jády B. E., Richard P., Bertrand E., Kiss T. (2006) Mol. Biol. Cell 17, 944–954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tomlinson R. L., Ziegler T. D., Supakorndej T., Terns R. M., Terns M. P. (2006) Mol. Biol. Cell 17, 955–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venteicher A. S., Meng Z., Mason P. J., Veenstra T. D., Artandi S. E. (2008) Cell 132, 945–957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang Z., Nie L., Xu M., Sun X. H. (2004) Mol. Cell. Biol. 24, 8951–8962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li X., Huang M., Zheng H., Wang Y., Ren F., Shang Y., Zhai Y., Irwin D. M., Shi Y., Chen D., Chang Z. (2008) J. Cell Biol. 181, 959–972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marques C., Guo W., Pereira P., Taylor A., Patterson C., Evans P. C., Shang F. (2006) FASEB J. 20, 741–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Young J. C., Agashe V. R., Siegers K., Hartl F. U. (2004) Nat. Rev. Mol. Cell Biol. 5, 781–791 [DOI] [PubMed] [Google Scholar]
- 51.Walker V. E., Wong M. J., Atanasiu R., Hantouche C., Young J. C., Shrier A. (2010) J. Biol. Chem. 285, 3319–3329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Luo W., Zhong J., Chang R., Hu H., Pandey A., Semenza G. L. (2010) J. Biol. Chem. 285, 3651–3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.