Abstract
Background
Collagen-induced arthritis (CIA) is an often-used murine model for human rheumatoid arthritis (RA). Earlier studies have shown potent anti-arthritic effects with the female sex hormone estradiol and the selective estrogen receptor modulator (SERM) raloxifene in CIA in DBA/1-mice. B10.Q-ncf1*/*mice are B10.Q mice with a mutated Ncf1 gene. In B10.Q-ncf1*/*mice, CIA develops as a chronic relapsing disease, which more accurately mimics human RA. We investigated the role of endogenous and exogenous sex steroids and raloxifene in the course of this model of chronic arthritis. We also examined whether treatment would prevent the development of inflammation-triggered generalized osteoporosis.
Methods
Female B10.Q-ncf1*/*mice were sham-operated or ovariectomized, and CIA was induced. 22 days later, when 30% of the mice had developed arthritis, treatment with raloxifene, estradiol or vehicle was started, and the clinical disease was evaluated continuously. Treatment was continued until day 56 after immunization. At termination of the experiment (day 73), bone mineral density (BMD) was analyzed, paws were collected for histological examination, and sera were analyzed for markers of cartilage turnover and pro-inflammatory cytokines.
Results
Raloxifene and estradiol treatment, as well as endogenous estrogen, decreased the frequency of arthritis, prevented joint destruction and countered generalized osteoporosis. These effects were associated with lower serum levels of the pro-inflammatory cytokine IL-6.
Conclusions
This is the first study to show that raloxifene and estradiol can ameliorate established erosive arthritis and inflammation-triggered osteoporosis in this chronic arthritis model. We propose that treatment with raloxifene could be a beneficial addition to the treatment of postmenopausal RA.
Background
Rheumatoid arthritis (RA) is a joint destructing autoimmune disease affecting 0.5-1% of the adult population [1,2]. The distribution between men and women is 1:3, with a peak incidence during menopause and in the post-partum period [3]. Several studies, including a population-based case-control study [4], have investigated whether the use of oral contraceptives could have an impact on the development of RA. Most of these studies found that current or ever use of oral contraceptives do have a protective effect (reviewed in [5]). The use of hormone replacement therapy (HRT) has been associated with some beneficial effects on disease activity [6-9]. For instance, a prospective two-year trial of 88 postmenopausal women with RA found that estrogen-containing HRT ameliorated clinical disease, retarded joint destruction, and increased bone mineral density (BMD) [6]. Estradiol-treatment of collagen-induced arthritis (CIA) in mice also suppressed disease progression [10,11], and blocking of the estrogen receptors enhanced the disease [12]. CIA is a model of human RA, and has been widely used to investigate disease mechanisms and treatments [13]. We have previously shown potent anti-arthritic effects of the selective estrogen receptor modulator (SERM) raloxifene in CIA in mice, when raloxifene was given as prophylaxis, therapy or in severe established disease [14,15]. Raloxifene hampered arthritis development, joint destruction and the development of generalized osteoporosis to the same degree as estradiol treatment. The rationale for using raloxifene instead of HRT is that estrogen treatment has been shown to increase the risk for cancer of the breast and uterus, as well as stroke, whereas raloxifene treatment does not have these side effects [16-18].
A polymorphism of the Ncf1 gene regulates the severity of arthritis in rats and mice, and has been shown to be caused by NADPH oxidase deficiency [19]. This results in a lower oxidative burst in macrophages, leading to spontaneous arthritis during the postpartum period, and to a more severe chronic relapsing collagen-induced arthritis disease in B10.Q mice with a mutated Ncf1 gene (B10.Q-ncf1*/*mice) [20,21]. The importance of reactive oxygen species in human RA was recently investigated in a Swedish case-control cohort consisting of 1842 RA cases and 1038 controls [22]. They found a genetic association between RA and the NADPH-oxidase complex, thus supporting the previous findings from animal models.
The role of endogenous and exogenous sex hormones in this chronic arthritis model has not previously been studied. We also wanted to investigate whether raloxifene would have a beneficial effect in this model. In addition we evaluated if treatment would prevent arthritis-induced osteoporosis, which is prominent in CIA [23] and postmenopausal RA [24,25], but has not previously been reported in arthritic B10.Q-ncf1*/* mice.
Methods
Animals and experimental procedures
The ethical committee for animal experiments at Göteborg University approved this study. Female B10.Q-ncf1*/* mice were generated as previously described [20]. Mice were electronically tagged and kept, 5 to 10 animals per cage, under standard environmental conditions, and fed standard laboratory chow and tap water ad libitum.
Ovariectomy and sham operations were performed at 7-19 weeks of age. Mice of different ages were mixed in each cage to avoid differences between the treatment groups. Ovaries were removed through a midline incision of the skin, and flank incisions of the peritoneum. The skin incision was closed with metallic clips. Sham-operated animals had their ovaries exposed but not removed. Surgery was performed after ketamine (PfizerAB, Täby, Sweden) and medetomidin (OrionPharma, Espoo, Finland) anesthesia. Carprofen (OrionPharma) was used post-operatively as a painkiller.
