Abstract
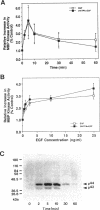
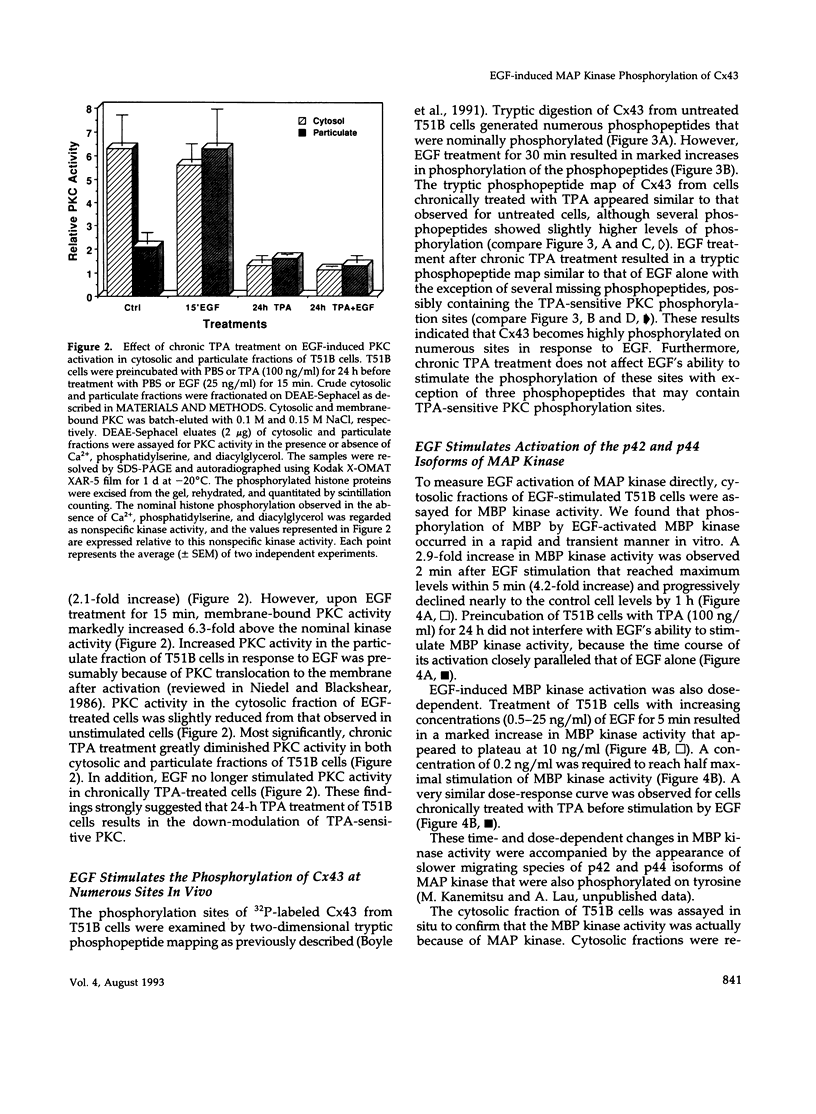
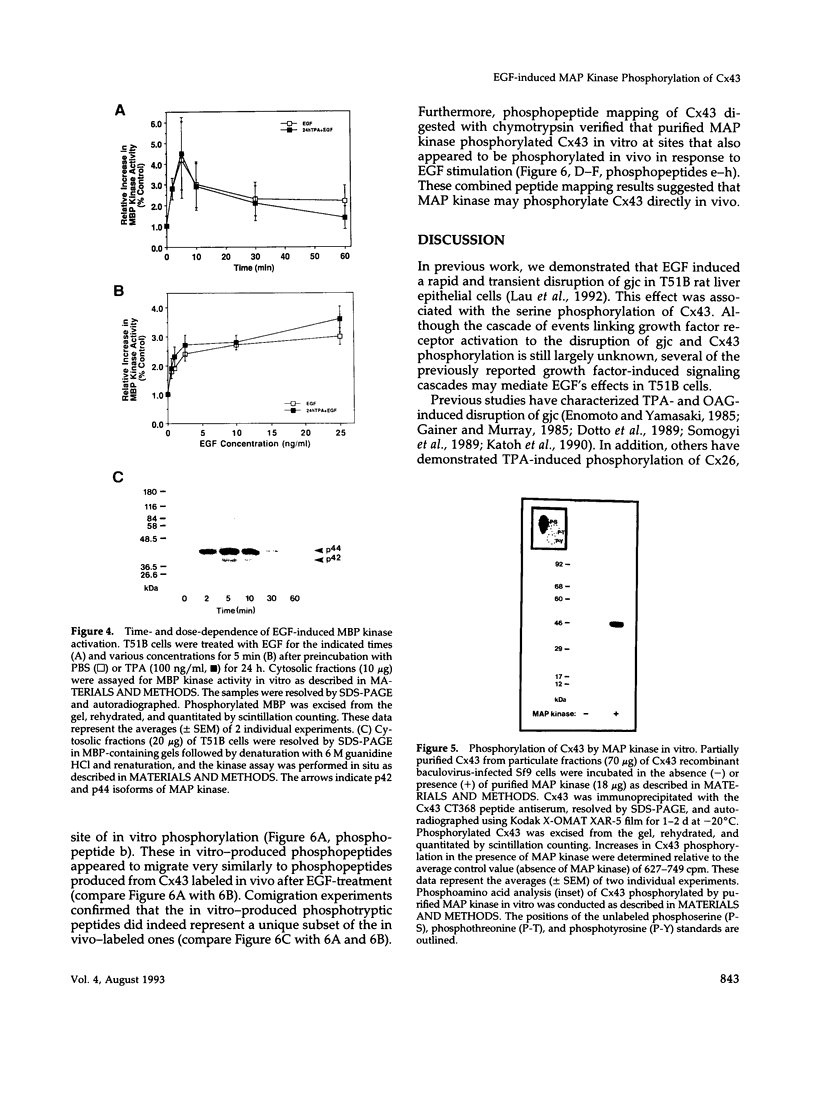
We previously reported that epidermal growth factor (EGF) induced the disruption of gap junctional communication (gjc) and serine phosphorylation of connexin43 (Cx43) in T51B rat liver epithelial cells. However, the cascade of events linking EGF receptor activation to these particular responses have not been fully characterized. Furthermore, the serine kinase(s) acting directly on Cx43 remain unidentified. In the current study, we demonstrate that downmodulation of 12-0-tetradecanoylphorbol 13-acetate (TPA)-sensitive protein kinase C (PKC) activity does not affect EGF's ability to reduce junctional permeability or phosphorylate Cx43 in T51B cells. EGF in the presence or absence of chronic TPA treatment stimulated marked increases in Cx43 phosphorylation on numerous sites as determined by two-dimensional tryptic phosphopeptide mapping. Computer-assisted sequence analysis of Cx43 identified several protein kinase phosphorylation consensus sites including two sites for mitogen-activated protein (MAP) kinase. EGF stimulated activation of MAP kinase in a time- and dose-dependent manner where the kinetics of kinase activity corroborated its possible involvement in mediating EGF's effects. Moreover, purified MAP kinase directly phosphorylated Cx43 on serine residues in vitro. Two-dimensional tryptic and chymotryptic phosphopeptide mapping demonstrated that the in vitro phosphopeptides represented a specific subset of the in vivo phosphopeptides produced in response to EGF after chronic TPA treatment. Therefore, EGF-induced disruption of gjc and phosphorylation of Cx43 may be mediated in part by MAP kinase in vivo.
