Abstract
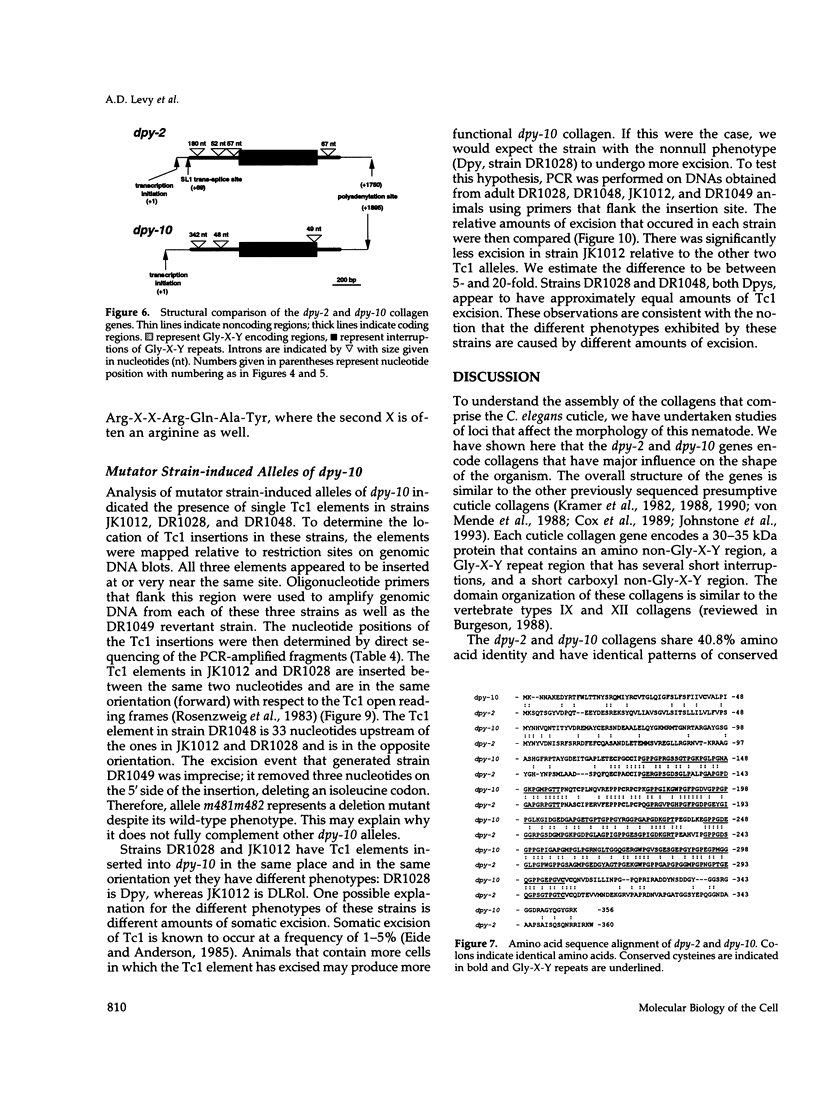
We have identified and cloned the Caenorhabditis elegans dpy-2 and dpy-10 genes and determined that they encode collagens. Genetic data suggested that these genes are important in morphogenesis and possibly other developmental events. These data include the morphologic phenotypes exhibited by mutants, unusual genetic interactions with the sqt-1 collagen gene, and suppression of mutations in the glp-1 and mup-1 genes. The proximity of the dpy-2 and dpy-10 genes (3.5 kilobase) and the structural similarity of their encoded proteins (41% amino acid identity) indicate that dpy-2 and dpy-10 are the result of a gene duplication event. The genes do not, however, appear to be functionally redundant, because a dpy-10 null mutant is not rescued by the dpy-2 gene. In addition, full complementation between dpy-2 and dpy-10 can be demonstrated with all recessive alleles tested in trans. Sequence analysis of several mutant alleles of each gene was performed to determine the nature of the molecular defects that can cause the morphologic phenotypes. Glycine substitutions within the Gly-X-Y portion of the collagens can result in dumpy (Dpy), dumpy, left roller (DLRol), or temperature-sensitive DLRol phenotypes. dpy-10(cn64), a dominant temperature-sensitive DLRol allele, creates an Arg-to-Cys substitution in the amino non-Gly-X-Y portion of the protein. Three dpy-10 alleles contain Tc1 insertions in the coding region of the gene. dpy-10(cg36) (DRLol) creates a nonsense codon near the end of the Gly-X-Y region. The nature of this mutation, combined with genetic data, indicates that DLRol is the null phenotype of dpy-10. The Dpy phenotype results from reduced function of the dpy-10 collagen gene. Our results indicate that a variety of molecular defects in these collagens can result in severe morphologic changes in C. elegans.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi M., Hornig H., Padgett R. A., Reiser J., Weissmann C. Sequence requirements for splicing of higher eukaryotic nuclear pre-mRNA. Cell. 1986 Nov 21;47(4):555–565. doi: 10.1016/0092-8674(86)90620-3. [DOI] [PubMed] [Google Scholar]
- Aroian R. V., Levy A. D., Koga M., Ohshima Y., Kramer J. M., Sternberg P. W. Splicing in Caenorhabditis elegans does not require an AG at the 3' splice acceptor site. Mol Cell Biol. 1993 Jan;13(1):626–637. doi: 10.1128/mcb.13.1.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin J., Kimble J. glp-1 is required in the germ line for regulation of the decision between mitosis and meiosis in C. elegans. Cell. 1987 Nov 20;51(4):589–599. doi: 10.1016/0092-8674(87)90128-0. [DOI] [PubMed] [Google Scholar]
- Barr P. J. Mammalian subtilisins: the long-sought dibasic processing endoproteases. Cell. 1991 Jul 12;66(1):1–3. doi: 10.1016/0092-8674(91)90129-m. [DOI] [PubMed] [Google Scholar]
- Barstead R. J., Waterston R. H. The basal component of the nematode dense-body is vinculin. J Biol Chem. 1989 Jun 15;264(17):10177–10185. [PubMed] [Google Scholar]