Induction and evaluation of arthritis
2 weeks after ovariectomy CIA was induced by immunization with 100 μg of chicken type II collagen (CII) (Sigma, St Louis, MO, USA) dissolved in 0.1 M acetic acid and emulsified with an equal volume of incomplete Freund's adjuvant (Sigma) supplemented with 0.5 mg/ml Mycobacterium tuberculosis (Sigma). A total volume of 100 μl was injected intradermally at the base of the tail. Mice had already developed arthritis three weeks after immunization. Arthritis frequency was evaluated continuously until termination of the experiment. Scoring was performed in a blinded way without knowledge of the treatment groups and previous scores, as described previously [26], scoring 1 to 3 in each paw (maximum of 12 points per mouse) as follows: 1, swelling or erythema in one joint; 2, swelling or erythema in two joints; 3, severe swelling of the entire paw or ankylosis. The experiment was terminated 73 days after immunization.
Treatment
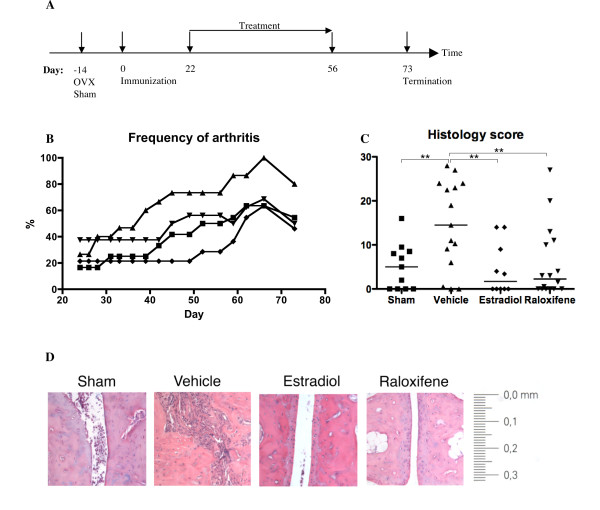
Mice were given subcutaneous injections at different locations 5 days per week of the raloxifene analogue LY117018 (generous gift from Eli Lilly, Indianapolis, IN, USA) (60 μg/mouse/day) or 17β-estradiol-3-benzoate (E2) (Sigma) (1.0 μg/mouse/day) dissolved in Miglyol812 (OmyaPeralta GmbH, Hamburg, Germany). Miglyol812 is a very pure neutral oil from fractionated plant fatty acids C8 and C10. OVX and sham control mice received Miglyol812 (100 μl/mouse/day). The dosages of raloxifene and E2 have previously been shown to equally well prevent osteoporosis in mice [27-29]. Treatment with raloxifene, estradiol or vehicle five days per week was started therapeutically, 3 weeks after immunization, when approximately 30% of the mice had developed arthritis. Treatment was ended on day 56 after immunization, and the mice were followed until day 73, when they were sacrificed (Figure 1A).
Figure 1.
Treatment with raloxifene or estradiol hampered arthritis development and joint destruction. Female B10.Q-ncf1*/* mice were sham-operated or ovariectomized, and immunized with collagen II and Freund's incomplete adjuvant supplemented with mycobacteria to induce CIA. Three weeks later treatment was started with raloxifene (60 μg/day; n = 15) (turned triangles), estradiol (1 μg/day; n = 11) (prisms), or vehicle control (Miglyol812) for sham-operated mice (n = 11; squares) and OVX controls (n = 15; triangles). Treatment was continued until day 56 after immunization, and the experiment was terminated on day 73. A: Diagram of the experimental timeline. OVX, ovariectomy; sham, sham operation; immunization with collagen II and Freund's complete adjuvant on day 0. B: Frequency of arthritis. Treatment with raloxifene in OVX mice delayed the onset and the frequency of arthritis compared to vehicle-treated OVX controls (P < 0.05 for the entire experiment). The presence of endogenous hormones (sham-operated mice) or estradiol treatment also delayed the onset of arthritis and decreased the frequency (P < 0.001 and P < 0.001, respectively). There was a significant difference between estradiol treatment and raloxifene treatment (P < 0.001). Kruskall-Wallis test with post hoc comparison was used. C: Histopathologic destruction scores of paw sections. Scores were evaluated in a blinded manner, with the proximal part of each paw graded 0-4, and the distal part graded 0-3, yielding a maximum score of 28 per mouse, as follows: 1 = synovial hypertrophy; 2 = pannus, erosions of cartilage and bone; 3 = severe erosions of cartilage and bone; 4 = complete ankylosis. The scatter plot shows the scores of individual mice, and lines show the median in each group. Kruskall-Wallis test with post hoc comparison was used, **P < 0.01. D: Representative images of paw tissue sections, revealing treatment effects on histologic features in each group.