Full text
PDF


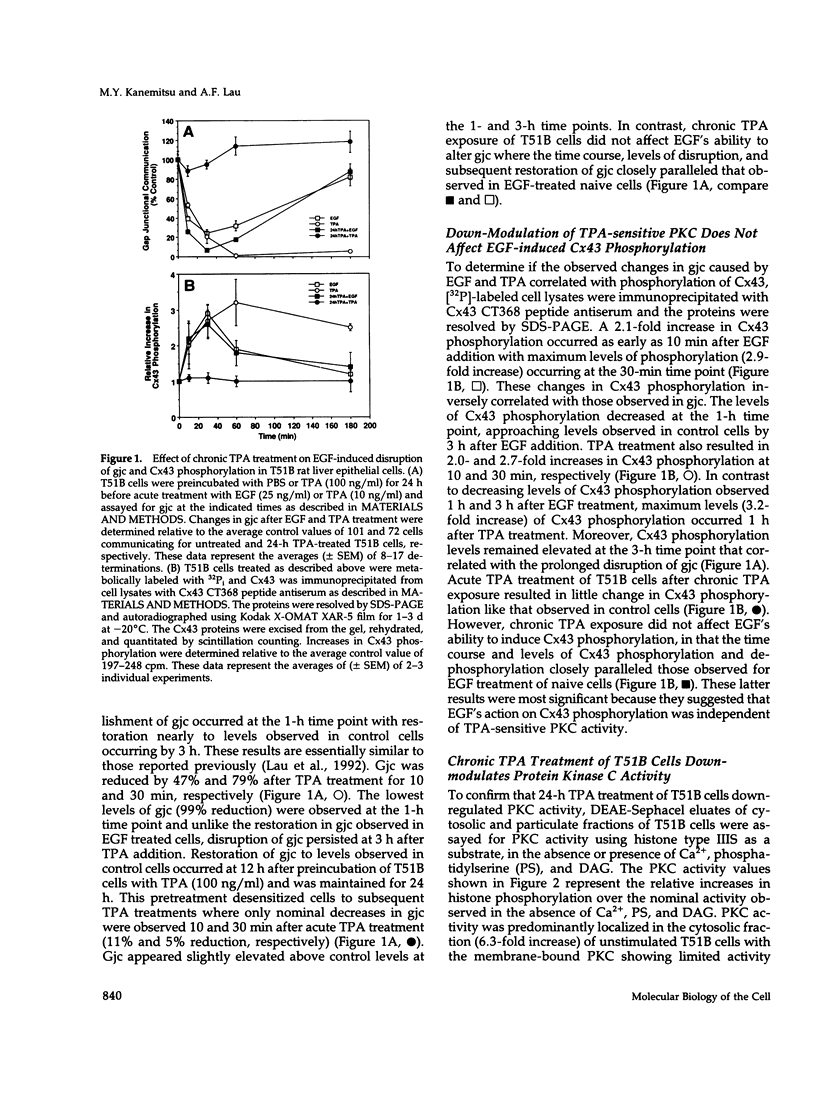






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. D., Parker P. J. Activation of mitogen-activated protein (MAP) kinase by a MAP kinase-kinase. J Biol Chem. 1992 Jul 5;267(19):13135–13137. [PubMed] [Google Scholar]
- Ahn N. G., Krebs E. G. Evidence for an epidermal growth factor-stimulated protein kinase cascade in Swiss 3T3 cells. Activation of serine peptide kinase activity by myelin basic protein kinases in vitro. J Biol Chem. 1990 Jul 15;265(20):11495–11501. [PubMed] [Google Scholar]
- Ahn N. G., Seger R., Bratlien R. L., Diltz C. D., Tonks N. K., Krebs E. G. Multiple components in an epidermal growth factor-stimulated protein kinase cascade. In vitro activation of a myelin basic protein/microtubule-associated protein 2 kinase. J Biol Chem. 1991 Mar 5;266(7):4220–4227. [PubMed] [Google Scholar]
- Ahn N. G., Weiel J. E., Chan C. P., Krebs E. G. Identification of multiple epidermal growth factor-stimulated protein serine/threonine kinases from Swiss 3T3 cells. J Biol Chem. 1990 Jul 15;265(20):11487–11494. [PubMed] [Google Scholar]
- Alvarez E., Northwood I. C., Gonzalez F. A., Latour D. A., Seth A., Abate C., Curran T., Davis R. J. Pro-Leu-Ser/Thr-Pro is a consensus primary sequence for substrate protein phosphorylation. Characterization of the phosphorylation of c-myc and c-jun proteins by an epidermal growth factor receptor threonine 669 protein kinase. J Biol Chem. 1991 Aug 15;266(23):15277–15285. [PubMed] [Google Scholar]
- Anderson N. G., Maller J. L., Tonks N. K., Sturgill T. W. Requirement for integration of signals from two distinct phosphorylation pathways for activation of MAP kinase. Nature. 1990 Feb 15;343(6259):651–653. doi: 10.1038/343651a0. [DOI] [PubMed] [Google Scholar]
- Atkinson M. M., Menko A. S., Johnson R. G., Sheppard J. R., Sheridan J. D. Rapid and reversible reduction of junctional permeability in cells infected with a temperature-sensitive mutant of avian sarcoma virus. J Cell Biol. 1981 Nov;91(2 Pt 1):573–578. doi: 10.1083/jcb.91.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarnia R., Loewenstein W. R. Intercellular communication and the control of growth: X. Alteration of junctional permeability by the src gene. A study with temperature-sensitive mutant Rous sarcoma virus. J Membr Biol. 1984;82(3):191–205. doi: 10.1007/BF01871629. [DOI] [PubMed] [Google Scholar]
- Azarnia R., Reddy S., Kmiecik T. E., Shalloway D., Loewenstein W. R. The cellular src gene product regulates junctional cell-to-cell communication. Science. 1988 Jan 22;239(4838):398–401. doi: 10.1126/science.2447651. [DOI] [PubMed] [Google Scholar]
- Berthoud V. M., Ledbetter M. L., Hertzberg E. L., Sáez J. C. Connexin43 in MDCK cells: regulation by a tumor-promoting phorbol ester and Ca2+. Eur J Cell Biol. 1992 Feb;57(1):40–50. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Gregory J. S., Cobb M. H. Purification and properties of extracellular signal-regulated kinase 1, an insulin-stimulated microtubule-associated protein 2 kinase. Biochemistry. 1991 Jan 8;30(1):278–286. doi: 10.1021/bi00215a038. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Brissette J. L., Kumar N. M., Gilula N. B., Dotto G. P. The tumor promoter 12-O-tetradecanoylphorbol-13-acetate and the ras oncogene modulate expression and phosphorylation of gap junction proteins. Mol Cell Biol. 1991 Oct;11(10):5364–5371. doi: 10.1128/mcb.11.10.5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao T. S., Byron K. L., Lee K. M., Villereal M., Rosner M. R. Activation of MAP kinases by calcium-dependent and calcium-independent pathways. Stimulation by thapsigargin and epidermal growth factor. J Biol Chem. 1992 Oct 5;267(28):19876–19883. [PubMed] [Google Scholar]
