This is a response to a letter by Falk and Sinning (1)
We recently identified the ankyrin region of cpSRP43 as the primary domain responsible for binding Alb3-Cterm during light-harvesting chlorophyll-binding protein (LHCP) targeting, an interaction shown to facilitate cpSRP43-dependent stimulation of cpSRP GTPases by Alb3-Cterm (2). Falk et al. (3), using only protein interaction assays, report that CD2CD3 of cpSRP43 forms the Alb3-Cterm binding interface, which appears inconsistent with the fact that CD3 is not required for LHCP integration (4) and that CD2 is not required for LHCP integration by a cpSRP54/cpFtsY-independent pathway that relies on cpSRP43/Alb3 (5).
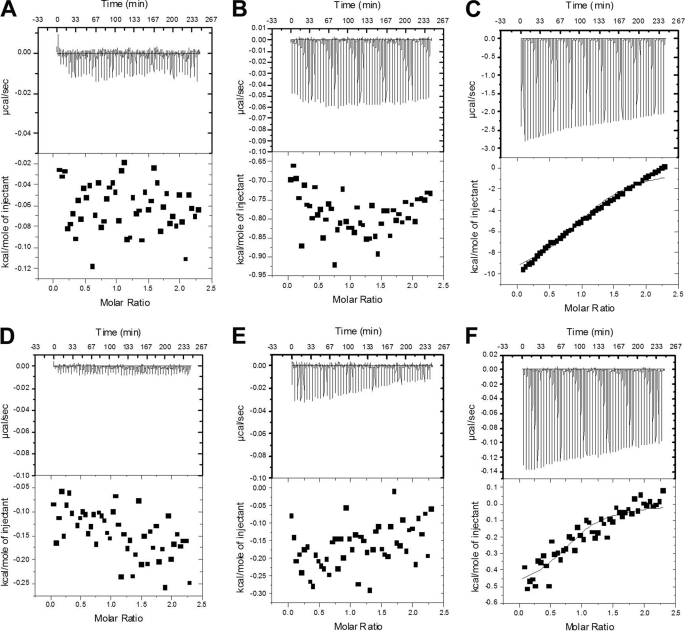
We suggested that buffer choice, including the use of glycerol, may play a role in why Falk et al. (3) observed μm rather than nm affinity for cpSRP43 constructs (2). The use of high concentrations of glycerol in isothermal titration calorimetry (ITC) is known to cause experimental artifacts (6). Our control experiments clearly show that the use of glycerol, even at 5% v/v, causes significant background heat changes (Fig. 1). In their letter, Sinning and Falk report a 13 μm affinity even in the absence of glycerol, suggesting glycerol may not be the primary cause for the reported differences. Although species-specific differences in Alb3-Cterm could explain the observed affinity differences, comparing GTPase stimulation by Arabidopsis and Pisum sativum Alb3-Cterm does not support this possibility (Fig. 2).
FIGURE 1.
Isothermal titration calorimetry investigation of the influence of glycerol in various buffers in buffer to buffer experiments. ITC was conducted by injecting a specific buffer/glycerol formulation into a sample well containing the same buffer/glycerol formulation. Buffers were: 10 mm phosphate, 100 mm NaCl, 50 mm AMS, pH 6.5 (A); 10 mm phosphate, 100 mm NaCl, 50 mm AMS, 2.5% glycerol (v/v), pH 6.5 (B); 10 mm phosphate, 100 mm NaCl, 50 mm AMS, 5% glycerol (v/v), pH 6.5 (C); ITC Buffer (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP (D); ITC Buffer with 2.5% glycerol (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP, 2.5% glycerol (v/v) (E); and ITC Buffer with 5% glycerol (Falk et al. (3)): 20 mm HEPES/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm TCEP, 5% glycerol (v/v) (F). Although polyols such as glycerol are frequently used to stabilize proteins, they cannot be assumed innocuous.