LY117018 differs from raloxifene at only one site on the molecule, with a pyrrolidine ring on the basic side chain instead of a piperidine ring. This small difference does not affect its biological properties [30].
Tissue collection and histologic examination
At termination of the experiment, mice were anaesthetized for blood withdrawal, and then killed by cervical dislocation. Sera were individually collected and stored at -20°C until used. One femur was placed in formaldehyde for 24 hours, and then in 70% ethanol, for analysis of bone mineral density. Paws were fixated in paraformaldehyde, decalcified for 30 hours in Parengy's decalcification solution (Histolab Products AB, Gothenburg, Sweden), and dehydrated over night in a vacuum infiltrator processor (Tissue-Tek V.I.P., Sakura Finetechnical Co. Ltd., Tokyo, Japan). After being embedded in paraffin they were sectioned in 4 μm sections on a Leica RM2255 (Leica Instruments GmbH, Nussloch, Germany). The sections were deparaffinated and stained in Mayer's hematoxylin followed by eosin. The sections were coded before examination. In sections from each animal, the proximal parts of all four paws were graded separately on a scale of 0-4, and the distal part was graded 0-3. This yielded a maximum histopathological destruction score of 28 points per mouse, assessed as follows: 1 = synovial hypertrophy, 2 = pannus, discrete erosions of cartilage and bone, 3 = severe erosions of cartilage and bone, 4 = complete ankylosis.
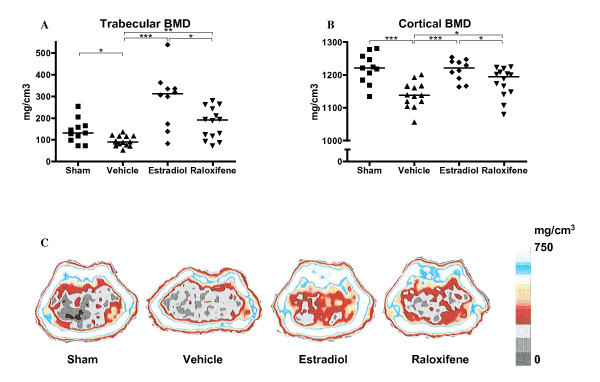
Assessment of BMD
One femur was subjected to a peripheral quantitative computed tomography (pQCT) scan with a Stratec pQCT XCT Research M, software version 5.4B (Norland, Fort Atkinson, WI) at a resolution of 70 μm, as described previously [31]. Trabecular BMD was determined with a metaphyseal scan at a point 3% of the length of the femur from the growth plate. The inner 45% of the area was defined as the trabecular bone compartment. Cortical BMD was determined with a mid-diaphyseal scan.
Identification of serologic markers of cartilage remodeling
As a marker of cartilage destruction, serum levels of COMP (cartilage oligomeric matrix protein) were determined with an Animal COMP® ELISA kit (AnaMar Medical AB, Uppsala, Sweden).
Serum IL-6 bioassay
A bioassay using the cell line B13.29, subclone B9 (which is dependent on IL-6 for growth) was used to measure serum levels of IL-6, as described previously [32,33].
Statistical analysis
For statistical evaluation, the Kruskall-Wallis test followed by a post hoc test were used for comparisons between all groups in each experiment. A P value ≤ 0.05 was considered significant.
Results
Endogenous and exogenous estrogen as well as raloxifene treatment hampered arthritis in B10.Q-ncf1*/* mice and protected joints from destruction
To examine the anti-arthritic properties of sex steroids, ovariectomized female B10.Q-ncf1*/* mice were treated therapeutically from day 22 until day 56 post immunization, with either raloxifene (60 μg/day), estradiol (1.0 μg/day) or vehicle (Miglyol 812). Sham operated controls received Miglyol 812. Arthritis development was evaluated continuously. Treatment was ended on day 56 to investigate how this would affect the disease.
The cages of mice were randomly divided into the different treatment groups in a blinded way, without knowledge of the arthritic score. As shown in Figure 1B, the raloxifene treatment group had a frequency of arthritis of 40% at the start of treatment, whereas the other groups only displayed 20% of mice with arthritis. In spite of this difference, our results clearly show that raloxifene treatment resulted in a slower onset of arthritis and a lower frequency of disease, compared to vehicle treated mice (P < 0.05 for the entire experiment). We also found a significantly slower onset of disease in sham-operated mice and in mice treated with estradiol compared to vehicle controls (P < 0.001 and P < 0.001, respectively), and between estradiol treatment and raloxifene treatment (P < 0.001).
Histological examination of sections from the paws revealed severe destruction of the joints in the vehicle treated controls. In contrast, examination of joints from raloxifene and estradiol treated mice revealed a lower destruction score (P < 0.01) (Figure 1C and 1D). Similarly, endogenous estrogen (sham-operated mice) protected the joints from destruction compared to OVX controls (P < 0.01).