- Clark S. G., Stern M. J., Horvitz H. R. C. elegans cell-signalling gene sem-5 encodes a protein with SH2 and SH3 domains. Nature. 1992 Mar 26;356(6367):340–344. doi: 10.1038/356340a0. [DOI] [PubMed] [Google Scholar]
- Countaway J. L., Northwood I. C., Davis R. J. Mechanism of phosphorylation of the epidermal growth factor receptor at threonine 669. J Biol Chem. 1989 Jun 25;264(18):10828–10835. [PubMed] [Google Scholar]
- Crow D. S., Beyer E. C., Paul D. L., Kobe S. S., Lau A. F. Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol. 1990 Apr;10(4):1754–1763. doi: 10.1128/mcb.10.4.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crow D. S., Kurata W. E., Lau A. F. Phosphorylation of connexin43 in cells containing mutant src oncogenes. Oncogene. 1992 May;7(5):999–1003. [PubMed] [Google Scholar]
- Dent P., Haser W., Haystead T. A., Vincent L. A., Roberts T. M., Sturgill T. W. Activation of mitogen-activated protein kinase kinase by v-Raf in NIH 3T3 cells and in vitro. Science. 1992 Sep 4;257(5075):1404–1407. doi: 10.1126/science.1326789. [DOI] [PubMed] [Google Scholar]
- Dermietzel R., Hwang T. K., Spray D. S. The gap junction family: structure, function and chemistry. Anat Embryol (Berl) 1990;182(6):517–528. doi: 10.1007/BF00186458. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., el-Fouly M. H., Nelson C., Trosko J. E. Similar and synergistic inhibition of gap-junctional communication by ras transformation and tumor promoter treatment of mouse primary keratinocytes. Oncogene. 1989 May;4(5):637–641. [PubMed] [Google Scholar]
- Enomoto T., Yamasaki H. Rapid inhibition of intercellular communication between BALB/c 3T3 cells by diacylglycerol, a possible endogenous functional analogue of phorbol esters. Cancer Res. 1985 Aug;45(8):3706–3710. [PubMed] [Google Scholar]
- Erickson A. K., Payne D. M., Martino P. A., Rossomando A. J., Shabanowitz J., Weber M. J., Hunt D. F., Sturgill T. W. Identification by mass spectrometry of threonine 97 in bovine myelin basic protein as a specific phosphorylation site for mitogen-activated protein kinase. J Biol Chem. 1990 Nov 15;265(32):19728–19735. [PubMed] [Google Scholar]
- Ferrell J. E., Jr, Martin G. S. Identification of a 42-kilodalton phosphotyrosyl protein as a serine(threonine) protein kinase by renaturation. Mol Cell Biol. 1990 Jun;10(6):3020–3026. doi: 10.1128/mcb.10.6.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filson A. J., Azarnia R., Beyer E. C., Loewenstein W. R., Brugge J. S. Tyrosine phosphorylation of a gap junction protein correlates with inhibition of cell-to-cell communication. Cell Growth Differ. 1990 Dec;1(12):661–668. [PubMed] [Google Scholar]
- Flagg-Newton J. L., Dahl G., Loewenstein W. R. Cell junction and cyclic AMP: 1. Upregulation of junctional membrane permeability and junctional membrane particles by administration of cyclic nucleotide or phosphodiesterase inhibitor. J Membr Biol. 1981;63(1-2):105–121. doi: 10.1007/BF01969452. [DOI] [PubMed] [Google Scholar]
- Flagg-Newton J., Simpson I., Loewenstein W. R. Permeability of the cell-to-cell membrane channels in mammalian cell juncton. Science. 1979 Jul 27;205(4404):404–407. doi: 10.1126/science.377490. [DOI] [PubMed] [Google Scholar]
- Gainer H. S., Murray A. W. Diacylglycerol inhibits gap junctional communication in cultured epidermal cells: evidence for a role of protein kinase C. Biochem Biophys Res Commun. 1985 Feb 15;126(3):1109–1113. doi: 10.1016/0006-291x(85)90300-6. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Sakai H. Okadaic acid activates microtubule-associated protein kinase in quiescent fibroblastic cells. Eur J Biochem. 1990 Nov 13;193(3):671–674. doi: 10.1111/j.1432-1033.1990.tb19385.x. [DOI] [PubMed] [Google Scholar]