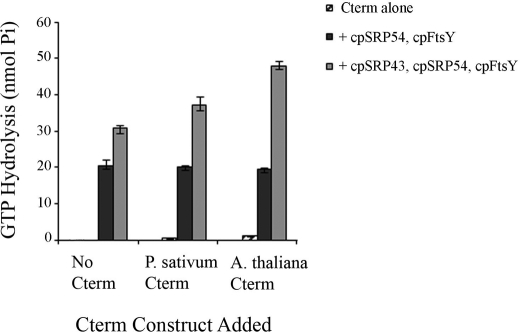
FIGURE 2.
Comparison of the ability of Pisum sativum and Arabidopsis thaliana Alb3-Cterm peptide to stimulate cpSRP43-dependent GTP hydrolysis by the cpSRP GTPases. The effect of Alb3-Cterm on the GTP hydrolysis activity of cpSRP54 and cpFtsY was examined in the presence or absence of cpSRP43. Assays contained 150 pmol (1 μm final concentration) of cpSRP43, cpSRP54, and cpFtsY and 4000 pmol (27 μm final) of P. sativum or A. thaliana Alb3-Cterm as indicated with 2 mm GTP. GTPase activity resulting in the release of inorganic phosphate (Pi) was determined according to Gonzalez and Romo (7) using known phosphate standards. The average and standard deviation were calculated from three separate experiments. In conclusion, peptides corresponding to both P. sativum and A. thaliana are able to increase GTP hydrolysis in a cpSRP43-dependent manner, which is as expected given that the heterologous system has been repeatedly shown to be fully functional in reconstituting LHCP integration (see also Fig. 3).
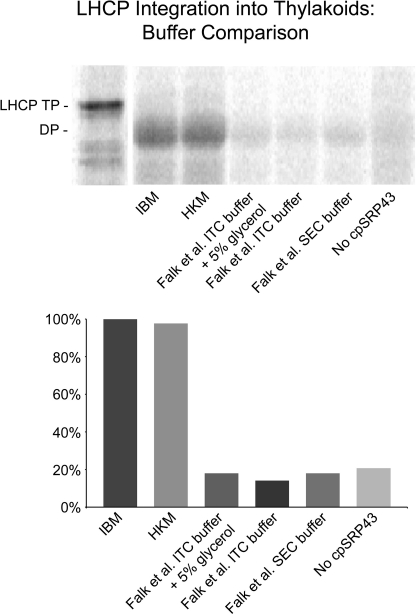
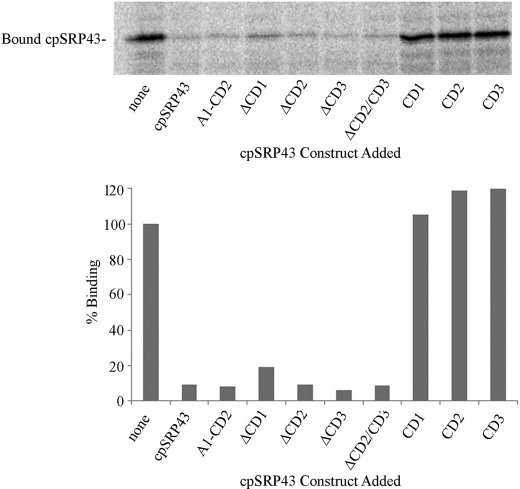
Published reports (2, 4, 5) supporting the physiological relevance of high affinity protein interactions still suggest that the low affinity of cpSRP43 for Alb3-Cterm reported by Falk et al. (3) stems from assay conditions unfavorable for observing the primary targeting interaction that takes place between Alb3-Cterm and the Ank region of cpSRP43. Importantly, buffers used by Falk et al. (3) in ITC and size-exclusion experiments do not support LHCP integration (Fig. 3). In addition, Ank-containing cpSRP43 constructs, including those that lack CD2 and/or CD3, are able to prevent binding of radiolabeled cpSRP43 to Alb3 in salt-washed thylakoids whereas chromodomains do not (Fig. 4).
FIGURE 3.