- Bateman J. F., Chan D., Walker I. D., Rogers J. G., Cole W. G. Lethal perinatal osteogenesis imperfecta due to the substitution of arginine for glycine at residue 391 of the alpha 1(I) chain of type I collagen. J Biol Chem. 1987 May 25;262(15):7021–7027. [PubMed] [Google Scholar]
- Bateman J. F., Lamande S. R., Dahl H. H., Chan D., Cole W. G. Substitution of arginine for glycine 664 in the collagen alpha 1(I) chain in lethal perinatal osteogenesis imperfecta. Demonstration of the peptide defect by in vitro expression of the mutant cDNA. J Biol Chem. 1988 Aug 25;263(24):11627–11630. [PubMed] [Google Scholar]
- Bird D. M. Sequence comparison of the Caenorhabditis elegans dpy-13 and col-34 genes, and their deduced collagen products. Gene. 1992 Oct 21;120(2):261–266. doi: 10.1016/0378-1119(92)90102-u. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974 May;77(1):71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstens R. P., Fenton W. A., Rosenberg L. R. Identification of RNA splicing errors resulting in human ornithine transcarbamylase deficiency. Am J Hum Genet. 1991 Jun;48(6):1105–1114. [PMC free article] [PubMed] [Google Scholar]
- Cladaras C., Hadzopoulou-Cladaras M., Felber B. K., Pavlakis G., Zannis V. I. The molecular basis of a familial apoE deficiency. An acceptor splice site mutation in the third intron of the deficient apoE gene. J Biol Chem. 1987 Feb 15;262(5):2310–2315. [PubMed] [Google Scholar]
- Coulson A., Sulston J., Brenner S., Karn J. Toward a physical map of the genome of the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1986 Oct;83(20):7821–7825. doi: 10.1073/pnas.83.20.7821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulson A., Waterston R., Kiff J., Sulston J., Kohara Y. Genome linking with yeast artificial chromosomes. Nature. 1988 Sep 8;335(6186):184–186. doi: 10.1038/335184a0. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Carr S., Kramer J. M., Hirsh D. Genetic mapping of Caenorhabditis elegans collagen genes using DNA polymorphisms as phenotypic markers. Genetics. 1985 Mar;109(3):513–528. doi: 10.1093/genetics/109.3.513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Fields C., Kramer J. M., Rosenzweig B., Hirsh D. Sequence comparisons of developmentally regulated collagen genes of Caenorhabditis elegans. Gene. 1989;76(2):331–344. doi: 10.1016/0378-1119(89)90173-x. [DOI] [PubMed] [Google Scholar]
- Cox G. N., Kramer J. M., Hirsh D. Number and organization of collagen genes in Caenorhabditis elegans. Mol Cell Biol. 1984 Nov;4(11):2389–2395. doi: 10.1128/mcb.4.11.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Kusch M., Edgar R. S. Cuticle of Caenorhabditis elegans: its isolation and partial characterization. J Cell Biol. 1981 Jul;90(1):7–17. doi: 10.1083/jcb.90.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Laufer J. S., Kusch M., Edgar R. S. Genetic and Phenotypic Characterization of Roller Mutants of CAENORHABDITIS ELEGANS. Genetics. 1980 Jun;95(2):317–339. doi: 10.1093/genetics/95.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. N., Shamansky L. M., Boisvenue R. J. Haemonchus contortus: evidence that the 3A3 collagen gene is a member of an evolutionarily conserved family of nematode cuticle collagens. Exp Parasitol. 1990 Feb;70(2):175–185. doi: 10.1016/0014-4894(90)90098-w. [DOI] [PubMed] [Google Scholar]