- Hattori S., Fukuda M., Yamashita T., Nakamura S., Gotoh Y., Nishida E. Activation of mitogen-activated protein kinase and its activator by ras in intact cells and in a cell-free system. J Biol Chem. 1992 Oct 5;267(28):20346–20351. [PubMed] [Google Scholar]
- Haystead T. A., Dent P., Wu J., Haystead C. M., Sturgill T. W. Ordered phosphorylation of p42mapk by MAP kinase kinase. FEBS Lett. 1992 Jul 13;306(1):17–22. doi: 10.1016/0014-5793(92)80828-5. [DOI] [PubMed] [Google Scholar]
- Hernández-Sotomayor S. M., Carpenter G. Epidermal growth factor receptor: elements of intracellular communication. J Membr Biol. 1992 Jun;128(2):81–89. doi: 10.1007/BF00231881. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Kamps M. P., Sefton B. M. Acid and base hydrolysis of phosphoproteins bound to immobilon facilitates analysis of phosphoamino acids in gel-fractionated proteins. Anal Biochem. 1989 Jan;176(1):22–27. doi: 10.1016/0003-2697(89)90266-2. [DOI] [PubMed] [Google Scholar]
- Katoh F., Fitzgerald D. J., Giroldi L., Fujiki H., Sugimura T., Yamasaki H. Okadaic acid and phorbol esters: comparative effects of these tumor promoters on cell transformation, intercellular communication and differentiation in vitro. Jpn J Cancer Res. 1990 Jun-Jul;81(6-7):590–597. doi: 10.1111/j.1349-7006.1990.tb02614.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennelly P. J., Krebs E. G. Consensus sequences as substrate specificity determinants for protein kinases and protein phosphatases. J Biol Chem. 1991 Aug 25;266(24):15555–15558. [PubMed] [Google Scholar]
- Klaunig J. E., Ruch R. J. Role of inhibition of intercellular communication in carcinogenesis. Lab Invest. 1990 Feb;62(2):135–146. [PubMed] [Google Scholar]
- Kyriakis J. M., App H., Zhang X. F., Banerjee P., Brautigan D. L., Rapp U. R., Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992 Jul 30;358(6385):417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lampe P. D., Johnson R. G. Phosphorylation of MP26, a lens junction protein, is enhanced by activators of protein kinase C. J Membr Biol. 1989 Feb;107(2):145–155. doi: 10.1007/BF01871720. [DOI] [PubMed] [Google Scholar]
- Lau A. F., Kanemitsu M. Y., Kurata W. E., Danesh S., Boynton A. L. Epidermal growth factor disrupts gap-junctional communication and induces phosphorylation of connexin43 on serine. Mol Biol Cell. 1992 Aug;3(8):865–874. doi: 10.1091/mbc.3.8.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loewenstein W. R. Junctional intercellular communication: the cell-to-cell membrane channel. Physiol Rev. 1981 Oct;61(4):829–913. doi: 10.1152/physrev.1981.61.4.829. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R., Rose B. The cell-cell channel in the control of growth. Semin Cell Biol. 1992 Feb;3(1):59–79. doi: 10.1016/s1043-4682(10)80008-x. [DOI] [PubMed] [Google Scholar]
- Lowenstein E. J., Daly R. J., Batzer A. G., Li W., Margolis B., Lammers R., Ullrich A., Skolnik E. Y., Bar-Sagi D., Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992 Aug 7;70(3):431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- Madhukar B. V., Oh S. Y., Chang C. C., Wade M., Trosko J. E. Altered regulation of intercellular communication by epidermal growth factor, transforming growth factor-beta and peptide hormones in normal human keratinocytes. Carcinogenesis. 1989 Jan;10(1):13–20. doi: 10.1093/carcin/10.1.13. [DOI] [PubMed] [Google Scholar]
- Maldonado P. E., Rose B., Loewenstein W. R. Growth factors modulate junctional cell-to-cell communication. J Membr Biol. 1988 Dec;106(3):203–210. doi: 10.1007/BF01872158. [DOI] [PubMed] [Google Scholar]