Endogenous and exogenous estrogen, and raloxifene treatment counteracted osteoporosis in arthritic mice
All mice in this experiment displayed low bone mineral density (BMD) compared to historic non-arthritic controls [23], where we have previously found the trabecular and cortical BMD to be 275 and 1270 mg/cm3, respectively. Vehicle treated OVX mice developed severe loss of trabecular and cortical BMD compared to sham-operated mice (P < 0.05 and P < 0.001), indicating a protective effect of endogenous sex hormones. In contrast, treatment with raloxifene and estradiol resulted in preserved trabecular and cortical BMD, with estradiol treated mice displaying the highest BMD (P < 0.05 and P < 0.001, respectively) (Figure 2).
Figure 2.
Treatment with raloxifene or estradiol protected arthritic mice from osteoporosis. Scatter plots showing the trabecular bone mineral density (BMD) (A) and the cortical BMD (B), measured with peripheral quantitative computer tomography (pQCT) in mice with CIA treated with raloxifene (60 μg/day), E2 (1 μg/day) or vehicle Miglyol812 (100 μl/day), n = 11-15 in each group. Lines represent medians. Kruskall-Wallis test with post hoc comparison was used, *P < 0.05, **P < 0.01, ***P < 0.001. C: Representative pQCT scans of cross-sections of the femur, showing the bone mineral density (BMD). The pictures show one representative mouse in each treatment group. The bar shows the density of the bone, from 0 (black) to >750 mg/cm3 (white).
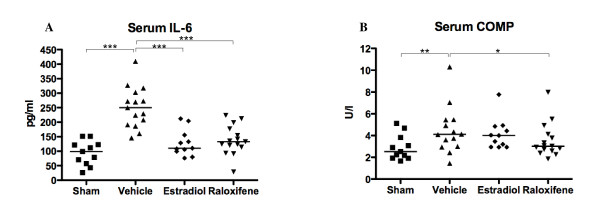
Endogenous and exogenous estrogen, and raloxifene treatment lowered the serum levels of IL-6
Increased serum IL-6 in arthritis is a marker of the general inflammatory disease. The serum levels of IL-6 were significantly lower (P < 0.001) in mice treated with raloxifene or estradiol, and in sham operated mice, than in vehicle controls (Figure 3A).
Figure 3.
Treatment with raloxifene decreased the serum levels of IL-6 and COMP. Scatter plots showing the serum levels of IL-6 (A) and cartilage oligomeric matrix protein (COMP) (B) in mice with CIA treated with raloxifene (60 μg/day), E2 (1 μg/day) or vehicle Miglyol812 (100 μl/day). n = 11-15 in each group. Lines represent medians. Kruskall-Wallis test with post hoc comparison was used, *P < 0.05, **P < 0.01, ***P < 0.001.
Endogenous estrogen and raloxifene treatment decreased cartilage resorption
The serum levels of COMP (cartilage oligomeric matrix protein) are elevated in arthritis due to increased destruction of joint cartilage. Treatment with raloxifene significantly decreased cartilage destruction, as indicated by lower serum COMP levels, compared to vehicle treated mice (Figure 3B). Interestingly, sham-operated mice also had lower levels of COMP, whereas estradiol-treated mice displayed similar levels as vehicle-treated controls.
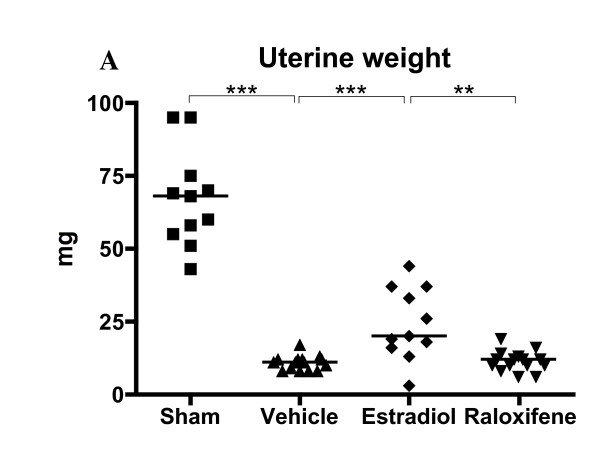
Raloxifene did not affect uterine weight
Treatment of the mice was ended on day 56 post immunization, and the experiment was terminated on day 73. As shown in Figure 4, treatment with estradiol resulted in partly preserved increased uterine weight compared with OVX mice, even 2.5 weeks after the last injection, whereas raloxifene did not. This may be due to the cessation of treatment 2.5 weeks before termination of the experiment. Previous studies have found differential effects of raloxifene in uterine tissue. We have previously found a slight proliferative effect in mice treated for 2.5-3 weeks [28,34], whereas in another study mice were treated with a higher dose for 5 days, and they found no proliferative effect [35]. No increase in uterine weight was found in rats [36-38]. Most importantly, raloxifene was found to have antiproliferative and proapoptotic effects in women [39].
Figure 4.