- Eide D., Anderson P. Transposition of Tc1 in the nematode Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1985 Mar;82(6):1756–1760. doi: 10.1073/pnas.82.6.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmons S. W., Yesner L., Ruan K. S., Katzenberg D. Evidence for a transposon in Caenorhabditis elegans. Cell. 1983 Jan;32(1):55–65. doi: 10.1016/0092-8674(83)90496-8. [DOI] [PubMed] [Google Scholar]
- Gelman R. A., Poppke D. C., Piez K. A. Collagen fibril formation in vitro. The role of the nonhelical terminal regions. J Biol Chem. 1979 Nov 25;254(22):11741–11745. [PubMed] [Google Scholar]
- Goh P. Y., Bogaert T. Positioning and maintenance of embryonic body wall muscle attachments in C. elegans requires the mup-1 gene. Development. 1991 Mar;111(3):667–681. doi: 10.1242/dev.111.3.667. [DOI] [PubMed] [Google Scholar]
- Guo X. D., Kramer J. M. The two Caenorhabditis elegans basement membrane (type IV) collagen genes are located on separate chromosomes. J Biol Chem. 1989 Oct 15;264(29):17574–17582. [PubMed] [Google Scholar]
- Higgins B. J., Hirsh D. Roller mutants of the nematode Caenorhabditis elegans. Mol Gen Genet. 1977 Jan 7;150(1):63–72. doi: 10.1007/BF02425326. [DOI] [PubMed] [Google Scholar]
- Hodgkin J. A., Brenner S. Mutations causing transformation of sexual phenotype in the nematode Caenorhabditis elegans. Genetics. 1977 Jun;86(2 Pt 1):275–287. [PMC free article] [PubMed] [Google Scholar]
- Johnstone I. L., Shafi Y., Barry J. D. Molecular analysis of mutations in the Caenorhabditis elegans collagen gene dpy-7. EMBO J. 1992 Nov;11(11):3857–3863. doi: 10.1002/j.1460-2075.1992.tb05478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Cox G. N., Hirsh D. Comparisons of the complete sequences of two collagen genes from Caenorhabditis elegans. Cell. 1982 Sep;30(2):599–606. doi: 10.1016/0092-8674(82)90256-2. [DOI] [PubMed] [Google Scholar]
- Kramer J. M., French R. P., Park E. C., Johnson J. J. The Caenorhabditis elegans rol-6 gene, which interacts with the sqt-1 collagen gene to determine organismal morphology, encodes a collagen. Mol Cell Biol. 1990 May;10(5):2081–2089. doi: 10.1128/mcb.10.5.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer J. M., Johnson J. J., Edgar R. S., Basch C., Roberts S. The sqt-1 gene of C. elegans encodes a collagen critical for organismal morphogenesis. Cell. 1988 Nov 18;55(4):555–565. doi: 10.1016/0092-8674(88)90214-0. [DOI] [PubMed] [Google Scholar]