- Margolis B. Proteins with SH2 domains: transducers in the tyrosine kinase signaling pathway. Cell Growth Differ. 1992 Jan;3(1):73–80. [PubMed] [Google Scholar]
- Matsuda S., Kosako H., Takenaka K., Moriyama K., Sakai H., Akiyama T., Gotoh Y., Nishida E. Xenopus MAP kinase activator: identification and function as a key intermediate in the phosphorylation cascade. EMBO J. 1992 Mar;11(3):973–982. doi: 10.1002/j.1460-2075.1992.tb05136.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Mehta P. P., Bertram J. S., Loewenstein W. R. Growth inhibition of transformed cells correlates with their junctional communication with normal cells. Cell. 1986 Jan 17;44(1):187–196. doi: 10.1016/0092-8674(86)90497-6. [DOI] [PubMed] [Google Scholar]
- Mehta P. P., Hotz-Wagenblatt A., Rose B., Shalloway D., Loewenstein W. R. Incorporation of the gene for a cell-cell channel protein into transformed cells leads to normalization of growth. J Membr Biol. 1991 Dec;124(3):207–225. doi: 10.1007/BF01994355. [DOI] [PubMed] [Google Scholar]
- Meier K. E., Licciardi K. A., Haystead T. A., Krebs E. G. Activation of messenger-independent protein kinases in wild-type and phorbol ester-resistant EL4 thymoma cells. J Biol Chem. 1991 Jan 25;266(3):1914–1920. [PubMed] [Google Scholar]
- Meier K. E., Weiel J. E., Bloom T. J., Krebs E. G. Regulation of S6 kinase activity in Madin-Darby canine kidney renal epithelial cells. J Biol Chem. 1990 Mar 15;265(8):4635–4645. [PubMed] [Google Scholar]
- Moreno A. P., Fishman G. I., Spray D. C. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J. 1992 Apr;62(1):51–53. doi: 10.1016/S0006-3495(92)81775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990 Nov;111(5 Pt 1):2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakielny S., Cohen P., Wu J., Sturgill T. MAP kinase activator from insulin-stimulated skeletal muscle is a protein threonine/tyrosine kinase. EMBO J. 1992 Jun;11(6):2123–2129. doi: 10.1002/j.1460-2075.1992.tb05271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. The molecular heterogeneity of protein kinase C and its implications for cellular regulation. Nature. 1988 Aug 25;334(6184):661–665. doi: 10.1038/334661a0. [DOI] [PubMed] [Google Scholar]
- Northwood I. C., Davis R. J. Signal transduction by the epidermal growth factor receptor after functional desensitization of the receptor tyrosine protein kinase activity. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6107–6111. doi: 10.1073/pnas.87.16.6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northwood I. C., Gonzalez F. A., Wartmann M., Raden D. L., Davis R. J. Isolation and characterization of two growth factor-stimulated protein kinases that phosphorylate the epidermal growth factor receptor at threonine 669. J Biol Chem. 1991 Aug 15;266(23):15266–15276. [PubMed] [Google Scholar]
- Oh S. Y., Grupen C. G., Murray A. W. Phorbol ester induces phosphorylation and down-regulation of connexin 43 in WB cells. Biochim Biophys Acta. 1991 Sep 3;1094(2):243–245. doi: 10.1016/0167-4889(91)90016-q. [DOI] [PubMed] [Google Scholar]
- Olivier A. R., Parker P. J. Identification of multiple PKC isoforms in Swiss 3T3 cells: differential down-regulation by phorbol ester. J Cell Physiol. 1992 Aug;152(2):240–244. doi: 10.1002/jcp.1041520204. [DOI] [PubMed] [Google Scholar]
- Olivier J. P., Raabe T., Henkemeyer M., Dickson B., Mbamalu G., Margolis B., Schlessinger J., Hafen E., Pawson T. A Drosophila SH2-SH3 adaptor protein implicated in coupling the sevenless tyrosine kinase to an activator of Ras guanine nucleotide exchange, Sos. Cell. 1993 Apr 9;73(1):179–191. doi: 10.1016/0092-8674(93)90170-u. [DOI] [PubMed] [Google Scholar]