Treatment with raloxifene did not increase uterine weight. Scatter plots showing the uterine weights in mice with CIA treated with raloxifene (60 μg/day), E2 (1 μg/day) or vehicle Miglyol812 (100 μl/day) from day 22 to day 56 after immunization, and terminated on day 73, n = 11-15 in each group. Lines represent medians. Kruskall-Wallis test with post hoc comparison was used, **P < 0.01, ***P < 0.001.
Discussion
This is the first study to evaluate the impact of endogenous ovarian hormones, as well as exogenous estradiol and raloxifene, in the chronic arthritis model of B10.Q-ncf1*/* mice. This model may more accurately relay the pathophysiologic process of RA than the widely used CIA model in DBA/1 mice, since it develops into a chronic active disease course [20]. We clearly show that the presence of ovarian hormones protects from development of arthritis and joint destruction. Interestingly, treatment with a physiological dose of estradiol or the selective estrogen receptor modulator raloxifene was equally efficient, although the treatment was given to already arthritic mice from day 22 to day 56 after collagen II immunization. The serum levels of COMP were significantly decreased in the raloxifene treated mice and mice with intact endogenous hormones compared to vehicle controls. This indicates a lower degree of on-going cartilage destruction. COMP was not decreased in the estradiol treated mice, although this group also displayed significantly less cartilage destruction. This finding is in accordance with the results in a recent study of CIA in rats demonstrating that estradiol exerts collagen type II and cartilage protective properties [40].
Women are more prone to develop RA than men, and the female peak incidence coincides with menopause, indicating that endogenous female sex hormones like estradiol have protective properties. Thus, we early proposed that treatment with estradiol would have disease-retarding effects in postmenopausal RA. The rationale for investigating the possible impact of raloxifene in this model is that previous studies have shown ameliorating effects of hormone replacement therapy containing estradiol in women with postmenopausal RA, but long-term therapy is no longer recommended due to the risk of side effects. We have previously shown, in the murine CIA model, that raloxifene treatment prophylactically or as therapy, or even when initiated in established disease, had a great ameliorating effect on arthritis, as well as on joint destruction and arthritis-induced osteoporosis [14,15].
Raloxifene binds with high affinity to estrogen receptor (ER) α, and acts as an estrogen agonist on bone tissue and blood lipids, but as an antagonist on breast and reproductive tissues [41]. We have recently shown that signaling through ERα (using propylpyrazoletriol) dramatically decreased the frequency and severity of arthritis, and prevented osteoporosis development, in CIA in DBA/1 mice, whereas signaling through the other estrogen receptors (ERβ and GPR30) did not affect arthritic disease or bone loss [42]. Raloxifene acts mainly via ERα, and its agonistic effects on this receptor were clearly seen in the current study, with the same anti-arthritic and anti-osteoporotic effects as estradiol.
We also recently showed that raloxifene activates the estrogen response element to the same extent as estradiol in bone, uterus and thymus, thus indicating that indeed the effects are mediated via the nuclear estrogen receptors [34]. Raloxifene has previously been shown to have a weak proliferative effect on mouse uterus, whereas estradiol displays a strong proliferative effect [28]. In the present study we found a lingering increase in uterus weight in mice treated with estradiol even though treatment had been halted 17 days before termination. No increase compared to vehicle treated mice was seen in the raloxifene treated group at termination, which may be due to the absence of treatment for the last 17 days of the experiment.
The serum levels of IL-6 were reduced in mice with intact endogenous sex hormones, or treated with estradiol or raloxifene. IL-6 targeting therapy is now being used to treat autoimmune diseases, including RA, and shows promising results with a significant clinical response and amelioration of joint damage (reviewed in [43]). In addition, it was recently shown that blockade of the IL-6 receptor both in vivo and in vitro directly affected osteoclast formation, suggesting a direct bone-sparing effect that is independent of the anti-inflammatory effects of anti-IL-6 treatment [44]. Thus, the lowering of serum IL-6 may be one mechanism by which raloxifene and estradiol exert their anti-osteoporotic and anti-arthritic effects.
Conclusions
Based on the results of this study in the chronic arthritis model and our previous results in the more acute CIA model, we suggest using raloxifene treatment at the dose already approved for the treatment of osteoporosis (60 mg/day) as an addition to the standard treatment of postmenopausal RA. This could be a beneficial adjuvant with benefits on both joint damage and osteoporosis, and without the side effects of hormone replacement therapy with estradiol. A clinical trial addressing this issue is planned.
Abbreviations
BMD: bone mineral density; CIA: collagen-induced arthritis; CII: type II collagen; COMP: cartilage oligomeric matrix protein; E2: estradiol; ELISA: enzyme-linked immunosorbent assay; ER: estrogen receptor; HRT: hormone replacement therapy; IL: interleukin; OVX: ovariectomy; pQCT: peripheral quantitative computed tomography; RA: rheumatoid arthritis; SERM: selective estrogen receptor modulator.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
HC, RH, CO and IG participated in study design, interpretation of data and manuscript preparation. UI aided with analysis of data and statistical analysis. ME, CE and ML aided with acquisition of data. The study was performed mainly by CJ. All authors read and approved the final manuscript
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Caroline Jochems, Email: caroline.jochems@rheuma.gu.se.