- Krause M., Hirsh D. A trans-spliced leader sequence on actin mRNA in C. elegans. Cell. 1987 Jun 19;49(6):753–761. doi: 10.1016/0092-8674(87)90613-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusch M., Edgar R. S. Genetic studies of unusual loci that affect body shape of the nematode Caenorhabditis elegans and may code for cuticle structural proteins. Genetics. 1986 Jul;113(3):621–639. doi: 10.1093/genetics/113.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamande S. R., Dahl H. H., Cole W. G., Bateman J. F. Characterization of point mutations in the collagen COL1A1 and COL1A2 genes causing lethal perinatal osteogenesis imperfecta. J Biol Chem. 1989 Sep 25;264(27):15809–15812. [PubMed] [Google Scholar]
- Maine E. M., Kimble J. Identification of genes that interact with glp-1, a gene required for inductive cell interactions in Caenorhabditis elegans. Development. 1989 May;106(1):133–143. doi: 10.1242/dev.106.1.133. [DOI] [PubMed] [Google Scholar]
- Mitchell P. J., Urlaub G., Chasin L. Spontaneous splicing mutations at the dihydrofolate reductase locus in Chinese hamster ovary cells. Mol Cell Biol. 1986 Jun;6(6):1926–1935. doi: 10.1128/mcb.6.6.1926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okkema P. G., Kimble J. Molecular analysis of tra-2, a sex determining gene in C.elegans. EMBO J. 1991 Jan;10(1):171–176. doi: 10.1002/j.1460-2075.1991.tb07933.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pack M., Constantinou C. D., Kalia K., Nielsen K. B., Prockop D. J. Substitution of serine for alpha 1(I)-glycine 844 in a severe variant of osteogenesis imperfecta minimally destabilizes the triple helix of type I procollagen. The effects of glycine substitutions on thermal stability are either position of amino acid specific. J Biol Chem. 1989 Nov 25;264(33):19694–19699. [PubMed] [Google Scholar]
- Parker-Thornburg J., Bonner J. J. Mutations that induce the heat shock response of Drosophila. Cell. 1987 Dec 4;51(5):763–772. doi: 10.1016/0092-8674(87)90099-7. [DOI] [PubMed] [Google Scholar]
- Priess J. R., Schnabel H., Schnabel R. The glp-1 locus and cellular interactions in early C. elegans embryos. Cell. 1987 Nov 20;51(4):601–611. doi: 10.1016/0092-8674(87)90129-2. [DOI] [PubMed] [Google Scholar]
- Prockop D. J., Constantinou C. D., Dombrowski K. E., Hojima Y., Kadler K. E., Kuivaniemi H., Tromp G., Vogel B. E. Type I procollagen: the gene-protein system that harbors most of the mutations causing osteogenesis imperfecta and probably more common heritable disorders of connective tissue. Am J Med Genet. 1989 Sep;34(1):60–67. doi: 10.1002/ajmg.1320340112. [DOI] [PubMed] [Google Scholar]
- Rosenbloom J., Endo R., Harsch M. Termination of procollagen chain synthesis by puromycin. Evidence that assembly and secretion require a COOH-terminal extension. J Biol Chem. 1976 Apr 10;251(7):2070–2076. [PubMed] [Google Scholar]