- Payne D. M., Rossomando A. J., Martino P., Erickson A. K., Her J. H., Shabanowitz J., Hunt D. F., Weber M. J., Sturgill T. W. Identification of the regulatory phosphorylation sites in pp42/mitogen-activated protein kinase (MAP kinase). EMBO J. 1991 Apr;10(4):885–892. doi: 10.1002/j.1460-2075.1991.tb08021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelicci G., Lanfrancone L., Grignani F., McGlade J., Cavallo F., Forni G., Nicoletti I., Grignani F., Pawson T., Pelicci P. G. A novel transforming protein (SHC) with an SH2 domain is implicated in mitogenic signal transduction. Cell. 1992 Jul 10;70(1):93–104. doi: 10.1016/0092-8674(92)90536-l. [DOI] [PubMed] [Google Scholar]
- Pomerance M., Schweighoffer F., Tocque B., Pierre M. Stimulation of mitogen-activated protein kinase by oncogenic Ras p21 in Xenopus oocytes. Requirement for Ras p21-GTPase-activating protein interaction. J Biol Chem. 1992 Aug 15;267(23):16155–16160. [PubMed] [Google Scholar]
- Pulverer B. J., Kyriakis J. M., Avruch J., Nikolakaki E., Woodgett J. R. Phosphorylation of c-jun mediated by MAP kinases. Nature. 1991 Oct 17;353(6345):670–674. doi: 10.1038/353670a0. [DOI] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Characterization of insulin-stimulated microtubule-associated protein kinase. Rapid isolation and stabilization of a novel serine/threonine kinase from 3T3-L1 cells. J Biol Chem. 1988 Sep 5;263(25):12721–12727. [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Insulin-stimulated microtubule-associated protein kinase is phosphorylated on tyrosine and threonine in vivo. Proc Natl Acad Sci U S A. 1988 Jun;85(11):3753–3757. doi: 10.1073/pnas.85.11.3753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray L. B., Sturgill T. W. Rapid stimulation by insulin of a serine/threonine kinase in 3T3-L1 adipocytes that phosphorylates microtubule-associated protein 2 in vitro. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1502–1506. doi: 10.1073/pnas.84.6.1502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossomando A. J., Payne D. M., Weber M. J., Sturgill T. W. Evidence that pp42, a major tyrosine kinase target protein, is a mitogen-activated serine/threonine protein kinase. Proc Natl Acad Sci U S A. 1989 Sep;86(18):6940–6943. doi: 10.1073/pnas.86.18.6940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozakis-Adcock M., McGlade J., Mbamalu G., Pelicci G., Daly R., Li W., Batzer A., Thomas S., Brugge J., Pelicci P. G. Association of the Shc and Grb2/Sem5 SH2-containing proteins is implicated in activation of the Ras pathway by tyrosine kinases. Nature. 1992 Dec 17;360(6405):689–692. doi: 10.1038/360689a0. [DOI] [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somogyi R., Batzer A., Kolb H. A. Inhibition of electrical coupling in pairs of murine pancreatic acinar cells by OAG and isolated protein kinase C. J Membr Biol. 1989 Jun;108(3):273–282. doi: 10.1007/BF01871742. [DOI] [PubMed] [Google Scholar]
- Sturgill T. W., Ray L. B., Erikson E., Maller J. L. Insulin-stimulated MAP-2 kinase phosphorylates and activates ribosomal protein S6 kinase II. Nature. 1988 Aug 25;334(6184):715–718. doi: 10.1038/334715a0. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Piwnica-Worms H., McNamee H., Paul D. L. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul. 1990 Dec;1(13):989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sáez J. C., Nairn A. C., Czernik A. J., Spray D. C., Hertzberg E. L., Greengard P., Bennett M. V. Phosphorylation of connexin 32, a hepatocyte gap-junction protein, by cAMP-dependent protein kinase, protein kinase C and Ca2+/calmodulin-dependent protein kinase II. Eur J Biochem. 1990 Sep 11;192(2):263–273. doi: 10.1111/j.1432-1033.1990.tb19223.x. [DOI] [PubMed] [Google Scholar]