Ulrika Islander, Email: ulrika.islander@rheuma.gu.se.
Malin Erlandsson, Email: malin.erlandsson@rheuma.gu.se.
Cecilia Engdahl, Email: cecilia.engdahl@rheuma.gu.se.
Marie Lagerquist, Email: marie.lagerquist@medic.gu.se.
Inger Gjertsson, Email: inger.gjertsson@rheuma.gu.se.
Claes Ohlsson, Email: claes.ohlsson@medic.gu.se.
Rikard Holmdahl, Email: rikard.holmdahl@ki.se.
Hans Carlsten, Email: hans.carlsten@rheuma.gu.se.
Acknowledgements
We thank Margareta Rosenkvist, Berit Eriksson, Anette Hansevi, and Maud Petersson for excellent technical assistance. This study was supported by grants from the Medical Faculty of Göteborg University (ALF), Göteborg Medical Society, King GustavV's 80 years' foundation, the Sahlgrenska Foundation, the NovoNordic Foundation, the Börje Dahlin foundation, the Association against Rheumatism, Reumaforskningsfond Margareta, the Swedish Research Council the Strategic Science Foundation (SSF) in Sweden, and EU (Masterswitch HEALTH-F2-2008-223404).
References
- Kvien TK, Glennas A, Knudsrod OG, Smedstad LM, Mowinckel P, Forre O. The prevalence and severity of rheumatoid arthritis in Oslo. Results from a county register and a population survey. Scand J Rheumatol. 1997;26:412–418. doi: 10.3109/03009749709065712. [DOI] [PubMed] [Google Scholar]
- Doran MF, Pond GR, Crowson CS, O'Fallon WM, Gabriel SE. Trends in incidence and mortality in rheumatoid arthritis in Rochester, Minnesota, over a forty-year period. Arthritis Rheum. 2002;46:625–631. doi: 10.1002/art.509. [DOI] [PubMed] [Google Scholar]
- Kvien TK, Uhlig T, Odegard S, Heiberg MS. Epidemiological aspects of rheumatoid arthritis: the sex ratio. Ann N Y Acad Sci. 2006;1069:212–222. doi: 10.1196/annals.1351.019. [DOI] [PubMed] [Google Scholar]
- Brennan P, Bankhead C, Silman A, Symmons D. Oral contraceptives and rheumatoid arthritis: results from a primary care-based incident case-control study. Semin Arthritis Rheum. 1997;26:817–823. doi: 10.1016/S0049-0172(97)80025-X. [DOI] [PubMed] [Google Scholar]
- Oliver JE, Silman AJ. Risk factors for the development of rheumatoid arthritis. Scand J Rheumatol. 2006;35:169–174. doi: 10.1080/03009740600718080. [DOI] [PubMed] [Google Scholar]
- Forsblad D'Elia H, Larsen A, Mattsson LA, Waltbrand E, Kvist G, Mellstrom D, Saxne T, Ohlsson C, Nordborg E, Carlsten H. Influence of hormone replacement therapy on disease progression and bone mineral density in rheumatoid arthritis. J Rheumatol. 2003;30:1456–1463. [PubMed] [Google Scholar]
- Bijlsma JW, Huber-Bruning O, Thijssen JH. Effect of oestrogen treatment on clinical and laboratory manifestations of rheumatoid arthritis. Ann Rheum Dis. 1987;46:777–779. doi: 10.1136/ard.46.10.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall GM, Daniels M, Huskisson EC, Spector TD. A randomised controlled trial of the effect of hormone replacement therapy on disease activity in postmenopausal rheumatoid arthritis. Ann Rheum Dis. 1994;53:112–116. doi: 10.1136/ard.53.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald AG, Murphy EA, Capell HA, Bankowska UZ, Ralston SH. Effects of hormone replacement therapy in rheumatoid arthritis: a double blind placebo-controlled study. Ann Rheum Dis. 1994;53:54–57. doi: 10.1136/ard.53.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Andersson M. Female sex hormones suppress development of collagen-induced arthritis in mice. Arthritis Rheum. 1986;29:1501–1509. doi: 10.1002/art.1780291212. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Oestrogen induced suppression of collagen arthritis. IV: Progesterone alone does not affect the course of arthritis but enhances the oestrogen-mediated therapeutic effect. J Reprod Immunol. 1989;15:141–150. doi: 10.1016/0165-0378(89)90033-8. [DOI] [PubMed] [Google Scholar]
- Jansson L, Holmdahl R. Enhancement of collagen-induced arthritis in female mice by estrogen receptor blockage. Arthritis Rheum. 2001;44:2168–2175. doi: 10.1002/1529-0131(200109)44:9<2168::AID-ART370>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Trentham DE, Townes AS, Kang AH. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977;146:857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems C, Islander U, Kallkopf A, Lagerquist M, Ohlsson C, Carlsten H. Role of raloxifene as a potent inhibitor of experimental postmenopausal polyarthritis and osteoporosis. Arthritis Rheum. 2007;56:3261–3270. doi: 10.1002/art.22873. [DOI] [PubMed] [Google Scholar]