- Rosenzweig B., Liao L. W., Hirsh D. Sequence of the C. elegans transposable element Tc1. Nucleic Acids Res. 1983 Jun 25;11(12):4201–4209. doi: 10.1093/nar/11.12.4201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro M. B., Senapathy P. RNA splice junctions of different classes of eukaryotes: sequence statistics and functional implications in gene expression. Nucleic Acids Res. 1987 Sep 11;15(17):7155–7174. doi: 10.1093/nar/15.17.7155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigurdson D. C., Spanier G. J., Herman R. K. Caenorhabditis elegans deficiency mapping. Genetics. 1984 Oct;108(2):331–345. doi: 10.1093/genetics/108.2.331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su T. S., Lin L. H. Analysis of a splice acceptor site mutation which produces multiple splicing abnormalities in the human argininosuccinate synthetase locus. J Biol Chem. 1990 Nov 15;265(32):19716–19720. [PubMed] [Google Scholar]
- Tromp G., Kuivaniemi H., Stolle C., Pope F. M., Prockop D. J. Single base mutation in the type III procollagen gene that converts the codon for glycine 883 to aspartate in a mild variant of Ehlers-Danlos syndrome IV. J Biol Chem. 1989 Nov 15;264(32):19313–19317. [PubMed] [Google Scholar]
- Tromp G., Prockop D. J. Single base mutation in the pro alpha 2(I) collagen gene that causes efficient splicing of RNA from exon 27 to exon 29 and synthesis of a shortened but in-frame pro alpha 2(I) chain. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5254–5258. doi: 10.1073/pnas.85.14.5254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyk T. K., Gatenby A. A., LaRossa R. A. Demonstration by genetic suppression of interaction of GroE products with many proteins. Nature. 1989 Nov 23;342(6248):451–453. doi: 10.1038/342451a0. [DOI] [PubMed] [Google Scholar]
- Vissing H., D'Alessio M., Lee B., Ramirez F., Godfrey M., Hollister D. W. Glycine to serine substitution in the triple helical domain of pro-alpha 1 (II) collagen results in a lethal perinatal form of short-limbed dwarfism. J Biol Chem. 1989 Nov 5;264(31):18265–18267. [PubMed] [Google Scholar]
- Wallis G. A., Starman B. J., Schwartz M. F., Byers P. H. Substitution of arginine for glycine at position 847 in the triple-helical domain of the alpha 1 (I) chain of type I collagen produces lethal osteogenesis imperfecta. Molecules that contain one or two abnormal chains differ in stability and secretion. J Biol Chem. 1990 Oct 25;265(30):18628–18633. [PubMed] [Google Scholar]
- Westerhausen A., Kishi J., Prockop D. J. Mutations that substitute serine for glycine alpha 1-598 and glycine alpha 1-631 in type I procollagen. The effects on thermal unfolding of the triple helix are position-specific and demonstrate that the protein unfolds through a series of cooperative blocks. J Biol Chem. 1990 Aug 15;265(23):13995–14000. [PubMed] [Google Scholar]
- Yochem J., Greenwald I. glp-1 and lin-12, genes implicated in distinct cell-cell interactions in C. elegans, encode similar transmembrane proteins. Cell. 1989 Aug 11;58(3):553–563. doi: 10.1016/0092-8674(89)90436-4. [DOI] [PubMed] [Google Scholar]
- von Mende N., Bird D. M., Albert P. S., Riddle D. L. dpy-13: a nematode collagen gene that affects body shape. Cell. 1988 Nov 18;55(4):567–576. doi: 10.1016/0092-8674(88)90215-2. [DOI] [PubMed] [Google Scholar]