- Takeda A., Hashimoto E., Yamamura H., Shimazu T. Phosphorylation of liver gap junction protein by protein kinase C. FEBS Lett. 1987 Jan 5;210(2):169–172. doi: 10.1016/0014-5793(87)81330-3. [DOI] [PubMed] [Google Scholar]
- Takishima K., Griswold-Prenner I., Ingebritsen T., Rosner M. R. Epidermal growth factor (EGF) receptor T669 peptide kinase from 3T3-L1 cells is an EGF-stimulated "MAP" kinase. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2520–2524. doi: 10.1073/pnas.88.6.2520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas S. M., DeMarco M., D'Arcangelo G., Halegoua S., Brugge J. S. Ras is essential for nerve growth factor- and phorbol ester-induced tyrosine phosphorylation of MAP kinases. Cell. 1992 Mar 20;68(6):1031–1040. doi: 10.1016/0092-8674(92)90075-n. [DOI] [PubMed] [Google Scholar]
- Tobe K., Kadowaki T., Tamemoto H., Ueki K., Hara K., Koshio O., Momomura K., Gotoh Y., Nishida E., Akanuma Y. Insulin and 12-O-tetradecanoylphorbol-13-acetate activation of two immunologically distinct myelin basic protein/microtubule-associated protein 2 (MBP/MAP2) kinases via de novo phosphorylation of threonine and tyrosine residues. J Biol Chem. 1991 Dec 25;266(36):24793–24803. [PubMed] [Google Scholar]
- Troppmair J., Bruder J. T., App H., Cai H., Liptak L., Szeberényi J., Cooper G. M., Rapp U. R. Ras controls coupling of growth factor receptors and protein kinase C in the membrane to Raf-1 and B-Raf protein serine kinases in the cytosol. Oncogene. 1992 Sep;7(9):1867–1873. [PubMed] [Google Scholar]
- Wang H. C., Erikson R. L. Activation of protein serine/threonine kinases p42, p63, and p87 in Rous sarcoma virus-transformed cells: signal transduction/transformation-dependent MBP kinases. Mol Biol Cell. 1992 Dec;3(12):1329–1337. doi: 10.1091/mbc.3.12.1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ways D. K., Cook P. P., Webster C., Parker P. J. Effect of phorbol esters on protein kinase C-zeta. J Biol Chem. 1992 Mar 5;267(7):4799–4805. [PubMed] [Google Scholar]
- Wood K. W., Sarnecki C., Roberts T. M., Blenis J. ras mediates nerve growth factor receptor modulation of three signal-transducing protein kinases: MAP kinase, Raf-1, and RSK. Cell. 1992 Mar 20;68(6):1041–1050. doi: 10.1016/0092-8674(92)90076-o. [DOI] [PubMed] [Google Scholar]
- Wu J., Michel H., Rossomando A., Haystead T., Shabanowitz J., Hunt D. F., Sturgill T. W. Renaturation and partial peptide sequencing of mitogen-activated protein kinase (MAP kinase) activator from rabbit skeletal muscle. Biochem J. 1992 Aug 1;285(Pt 3):701–705. doi: 10.1042/bj2850701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Enomoto T. Role of intercellular communication in BALB/c 3T3 cell transformation. Carcinog Compr Surv. 1985;9:179–194. [PubMed] [Google Scholar]
- Yamasaki H. Gap junctional intercellular communication and carcinogenesis. Carcinogenesis. 1990 Jul;11(7):1051–1058. doi: 10.1093/carcin/11.7.1051. [DOI] [PubMed] [Google Scholar]
- de Vries-Smits A. M., Burgering B. M., Leevers S. J., Marshall C. J., Bos J. L. Involvement of p21ras in activation of extracellular signal-regulated kinase 2. Nature. 1992 Jun 18;357(6379):602–604. doi: 10.1038/357602a0. [DOI] [PubMed] [Google Scholar]
- el-Fouly M. H., Trosko J. E., Chang C. C., Warren S. T. Potential role of the human Ha-ras oncogene in the inhibition of gap junctional intercellular communication. Mol Carcinog. 1989;2(3):131–135. doi: 10.1002/mc.2940020305. [DOI] [PubMed] [Google Scholar]