Buffer influence on LHCP integration. Salt-washed thylakoids in IBM were incubated with 5 mm ATP, 1 mm GTP, 12.5 μl of radiolabeled pLHCP translation product, and recombinant cpSRP43, cpSRP54, and cpFtsY (2). The final volume was brought to 150 μl in IBM or to 150 μl with a final concentration matching the buffer listed: 50 mm Hepes/KOH pH 8.0, 330 mm sorbitol, 10 mm MgCl2 (IBM); 10 mm Hepes/KOH pH 8.0, 10 mm MgCl2 (HKM); 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 5% (w/v) glycerol, 0.25 mm tris(2-carboxyethyl)phosphine) (ITC Buffer + 5% glycerol (Falk et al. (3)); 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 0.25 mm tris(2-carboxyethyl)phosphine) (ITC Buffer (Falk et al. (3)); and 20 mm Hepes/NaOH pH 7.5, 200 mm NaCl, 2 mm MgCl2, 1 mm EDTA, 1 mm DTT (SEC Buffer (Falk et al. (3)). Reactions were incubated at 25 °C for 30 min under light. Membranes were collected by centrifugation at 3200 × g for 6 min at 4 °C and protease-treated with thermolysin. Protease-treated membranes were solubilized in SDS buffer, heated, and analyzed by SDS-PAGE and phosphorimaging. IQ Solutions software (Molecular Dynamics) was used to quantify pLHCP degradation product (indicated as DP), which is indicative of properly inserted LHCP. Each integration assay was compared with the level of integration in IBM (set to 100%). Integration in HKM, used by Lewis et al. (2) for ITC and protein interaction/function assays, is equally as efficient as IBM. Buffers used by Falk et al. (3) do not support integration at a level above the negative control, which lacked cpSRP43.
FIGURE 4.
Competition for cpSRP43 binding to the C terminus of Alb3 in salt-washed thylakoids. Salt-washed thylakoids (equivalent to 75 μg of chlorophyll) and 8 nmol of recombinant cpSRP43 construct as indicated were incubated for 15 min at 25 °C in light. Equal amounts of radiolabeled cpSRP43 were added to each tube and incubated an additional 30 min under the same conditions. Samples were pelleted, washed, and analyzed via SDS-PAGE and phosphorimaging for radiolabeled cpSRP43. The graph depicts the amount of radiolabeled cpSRP43 bound to salt-washed thylakoids relative to the amount recovered when no recombinant protein was added. It has been previously demonstrated that cpSRP43 binding to salt-washed thylakoids takes place through a cpSRP43/Alb3-Cterm interaction (2). As shown, all of the ankyrin region-containing constructs (including cpSRP43 lacking CD2CD3) were able to prevent cpSRP43 binding to the C terminus of Alb3 in salt-washed thylakoids whereas chromodomains 1, 2, and 3 did not prevent cpSRP43 binding to thylakoids.
References
- 1.Falk S., Sinning I. (2010) J. Biol. Chem. http://www.jbc.org/cgi/content/full/285/53/le25 [DOI] [PMC free article] [PubMed]
- 2.Lewis N. E., Marty N. J., Kathir K. M., Rajalingam D., Kight A. D., Daily A., Kumar T. K., Henry R. L., Goforth R. L. (2010) J. Biol. Chem. 285, 34220–34230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Falk S., Ravaud S., Koch J., Sinning I. (2010) J. Biol. Chem. 285, 5954–5962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goforth R. L., Peterson E. C., Yuan J., Moore M. J., Kight A. D., Lohse M. B., Sakon J., Henry R. L. (2004) J. Biol. Chem. 279, 43077–43084 [DOI] [PubMed] [Google Scholar]
- 5.Tzvetkova-Chevolleau T., Hutin C., Noel L. D., Goforth R., Carde J.-P., Caffarri S., Sinning I., Groves M., Teulon J.-M., Hoffman N. E., Henry R., Havaux M., Nussaume L. (2007) Plant Cell 19, 1635–1648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milev S., Bosshard H.R., Jelesarov I. (2005) Biochemistry 44, 285–293 [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Romo P., Sanchez-Nieto S., Gavilanes-Ruiz M. (1992) Anal. Biochem. 200, 235–238 [DOI] [PubMed] [Google Scholar]