- Jochems C, Lagerquist M, Hakansson C, Ohlsson C, Carlsten H. Long-term anti-arthritic and anti-osteoporotic effects of raloxifene in established experimental postmenopausal polyarthritis. Clin Exp Immunol. 2008;152:593–597. doi: 10.1111/j.1365-2249.2008.03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossouw JE, Anderson GL, Prentice RL, LaCroix AZ, Kooperberg C, Stefanick ML, Jackson RD, Beresford SA, Howard BV, Johnson KC. et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. Jama. 2002;288:321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- Beral V. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–427. doi: 10.1016/S0140-6736(03)14596-5. [DOI] [PubMed] [Google Scholar]
- Anderson GL, Limacher M, Assaf AR, Bassford T, Beresford SA, Black H, Bonds D, Brunner R, Brzyski R, Caan B. et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. Jama. 2004;291:1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- Olofsson P, Holmberg J, Tordsson J, Lu S, Akerstrom B, Holmdahl R. Positional identification of Ncf1 as a gene that regulates arthritis severity in rats. Nat Genet. 2003;33:25–32. doi: 10.1038/ng1058. [DOI] [PubMed] [Google Scholar]
- Hultqvist M, Olofsson P, Holmberg J, Backstrom BT, Tordsson J, Holmdahl R. Enhanced autoimmunity, arthritis, and encephalomyelitis in mice with a reduced oxidative burst due to a mutation in the Ncf1 gene. Proc Natl Acad Sci USA. 2004;101:12646–12651. doi: 10.1073/pnas.0403831101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelderman KA, Hultqvist M, Pizzolla A, Zhao M, Nandakumar KS, Mattsson R, Holmdahl R. Macrophages suppress T cell responses and arthritis development in mice by producing reactive oxygen species. J Clin Invest. 2007;117:3020–3028. doi: 10.1172/JCI31935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson LM, Lindqvist AK, Kallberg H, Padyukov L, Burkhardt H, Alfredsson L, Klareskog L, Holmdahl R. A case-control study of rheumatoid arthritis identifies an associated single nucleotide polymorphism in the NCF4 gene, supporting a role for the NADPH-oxidase complex in autoimmunity. Arthritis Res Ther. 2007;9:R98. doi: 10.1186/ar2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jochems C, Islander U, Erlandsson M, Verdrengh M, Ohlsson C, Carlsten H. Osteoporosis in experimental postmenopausal polyarthritis: the relative contributions of estrogen deficiency and inflammation. Arthritis Res Ther. 2005;7:R837–843. doi: 10.1186/ar1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinigaglia L, Nervetti A, Mela Q, Bianchi G, Del Puente A, Di Munno O, Frediani B, Cantatore F, Pellerito R, Bartolone S. et al. A multicenter cross sectional study on bone mineral density in rheumatoid arthritis. Italian Study Group on Bone Mass in Rheumatoid Arthritis. J Rheumatol. 2000;27:2582–2589. [PubMed] [Google Scholar]
- Forsblad D'Elia H, Larsen A, Waltbrand E, Kvist G, Mellstrom D, Saxne T, Ohlsson C, Nordborg E, Carlsten H. Radiographic joint destruction in postmenopausal rheumatoid arthritis is strongly associated with generalised osteoporosis. Ann Rheum Dis. 2003;62:617–623. doi: 10.1136/ard.62.7.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmdahl R, Jansson L, Larsson E, Rubin K, Klareskog L. Homologous type II collagen induces chronic and progressive arthritis in mice. Arthritis Rheum. 1986;29:106–113. doi: 10.1002/art.1780290114. [DOI] [PubMed] [Google Scholar]
- Erlandsson MC, Gomori E, Taube M, Carlsten H. Effects of raloxifene, a selective estrogen receptor modulator, on thymus, T cell reactivity, and inflammation in mice. Cell Immunol. 2000;205:103–109. doi: 10.1006/cimm.2000.1719. [DOI] [PubMed] [Google Scholar]
- Erlandsson MC, Jonsson CA, Lindberg MK, Ohlsson C, Carlsten H. Raloxifene- and estradiol-mediated effects on uterus, bone and B lymphocytes in mice. J Endocrinol. 2002;175:319–327. doi: 10.1677/joe.0.1750319. [DOI] [PubMed] [Google Scholar]
- Onoe Y, Miyaura C, Ito M, Ohta H, Nozawa S, Suda T. Comparative effects of estrogen and raloxifene on B lymphopoiesis and bone loss induced by sex steroid deficiency in mice. J Bone Miner Res. 2000;15:541–549. doi: 10.1359/jbmr.2000.15.3.541. [DOI] [PubMed] [Google Scholar]
- Levine L. Tamoxifen and the Rafoxifene analog LY117018: their effects on arachidonic acid release from cells in culture and on prostaglandin I2 production by rat liver cells. BMC Cancer. 2004;4:49. doi: 10.1186/1471-2407-4-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windahl SH, Vidal O, Andersson G, Gustafsson JA, Ohlsson C. Increased cortical bone mineral content but unchanged trabecular bone mineral density in female ERbeta(-/-) mice. J Clin Invest. 1999;104:895–901. doi: 10.1172/JCI6730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bremell T, Abdelnour A, Tarkowski A. Histopathological and serological progression of experimental Staphylococcus aureus arthritis. Infect Immun. 1992;60:2976–2985. doi: 10.1128/iai.60.7.2976-2985.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helle M, Boeije L, Aarden LA. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988;18:1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Engdahl C, Jochems C, Gustafsson JA, van der Saag PT, Carlsten H, Lagerquist MK. In vivo activation of gene transcription via oestrogen response elements by a raloxifene analogue. J Endocrinol. 2009;203:349–356. doi: 10.1677/JOE-09-0012. [DOI] [PubMed] [Google Scholar]
- Al-Jamal JH, Dubin NH. The effect of raloxifene on the uterine weight response in immature mice exposed to 17beta-estradiol, 1,1,1-trichloro-2, 2-bis(p-chlorophenyl)ethane, and methoxychlor. Am J Obstet Gynecol. 2000;182:1099–1102. doi: 10.1067/mob.2000.105407. [DOI] [PubMed] [Google Scholar]
- Black LJ, Sato M, Rowley ER, Magee DE, Bekele A, Williams DC, Cullinan GJ, Bendele R, Kauffman RF, Bensch WR. et al. Raloxifene (LY139481 HCI) prevents bone loss and reduces serum cholesterol without causing uterine hypertrophy in ovariectomized rats. J Clin Invest. 1994;93:63–69. doi: 10.1172/JCI116985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M, Bryant HU, Iversen P, Helterbrand J, Smietana F, Bemis K, Higgs R, Turner CH, Owan I, Takano Y, Burr DB. Advantages of raloxifene over alendronate or estrogen on nonreproductive and reproductive tissues in the long-term dosing of ovariectomized rats. J Pharmacol Exp Ther. 1996;279:298–305. [PubMed] [Google Scholar]
- Sato M, Rippy MK, Bryant HU. Raloxifene, tamoxifen, nafoxidine, or estrogen effects on reproductive and nonreproductive tissues in ovariectomized rats. Faseb J. 1996;10:905–912. doi: 10.1096/fasebj.10.8.8666168. [DOI] [PubMed] [Google Scholar]
- Palomba S, Orio F, Russo T, Falbo A, Tolino A, Lombardi G, Cimini V, Zullo F. Antiproliferative and proapoptotic effects of raloxifene on uterine leiomyomas in postmenopausal women. Fertil Steril. 2005;84:154–161. doi: 10.1016/j.fertnstert.2004.12.058. [DOI] [PubMed] [Google Scholar]
- Nielsen RH, Christiansen C, Stolina M, Karsdal MA. Oestrogen exhibits type II collagen protective effects and attenuates collagen-induced arthritis in rats. Clin Exp Immunol. 2008;152:21–27. doi: 10.1111/j.1365-2249.2008.03594.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Eckert S, Krueger KA, Grady D, Powles TJ, Cauley JA, Norton L, Nickelsen T, Bjarnason NH, Morrow M. et al. The effect of raloxifene on risk of breast cancer in postmenopausal women: results from the MORE randomized trial. Multiple Outcomes of Raloxifene Evaluation. Jama. 1999;281:2189–2197. doi: 10.1001/jama.281.23.2189. [DOI] [PubMed] [Google Scholar]
- Engdahl C, Jochems C, Windahl SH, Börjesson AE, Ohlsson C, Carlsten H, Lagerquist MK. Amelioration of Collagen-Induced Arthritis and Immune-Associated Bone Loss Through Signaling via Estrogen Receptor. Arthritis and Rheumatism. 2010;62(2):524–33. doi: 10.1002/art.25055. [DOI] [PubMed] [Google Scholar]
- Ding C, Cicuttini F, Li J, Jones G. Targeting IL-6 in the treatment of inflammatory and autoimmune diseases. Expert Opin Investig Drugs. 2009;18:1457–1466. doi: 10.1517/13543780903203789. [DOI] [PubMed] [Google Scholar]
- Axmann R, Bohm C, Kronke G, Zwerina J, Smolen J, Schett G. Inhibition of interleukin-6 receptor directly blocks osteoclast formation in vitro and in vivo. Arthritis Rheum. 2009;60:2747–2756. doi: 10.1002/art.24781. [DOI] [PubMed] [Google Scholar]